Abstract
The ability to examine genetically engineered mice in a chronic intravenous (IV) nicotine self-administration paradigm will be a powerful tool for investigating the contribution of specific genes to nicotine reinforcement and more importantly, to relapse behavior. Here we describe a reliable model of nicotine-taking and -seeking behavior in male C57BL/6J mice without prior operant training or food restriction. Mice were allowed to self-administer either nicotine (0.03 mg/kg/infusion) or saline in 2-hr daily sessions under fixed ratio 1 (FR1) followed by FR2 schedules of reinforcement. In the nicotine group, a dose-response curve was measured after the nose-poke behavior stabilized. Subsequently, nose-poke behavior was extinguished and ability of cue presentations, priming injections of nicotine, or intermittent footshock to reinstate responding was assessed in both groups. C57BL/6J mice given access to nicotine exhibited high levels of nose-poke behavior and self-administered a high number of infusions as compared to mice given access to saline. After this acquisition phase, changing the unit-dose of nicotine resulted in a flat dose-response curve for nicotine-taking and subsequently reinstatement of nicotine-seeking behavior was achieved by both nicotine-associated light cue presentation and intermittent footshock. Nicotine priming injections only triggered significant reinstatement on the second consecutive day of priming. In contrast, mice previously trained to self-administer saline did not increase their responding under those conditions.. These results demonstrate the ability to produce nicotine-taking and nicotine-seeking behavior in naive C57BL/6J mice without both prior operant training and food restriction. Future work will utilize these models to evaluate nicotine-taking and relapsing behavior in genetically-altered mice.
Keywords: nicotine, self-administration, seeking behavior, mice, relapse, dependence
1. INTRODUCTION
Nicotine is a primary reinforcing and addictive component in tobacco smoke [1]. Animal models of intravenous (IV) nicotine self-administration and reinstatement behavior provide the opportunity to study the mechanisms underlying nicotine reinforcement and relapse. IV nicotine self-administration has been well demonstrated in human subjects [2] and larger laboratory animal species such as squirrel monkeys [3, 4], beagle dogs [5], and rats [6–9]. Chronic IV nicotine self-administration in the rat has been used to examine the effects of environmental stimuli [10, 11], food restriction [12], pharmacological manipulations [13–20], sex [21], strain [22, 23] and even cortical inhibition [24] on nicotine intake. In the last decade, rat models of the reinstatement of drug-seeking behavior have been used extensively to study the mechanisms of drug relapse and screen potential therapeutics for preventing drug relapse [13–15]. One major limitation of a rat chronic IV nicotine self-administration paradigm is the inability to examine the contributions of specific gene products to nicotine reinforcement and relapse behavior through a knockout or transgenic strategy. Mouse models of chronic IV nicotine self-administration and relapse behavior would help identify the genes and encoded molecules necessary for nicotine reinforcement and relapsing behavior through the testing of knockout and transgenic mice available worldwide. To this end, scientists have established IV nicotine self-administration in mice through acute catheterization of the lateral tail vein [25–30]. Due to the short duration inherent of tail catheterization, this approach is not appropriate for studying chronic nicotine-taking behavior. Drug relapse, even after prolonged abstinence, is the greatest challenge for the treatment of drug addiction including tobacco smoking. Recently, several groups have examined IV nicotine self-administration and relapse behavior through catheterization of the mouse jugular vein [31–41]. This approach is desirable for studying both chronic nicotine-taking and relapsing behavior. Chronic IV nicotine self-administration paradigms can differ in many important aspects, including limited or continuous access to drug, time of day of drug access, environmental stimuli paired with the infusion, rate of drug delivery, whether the animals have restricted access to food, and whether the animals receive any prior operant training or drug exposure. Most of the studies available on chronic IV nicotine self-administration and relapse behavior have used mice with a history of prior operant training, food/water restriction, or both [31–34, 38, 40]. The characterization of IV nicotine self-administration and relapse behavior in mice with neither prior food training nor food restriction remains somewhat preliminary. Previously, we characterized methamphetamine IV self-administration and reinstatement behavior in naive C57BL/6J mice without either prior operant training or food restriction [42]. Using similar procedures, in the current set of experiments, naive male C57BL/6J mice (no prior operant training, no drug pre-exposure, and no food/water restriction) were used to establish behavioral models of a limited access (2-hr session), chronic IV nicotine self-administration for 10 days followed by extinction and reinstatement of nicotine-seeking behavior induced by either environmental cues (cue light), nicotine priming injections (i.p.), or stress (intermittent footshock).
