Abstract
Stimulant drugs have been shown either to increase or decrease rates of delay discounting (impulsive choice). These mixed findings may result from genetic, neurochemical, or environmental factors. Lewis (LEW) and Fischer 344 (F344) rats have neurochemical and behavioral differences that may be relevant to delay discounting and were used to examine effects of acute and chronic administration of d-amphetamine (d-AMP) on impulsive choice using a within-session delay-discounting procedure. Male LEW (n=8) and F344 (n=8) rats chose between one food pellet delivered immediately and three food pellets delivered after an increasing delay. Saline and d-AMP (0.1, 0.3, 1.0, and 1.7 mg/kg) were tested acutely and during chronic d-AMP exposure. Choice for the larger reinforcer decreased as the delay to its presentation increased for both strains at baseline. LEW rats made more impulsive choices than F344 rats as indicated by shorter indifference points, and this is consistent with previous research. Acute administration of d-AMP dose dependently increased larger-reinforcer choice and area under the curve (AUC) for LEW, but not F344 rats. During chronic exposure to d-AMP, larger-reinforcer choice and AUC increased relative to acute administration for F344 rats responding in shorter delay series, but not for F344 rats responding in longer delay series or for LEW rats. Differential effects of acute and chronic administration of d-AMP on impulsive choice in LEW and F344 rats may be a result of various factors, including genetic, neurochemical, and environmental variables. Future research should attempt to tease apart the relative contribution of each of these factors on impulsive choice.
Keywords: d-Amphetamine, Delay discounting, Fischer 344, Impulsivity, Lewis, Rat, Self-control
1. Introduction
Behavior identified as impulsive is related to many behavioral and psychological disorders, e.g., gambling, substance abuse, attention-deficit/hyperactivity disorder (ADHD), and others (Evenden, 1999; Evenden and Ryan, 1996; Perry and Carroll, 2008). Identifying specific and relative contributions of neurological, genetic, and behavioral mechanisms underlying impulsive behavior may enhance understanding and treatment of problem disorders. A delay-discounting procedure is often used to evaluate impulsive choice in humans and animals (Mazur, 1987; Perry and Carroll, 2008). Within this procedure, choice is between two reinforcers of different magnitudes presented at varying delays. Larger-reinforcer choice generally decreases as delay to its presentation increases. Impulsive choice is defined as choosing the smaller, more immediate reinforcer at shorter delays, and self-controlled choice is defined as choosing the larger, more delayed reinforcer at longer delays (e.g., Mazur, 1987).
Using a delay-discounting procedure with different rat strains may enhance understanding of genetic factors contributing to impulsive choice. Lewis (LEW) and Fischer 344 (F344) rats have behavioral and neurochemical differences relevant to delay discounting that are outlined in Table 1. For example, LEW rats chose the impulsive option in a delay-discounting task more often than F344 rats (Anderson and Diller, 2010; Anderson and Woolverton, 2005; Garcia-Lecumberri et al., 2010; Madden et al., 2008; but see Wilhelm and Mitchell, 2009). LEW rats also have lower levels of the monoamines dopamine (DA) and serotonin (5-HT) in various brain regions compared to F344 rats (see Table 1 for specific receptor subtypes, brain regions, and exceptions). Low levels of DA and 5-HT are correlated with more impulsive choices (for a review, see Cardinal et al., 2003), and rats depleted of DA (Kheramin et al., 2004) and 5-HT in specific brain regions (Mobini et al., 2000; Wogar et al., 1993) have steeper discounting functions compared to controls.