2. MATERIALS AND METHODS
Naive male C57BL/6J mice were purchased from Toronto Centre for Phenogenomics (TCP) inhouse strains at 8 weeks old and weighed 25–30 g at the beginning of the experiments. All animals were kept in a regulated environment (21–22°C) with a 12-hr light/dark cycle (lights on at 7:00 A.M.). Both water and food were available ad libitum except during the experimental sessions (daily 2-hr session). All the experimental procedures described in this study were carried out in compliance with the guidelines of the Canadian Council on Animal Care and National Institute of Health (USA) guidelines. (−)Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline, and the pH of solution was adjusted to 7.0 ± 0.2. Nicotine solution was freshly prepared and filtered through a 0.22-mm syringe filter (Fisher Scientific, Pittsburgh, PA, USA) to minimize risk of infection. All nicotine doses are described as free base concentrations. Nicotine self-administered at the dose of 0.03 mg/kg/infusion over 1 s (infusion volume, 20 µl).
After a week of habituation to the colony room, naive male C57BL/6J mice were anesthetized with combination of xylazine (10 mg/kg, i.p.) and ketamine hydrochloride (75 mg/kg, i.p.). Indwelling catheters were constructed of micro-silicone tubing (inner diameter, 0.50 mm; outer diameter, 0.7 mm; IMG, Imamura Co., Ltd., Tokyo, Japan) and polyethylene tubing (inner diameter, 0.50 mm; outer diameter, 0.8 mm). Incisions were made on the skin of the head and ventral neck, and the right jugular vein was externalized. The end of the catheter was inserted into the jugular vein via a small incision and was secured to the vein and surrounding tissue with silk sutures. The exit port of the catheter passed subcutaneously to the top of the skull where it was attached to a modified 24-gauge cannula, which was secured to the mouse's skull with all-purpose Instant Krazy Glue (Krazy Glue, Columbus, OH USA). Metacam (meloxicam) was given for postoperative analgesia (0.13 ml/30g, s.c.) for at least three days. To extend catheter patency, the catheters were flushed once a day immediately after surgery or nicotine self-administration training with 0.05 ml of heparin in saline (30 Unit/ml; Fisher Scientific, Pittsburgh, PA, USA).
Nicotine and saline self-administration were conducted in standard mouse operant conditioning chambers (ENV–307A, Med Associates, Georgia, VT) located within ventilated sound attenuation cubicles. Briefly, the chambers were equipped with nose-poke sensors (ENV-313M, Med Associates) in two holes located on one side of the chamber 1.0 cm above the floor, cue- and hole-lamps located, respectively, above and in each hole, and a house light located on the top of the chamber opposite the holes. During self-administration training, one hole was defined as active, and the other, as inactive. Nose-poke responses in the active hole resulted in the activation of the infusion pump (PHM-100, Med Associates) and activation of the cue-lamp and hole-lamp (10 s). Nose-poke responses in the inactive hole, and in the active hole during the timeout period (10 s), had no programmed consequences but were recorded. The components of the infusion line were connected from the injector to the exit port of the mouse's catheter by PE20 tubing (Instech, Plymouth Meeting, PA), which was encased in steel spring leashes (Instech, Plymouth Meeting, PA). Swivels were suspended above the chamber. One pump/syringe set was used for each chamber located inside of the cubicle. The infusion pump/syringe set was outside of the cubicles.
After recovery from the catheterization (4–7 days), naive male C57BL/6J mice were initially subjected to daily 2-hr sessions of nicotine or saline self-administration under an FR1 schedule for 6 days, and the nicotine reinforcement schedule was then changed to FR2 for a period of 4 days. Based on previous reports of nicotine IV self-administration in C57BL/6J mice without prior operant training [38, 40, 41], 0.03 mg/kg/infusion (20 µl/infusion) was selected as the unit dose of nicotine for chronic IV self-administration in our study.
The animals that were judged to have acquired stable nicotine self-administration (the criteria were an average of at least 10 active nose-poke responses on the last 3 days of self-administration; 3 of 17 animals were excluded) were then given access to different unit-dose of nicotine in order to establish a dose-response curve (from higher to lower doses and saline, each dose for two consecutive daily 2-hr sessions). After establishment of the dose-response curve, the mice were subjected to daily 2-hr sessions of extinction training. Throughout the extinction session, the house light was on. The nicotine-associated cue- and hole-lamps, and the pump for nicotine infusions, were turned off. Therefore, nose-poke responses into the previously active hole resulted in neither an infusion of nicotine nor nicotine-associated cues (cue- and hole-lamps) though responding was recorded.
Once the extinction criterion was met (less than 30% of active responses in the stable phase of self-administration in 2 consecutive sessions), mice were subjected to a 2-hr session of cue-induced reinstatement testing on the next day. Animals generally required between 5–12 sessions before criterion were met though one animal (out of 13) failed to reach criterion throughout training. The cue-induced reinstatement tests were conducted under the same conditions as the nicotine self-administration under the FR2 schedule, except that nicotine was unavailable throughout the testing session. A non-contingent cue presentation was made at the start of the session along with contingent presentations made according to a FR2 schedule. Nose-poke responses in the previously active or inactive hole were counted as active and inactive, respectively.
After reinstatement testing was completed with cues, the animals were given saline injections (s.c.) 5 min prior to the start of all extinction sessions. Once extinction criteria were again achieved for 2 consecutive sessions, animals were tested for nicotine priming-induced reinstatement by being given a 0.15 mg/kg nicotine injection (s.c.) 5 min prior to the start of a regular extinction session. The timing and dose of the nicotine priming injection was based on a previous report using C57BL/6J mice [41] and our recent studies with rats [16, 18, 20, 24, 43]. This testing was repeated for a second consecutive day.
Following the completion of reinstatement testing with nicotine priming, all saline injections were ceased and the animals continued to undergo regular daily extinction sessions. Once extinction criteria were again achieved for 2 consecutive sessions, the animals were tested with intermittent footshock (0.22 mA intensity, 2-s shock duration, 5 shocks total) in the self-administration chamber for 5 min [41]. Immediately after the 5 min period of footshock, the houselight was turned on indicating the start of the session. The session was exactly similar to a normal extinction session.
A separate control group of mice underwent the same procedures as those conducted above, with the exception of the replacement of nicotine with saline as the self-administered solution and no dose-response testing.
Statistical analyses were conducted using all subjects. A two-way ANOVA with repeated measures was used for analysis of acquisition data, followed by Bonferroni post-hoc tests comparing active to inactive nose-pokes over the sessions. Paired t-tests were conducted for the cue- and footshock-induced reinstatement tests. A one-way ANOVA with repeated measures was used for analysis of nicotine priming-induced reinstatement tests, followed by Dunnett’s multiple comparison test used for post-hoc analysis. A statistical significance level of p<0.05 was used for all comparisons.
3. RESULTS
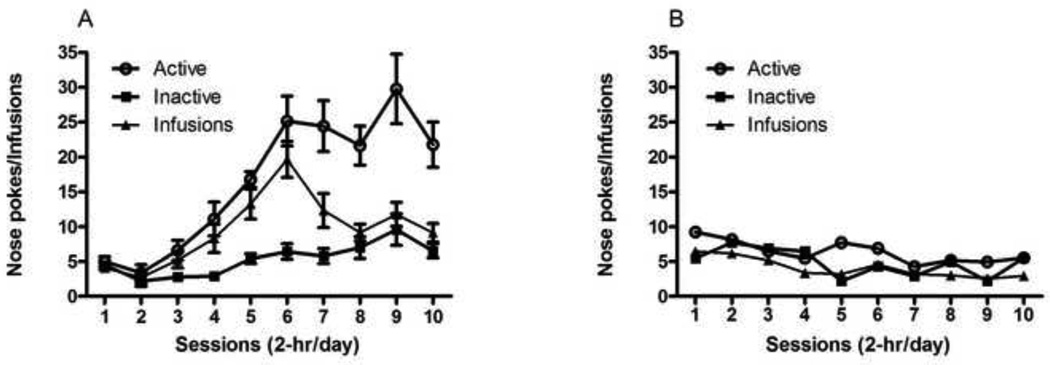
A two-way repeated measures ANOVA for nose-poke responses during nicotine self-administration (Fig. 1, A) revealed a significant effect of session (F(9,540) = 19.85), response type (F(1,540) = 78.16) and interaction (F(17,540) = 4.49). Bonferroni post hoc analysis revealed significant differences between active and inactive responses for session 4 (p < 0.01) and all subsequent sessions (p < 0.001). A similar ANOVA for nose-poke responses during saline self-administration (Fig. 1, B) revealed no significant effects of session (F(9,210) = 2.70), response type (F(1,210) = 6.44) or interaction (F(17,210) = 0.58). Bonferroni post hoc analysis revealed no significant difference between active and inactive responses for saline on any session.
Figure 1. Acquisition of nicotine or saline self-administration.
After catheter implantation and recovery, mice were trained to nose-poke for infusions of nicotine (0.03 mg/kg/infusion) (A) or saline (B). The schedule of reinforcement was maintained at a fixed ratio 1 (FR1) for a period of 6 sessions, followed by a FR2 schedule for a period of 4 sessions. Data is shown as mean (±SEM) number of nose-pokes or infusions. *p<0.05, **p<0.01, ***p<0.001, a two-way ANOVA followed by Bonferroni post hoc analysis.
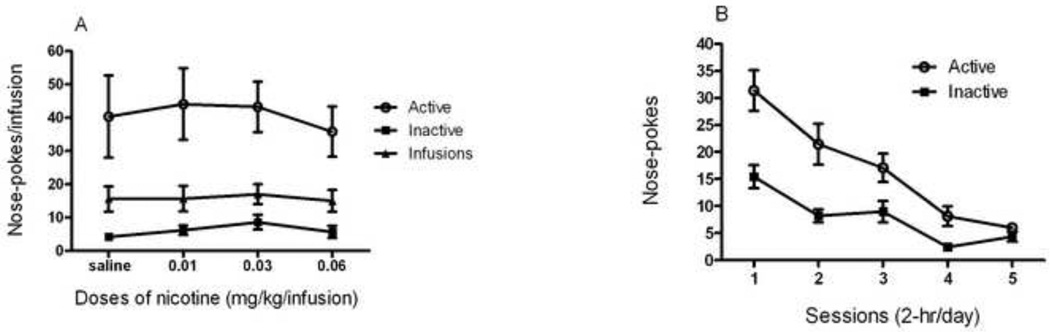
A two-way repeated measures ANOVA conducted for the nicotine self-administration dose-response curve (Fig. 2, A) revealed a significant effect of response type (F(1,123) = 35.61), but no significant effect of dose (F(3,123) = 0.32) or interaction (F(5,123) = 0.10). Bonferroni post hoc analysis revealed significant differences between active and inactive responses for 0.6 mg/kg/infusion of nicotine (p < 0.01), and for 0.03, 0.01 and 0.0 (saline) mg/kg/infusion of nicotine (p < 0.001). There was no significant difference in active nose-poke responses or number of infusions between the different doses of nicotine or between nicotine and saline.
Figure 2. Dose-response curve for nicotine self-administration and extinction training.
(A) After 10 daily 2-hr sessions of nicotine self-administration, mice were exposed to different doses of nicotine for self-administration from highest dose to saline, with each dose tested in two consecutive sessions. Data is shown as mean (±SEM) number of nose-pokes or infusions during two consecutive sessions. (B) Following dose-response testing, animals underwent 5–12 sessions of extinction (the first 5 sessions shown here) in which both nicotine reinforcements and associated cues were withheld. Data is shown as mean (±SEM) number of nose-pokes.
A one-way repeated measures ANOVA conducted for responding during the extinction of nicotine-seeking behaviour (Fig. 2, B) revealed a significant effect of response type (F(1,120) = 39.15), day (F(4,120) = 22.71) and interaction (F(4,120) = 3.259). Bonferroni post hoc analysis revealed significant differences between active and inactive responses for days 1 and 2 which became insignificant for days 3–5.
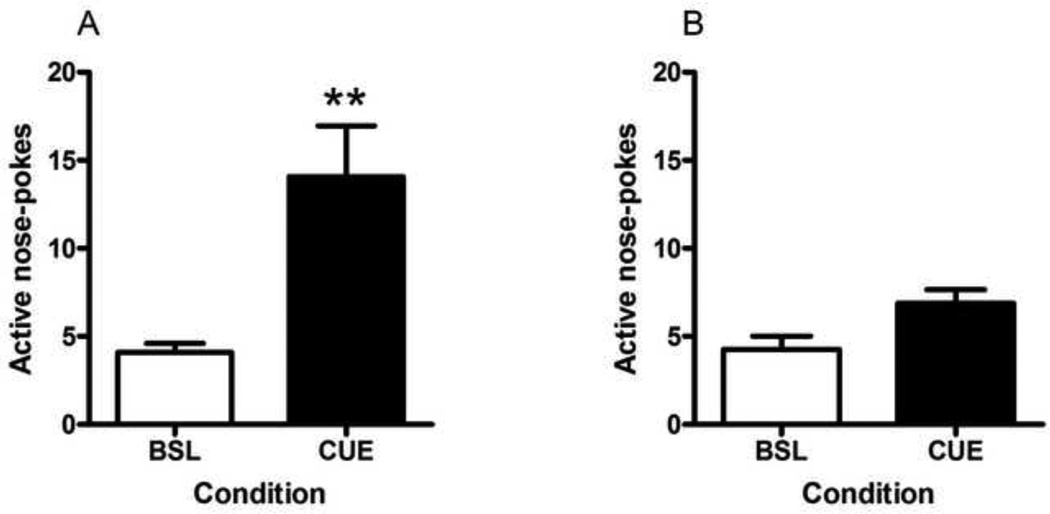
The paired t-test conducted for nicotine-associated cue-induced reinstatement (Fig. 3, A) revealed a significant difference (t(11) = 3.809, p = 0.0029) in active nose-pokes between baseline (average of two extinction sessions prior to testing) and cue-induced reinstatement. In contrast, there was no significant difference (t(11) = 2.012, p = 0.0770) in active nose-poke responses between baseline (average of two extinction sessions prior to testing) and cue-induced reinstatement in the saline control group (Fig. 3, B).
Figure 3. Nicotine-associated cue-induced reinstatement of nicotine-seeking behavior.
Following the acquisition and extinction of nicotine- (A) or saline (B)-seeking behavior, this behavior was reinstated by the presentation of nicotine- or saline-associated light cues from the active nose-poke hole. One non-contingent cue presentation was given at the start of the session and subsequent cue presentations were given contingently for nose-pokes on an FR2 schedule (CUE condition). This reinstatement session was compared to the average of the two extinction sessions preceding testing (BSL condition). Data is shown as mean (±SEM) number of nose-pokes in the active hole. **p<0.01, Student’s t-test.
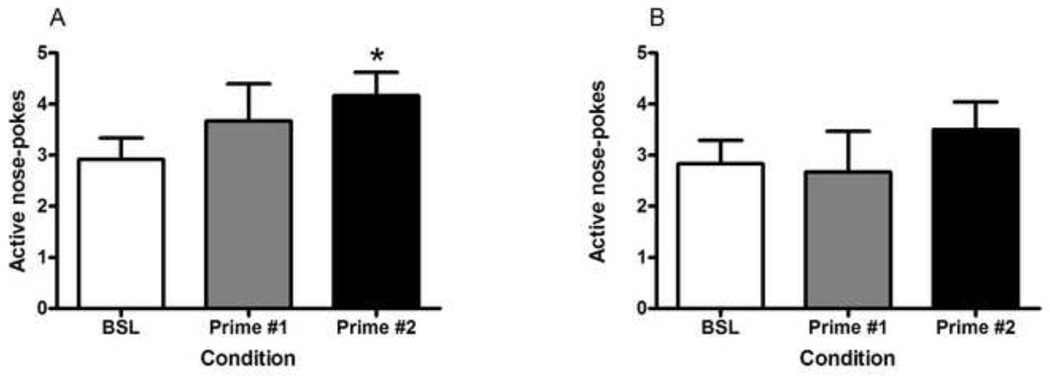
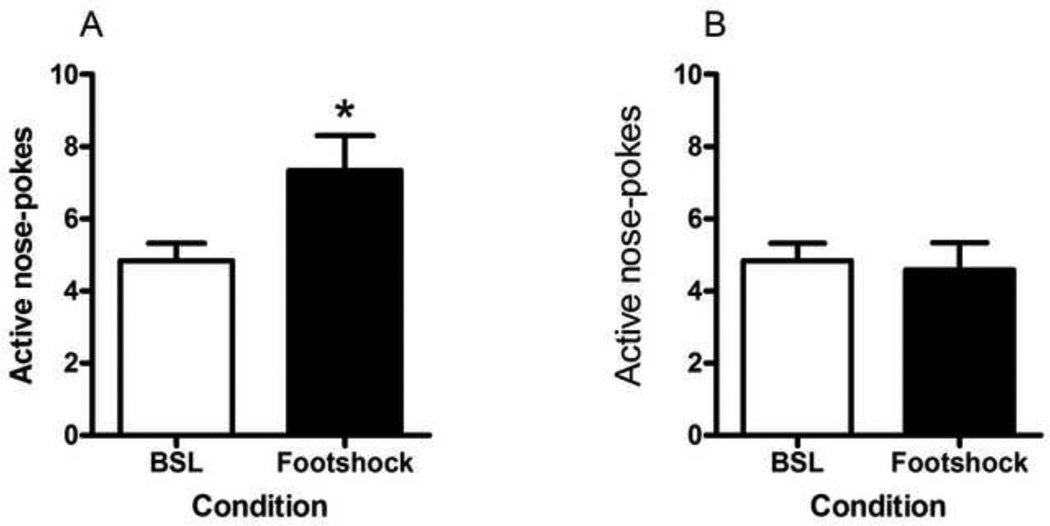
A one-way repeated measures ANOVA revealed no significant differences in nicotine primed reinstatement (Fig. 4, A; F(2,22) = 3.151, p = 0.0626); however, Dunnett’s post hoc analysis revealed a significant difference in active nose-pokes between baseline and the second session of nicotine priming (p < 0.05). A similar ANOVA conducted on primed reinstatement in the saline group (Fig. 4, B) revealed no effects (F(2,22) = 0.5423, p = 0.5890) even with Dunnett’s post hoc analysis. The paired t-test conducted for intermittent footshock-induced reinstatement of nicotine-seeking (Fig. 5, A) revealed a significant difference (t(11) = 2.751, p = 0.0189) in active nose-pokes between baseline and footshock-induced reinstatement. In contrast, there was no significant difference (t(11) = 2.736, p = 0.7895) in active nose-poke responses between baseline (average of two extinction sessions prior to testing) and footshock-induced reinstatement in the saline control group (Fig. 5, B). Inactive nose-pokes were statistically analyzed for all reinstatement testing; however, no significant differences were observed (Table 1).
Figure 4. Nicotine priming-induced reinstatement of nicotine-seeking behavior.
Following the testing of cue-induced reinstatement of nicotine- or saline-seeking behavior, the mice were given interperitoneal (i.p.) injections of saline prior to extinction session in order to habituate them to the injection procedure. After two days of saline injections, if extinction criteria were reached, the animals were given a priming injection of nicotine (0.15 mg/kg, i.p.) (A) or saline (B) 5 min prior to their extinction session (Prime #1 condition). The priming injection was repeated prior to the next session as well (Prime #2 condition). These reinstatement sessions were compared to the average of the two extinction sessions preceding testing (BSL condition). Data is shown as mean (±SEM) number of nose-pokes in the active hole. #p<0.05, Dunnett’s post hoc test (however, one-way ANOVA was insignificant).
Figure 5. Stress-induced reinstatement of nicotine-seeking behavior.
Following the testing of nicotine priming-induced reinstatement of nicotine- or saline-seeking behavior, once criteria of extinction were reached once again, the mice were tested with footshock stress to reinstate nicotine-(A) or saline-(B) seeking behavior. Intermittent footshock (0.22mA) was given during the 5 min prior to the extinction session (Footshock condition). This reinstatement session was compared to the average of the two extinction sessions preceding testing (BSL condition). Data is shown as mean (±SEM) number of nose-pokes in the active hole. *p<0.05, Student’s t-test.
Table 1.
Inactive Lever Data for All Groups Under Baseline and Test Conditions in Nicotine or Saline Reinstatement
| Nicotine- associated cue |
Nicotine priming |
Nicotine footshock |
Saline- associated cue |
Saline priming |
Saline footshock |
|
|---|---|---|---|---|---|---|
| Baseline (Extinction) | 4.1 ± 1.1 | 3.7 ± 1.6 | 3.9 ± 1.8 | 5.2 ± 1.2 | 2.4 ± 1.9 | 3.4 ± 0.9 |
| Reinstatement | 3.4 ± 1.2 | 3.8 ± 1.2 | 4.9 ± 1.5 | 4.5 ± 1.3 | 4.2 ± 2.4 | 4.2 ± 2.4 |
| 2nd Reinstatement | 4.8 ± 2.4 | 5.2 ± 3.2 |
Data are expressed as mean (±SEM) number of inactive lever presses
4. DISCUSSION
Due to early technical difficulties with maintaining the patency of catheters implanted into the jugular vein, IV self-administration through catheterization into lateral tail vein was previously the primary method for analyzing IV nicotine intake in mice [25–30]. However, this approach allows for only one session of analysis and therefore cannot be used to examine acquisition rates or maintenance of drug self-administration, let alone reinstatement of nicotine-seeking behavior, a big challenge for the treatment of drug addiction. On the other hand, a series of previous studies have tried to establish chronic nicotine IV self-administration in mice through catheterization of jugular vein [31–34, 38, 39]. These studies used prior operant training with cocaine [31, 32], water [33], or food pellets [33, 38, 39] to facilitate acquisition of chronic nicotine IV self-administration. It has been reported previously that either food restriction or prior operant training with food, water, or drugs results in neuroadaptations in brain reward circuitry and affects IV drug self-administration and the reinstatement of drug-seeking behavior [44–46].
To overcome the above-mentioned caveats, three research groups have recently attempted to characterize chronic IV nicotine self-administration through catheterization of the jugular vein of naive C57BL/6J mice [38, 40, 41], although two still employed mild food restriction in their studies [38, 40]. Consistent with these reports, our results once again showed that nicotine at 0.03 mg/kg/infusion supports reliable nicotine IV self-administration in naïve C57BL/6J mice. Although Fowler and Kenny (2011) showed that initial exposure to a lower unit dose of (0.03 mg/kg/infusion) before access to higher doses (e.g. 0.1 mg/kg/infusion) facilitated chronic nicotine IV self-administration [38], it should be noted that 0.03 mg/kg/infusion of nicotine for IV self-administration training was used for chronic IV self-administration training in naive C57BL/6J mice without prior operant training [41] and was also the dose used for wildtype littermates of α5 nicotinic acetylcholine receptor knockout mice with prior operant training[39]. Importantly, in our study, naive C57BL/6J mice failed to discriminate active from inactive nose-poke response for saline IV self-administration under the same conditions, suggesting that the increased active nose-pokes for nicotine-taking were largely due to reinforcing effects of nicotine and not the contingent presentation of light cues. This is consistent with the finding of Fowler and Kenny (2011) that after acquired stable nicotine IV self-administration, lever responding of C57BL/6J mice to nicotine-associated cues alone decreased gradually to a relatively lower level [38] and our previous report in which C57BL/6J mice failed to acquire stable IV saline self-administration [47]. However, these findings seem discrepant with the finding of Contet et al. (2010), who showed that the visual stimulus alone is able to support high level of lever-pressing in naive C57BL/6J mice [40]. With regards to stimulus duration in the present study the visual stimulus was limited to a 10 s duration (compared to a 20 s duration used by Contet and colleagues); however, it should be noted that the work of Fowler and Kenney (2011) used a 20 s visual stimulus similar to that of Contet et al. (2010).
In our study, there is no clear dose-dependent change in the response curve for nicotine self-administration within the dose range tested of 0.01–0.06 mg/kg/infusion. Also, there is no significant difference in active nose-pokes or number of infusions between nicotine and saline sessions during dose-response testing. The flat dose-response curve for nicotine self-administration was also reported in naive C57BL/6J mice in the dose range of 0.01–0.1 mg/kg/infusion by Contet et al. (2010) [40]. In addition, Galeote et al. (2009) also reported a flat dose-response curve for nicotine IV self-administration in wild-type littermates of prodynorphin knockout mice in the dose range of 0.0052–0.085 mg/kg/infusion [35]. Note that Fowler and colleagues (2011) reported a typical inverted U-shape dose-dependent response curve for nicotine IV self-administration in C57BL/6J mice and wild-type littermates of α5 nicotinic acetylcholine receptor knockout mice; however both groups of mice had a history of prior operant training [38, 39]. It remains unclear how prior operant training could contribute to the establishment of this typical inverted U-shape dose-dependent response curve for nicotine IV self-administration in mice.
Apart from that noted above, the particular methods under which the dose-response curve was established in this study is quite different from those conducted by Fowler and colleagues (2011) where doses of nicotine were tested in ascending order apart from saline which was tested lasted [38] while the current study utilized a descending order. More importantly, in that study, access to each testing dose was for a minimum of 5 days and continued until intake was considered stable with the data being reported as an average of the last three sessions [38]. In the current study, access was limited to only two consecutive sessions while in the study by Contet et al. (2010), where a flat dose-response curve was also observed, access was limited to only a single session [40]. It should also be noted that in previous experiments, we have found high level of responding at the first saline substitution in non-human primates, a phenomenon that could reflect the acquisition of motivational properties by the light cues after repeated pairing [4]. After repeated session of extinction in saline, a clear typical inverted U curve was obtained in non-human primates, again suggesting that the method in which the dose response curve is conducted may have important consequences. It is possible that if the animals in the current study had been allowed to stabilize on each testing dose a typical inverted U-shape dose response curve may have been established.
It has been shown that both nicotine-associated cues and stress can trigger reinstatement of nicotine-seeking behavior in C57BL/6J mice with prior operant training [34, 38] or without both prior operant training and pre-exposure to drug [40, 41]. In our study, male C57BL/6J mice were subjected to neither prior operant training with drugs, food, or water nor food or water restriction. Consistent with prior findings, nicotine-associated cues and stress (intermittent footshock) triggered a reliable reinstatement of nicotine-seeking behavior in naive C57BL/6J mice. In contrast, neither saline-associated cues nor stress was able to trigger reinstatement of saline-seeking behavior in our saline control group. Taken together, both drug-associated cues and stress reliably reinstate drug-seeking behavior in naive C57BL/6J mice regardless of a history of prior operant training or food restriction.
Priming injections of nicotine (0.15 mg/kg, i.p.) were able to induce reinstatement of nicotine-seeking behavior in C57BL/6J mice, but the relative magnitude of this reinstatement was weak and similar to that observed in the saline control group (Fig. 4). This magnitude is also similar to that induced by a priming injection of nicotine (0.18 mg/kg, i.p.) in the report from Martin-Garcia and colleagues (2009) [41]. In addition, previous reports have shown that the ability of drug priming injections to trigger drug-seeking behavior is dependent on the route of injection (intravenously, intraperitoneally, or subcutaneously) and the pretreatment time [42, 47–51]. It should be noted that only one dose of nicotine (0.15 mg/kg) through one route (s.c.) was tested. Future studies should attempt a dose-response curve and time course for nicotine-primed reinstatement of nicotine-seeking behavior. It may also be interesting to attempt nicotine priming-induced reinstatement with different routes of administration.
Chronic IV nicotine self-administration paradigms in the rat support nicotine intake, approximately 0.3 mg/kg/2-hr session [52] to 0.6 mg/kg/1-hr session [11], that results in plasma nicotine levels at or above the levels found in human smokers [52]. Acute tail vein self-administration paradigms demonstrate a range of nicotine intake rates from 0.4 mg/kg/hr [29] to 2.6 mg/kg/hr [28]. The mouse paradigm of chronic IV nicotine self-administration described here results in total daily intake of nicotine similar to chronic nicotine IV self-administration through the jugular vein in the rat and nicotine IV self-administration through tail vein in the mouse. The rate of nicotine metabolism is significantly faster in mice than that in rats or humans [53]. It remains unclear whether plasma nicotine level in C57BL/6J mice in our study is comparable with those in human smokers.
The data shown here demonstrate that naive C57BL/6J mice that are neither food restricted nor previously operant trained with drugs, water or food pellets reliably self-administer nicotine in a chronic paradigm, and that the dose-response curve for these mice is flat. Both nicotine-associated cues and stress (footshock) reliably reinstate nicotine-seeking behavior in C57BL/6J mice, but future study is needed to determine whether nicotine priming injections can also reinstate this behavior. Chronic nicotine IV self-administration and reinstatement can be used to examine behavior supported by nicotine in knockout and transgenic mouse lines, increasing the techniques available to identify the molecular basis for behaviors related to nicotine addiction.
-
-
We examined a model of nicotine self administration and relapse in C57Bl/6J mice
-
-
Reinstatement of drug-seeking behaviour was attempted by nicotine-associated cues, nicotine priming injections and footshock
-
-
The methodological factors of the current study are compared to those of previous publications
Acknowledgements
YY was supported by a fellowship award from the CIHR-TUSP program and by a CIHR post-doctoral fellowship award. MRP and RCS were supported by DA14241 and DA10455 from the National Institutes of Health. The Translational Addiction Research Laboratory of BLF was supported by LOF940707 (Project #15819) from the Canadian Foundation for Innovation’s Leaders Opportunity Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology(Berl.) 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- 2.Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav. 1983;19:1021–1026. doi: 10.1016/0091-3057(83)90409-4. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- 4.Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS One. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- 6.Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. Br J Pharmacol. 1984;83:49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berlin) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 8.Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berlin) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- 9.Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacology. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- 10.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacology Biochemistry and Behavior. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 11.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- 12.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 13.Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- 14.Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2000;152:289–294. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- 15.Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 16.Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, et al. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- 18.Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration-- comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- 19.Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking induced by nicotine associated cues and nicotine priming, but does not affect nicotine-intake. Br J Pharmacol. 2011;164:1652–1660. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 22.Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- 23.Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- 24.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Martellotta MC, Kuzmin A, Zvartau E, Cossu G, Gessa GL, Fratta W. Isradipine inhibits nicotine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1995;52:271–274. doi: 10.1016/0091-3057(95)00096-f. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen T, Swedberg MD. Reinforcing effects of nicotinic compounds: intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1998;60:567–573. doi: 10.1016/s0091-3057(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 27.Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 28.Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- 29.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 30.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 32.Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- 33.Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- 34.Bilkei-Gorzo A, Racz I, Michel K, Darvas M, Maldonado R, Zimmer A. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biol Psychiatry. 2008;63:164–171. doi: 10.1016/j.biopsych.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- 36.Metaxas A, Bailey A, Barbano MF, Galeote L, Maldonado R, Kitchen I. Differential region-specific regulation of alpha4beta2* nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice. Addict Biol. 2010;15:464–479. doi: 10.1111/j.1369-1600.2010.00246.x. [DOI] [PubMed] [Google Scholar]
- 37.Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contet C, Whisler KN, Jarrell H, Kenny PJ, Markou A. Patterns of responding differentiate intravenous nicotine self-administration from responding for a visual stimulus in C57BL/6J mice. Psychopharmacology (Berl) 2010;212:283–299. doi: 10.1007/s00213-010-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Garcia E, Barbano MF, Galeote L, Maldonado R. New operant model of nicotine-seeking behaviour in mice. Int J Neuropsychopharmacol. 2009;12:343–356. doi: 10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- 42.Yan Y, Yamada K, Nitta A, Nabeshima T. Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res. 2007;177:261–268. doi: 10.1016/j.bbr.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking induced by nicotine associated cues and nicotine priming, but does not affect nicotine-intake. Br J Pharmacol. 2011;164:1652–1660. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003 doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- 45.Shalev U, Robarts P, Shaham Y, Morales M. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav Brain Res. 2003;145:79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 46.Carr KD. Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav. 2011;104:162–167. doi: 10.1016/j.physbeh.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res. 2006;168:137–143. doi: 10.1016/j.bbr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl) 2002;161:417–424. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs RA, See RE, Middaugh LD. Conditioned stimulus-induced reinstatement of extinguished cocaine seeking in C57BL/6 mice: a mouse model of drug relapse. Brain Res. 2003;973:99–106. doi: 10.1016/s0006-8993(03)02560-5. [DOI] [PubMed] [Google Scholar]
- 50.Kruzich PJ. Does response-contingent access to cocaine reinstate previously extinguished cocaine-seeking behavior in C57BL/6J mice? Brain Res. 2007;1149:165–171. doi: 10.1016/j.brainres.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 51.Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology (Berl) 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- 52.Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- 53.Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]