Table 1. Neurochemical and behavioral differences between Lewis (LEW) and Fischer 344 (F344) rats.
| Category | Reference | ||
|---|---|---|---|
| Delay Discounting | Indifference Point (↓ value = more impulsive) | LEW < F344 |
Anderson and Diller, 2010 Anderson and Woolverton, 2005 Garcia-Lecumberri et al., 2010 Madden et al., 2008 |
| k value (↑ value = more impulsive) | LEW = F344 | Wilhelm and Mitchell, 2009 | |
|
| |||
| Neurochemical differences | |||
| Dopamine (DA) | D2 receptors in striatum and accumbens core | LEW < F344 | Flores et al., 1998 |
| D3 receptors in accumbens shell and olfactory tubercle |
LEW < F344 | ||
| DA transporters in striatum, accumbens core and shell, and ofactory tubercle |
LEW < F344 | ||
| D2 receptors in accumbens shell and olfactory tubercle |
LEW = F344 | ||
| D3 receptors in striatum and accumbens core |
LEW = F344 | ||
| Extracellular DA in Nucleus accumbens | LEW = F344 | Mocsary and Bradberry, 1996 | |
| Serotonin (5-HT) | 5-HT1A receptors in frontal cortex and hippocampus | LEW < F344 | Burnet el al., 1992 |
| 5-HT1A receptors in hypothalamus, midbrain, and brainstem |
LEW = F344 | ||
| Extracellular levels of 5-HT in nucleus accumbens | LEW < F344 | Selim and Bradberry, 1996 | |
Administration of dopaminergic and serotonergic drugs also affect choice on delay-discounting tasks, but results have been mixed. Acute methamphetamine, caffeine, methylphenidate, and d-amphetamine (d-AMP) administration, all of which have indirect effects on DA, and dexfenfluramine, a 5-HT releaser, increased larger-reinforcer choice (Barbelivien et al., 2008; Cardinal et al., 2000; Diller et al., 2008; Perry et al., 2008; Pitts and McKinney, 2005; Poulos et al., 1996; Richards et al., 1999; Slezak and Anderson, 2011; Wade et al., 2000; Winstanley et al., 2003; 2005). Conversely, acute d-AMP and DOI (5-HT2 agonist) administration decreased larger-reinforcer choice (Cardinal et al., 2000; Hand et al., 2009; Evenden and Ryan, 1996; 1999; Perry et al., 2008; Slezak and Anderson, 2009; Stanis et al., 2008). DA antagonists, flupenthixol and raclopride, decreased larger-reinforcer choice, and SCH 23390, a D1 antagonist, had no effect (Cardinal et al., 2000; Wade et al., 2000). The interaction between acute drug administration and delay discounting requires additional research.
Discrepancies in choice following administration of dopaminergic and serotonergic drugs may result from several factors, including the presence or absence of signals during the delay (Cardinal et al., 2000; Zeeb et al., 2010), strain differences (Hand et al., 2009), order of delay presentation (Slezak and Anderson, 2009), and different baseline rates of discounting (Barbelivien et al., 2008; Perry et al., 2008; Stanis et al., 2008). For example, rats raised in isolated environments made more impulsive choices at baseline compared to those raised in enriched environments (Perry et al., 2008). Subsequent methylphenidate and d-AMP administration increased larger-reinforcer choice in rats with higher baseline rates of impulsive choices (isolated environment). Methylphenidate had no effect and d-AMP decreased larger-reinforcer choice in rats with lower baseline rates of impulsive choices (enriched environment). Acute stimulant administration may yield differential effects on impulsive choice in LEW and F344 rats with different baseline rates of choice.
LEW and F344 rats also show different neurochemical responses to acute stimulant-drug administration, and stimulant-drug administration may differentially affect choice in these rats. Cocaine and methamphetamine administration increased extracellular DA in the ventral striatum of both strains; this increase was more pronounced and, with methamphetamine, lasted longer in LEW rats (Camp et al., 1994). Cocaine and amphetamine administration also increased basal levels of DA in the nucleus accumbens shell and core to a greater extent in LEW compared to F344 rats (Cadoni and Di Chiara, 2007). In vivo examination of DA transporters in the dorsal striatum and nucleus accumbens revealed that LEW cleared locally applied DA at a slower rate than F344 rats; however, DA clearance following amphetamine administration was inhibited more in F344 compared to LEW rats (Gulley et al., 2007).
In comparison to acute drug effects, effects of chronic drug exposure on delay discounting have received relatively little attention. Some evidence suggests that chronic drug exposure can affect choice (e.g., Diller et al., 2008; Gipson and Bardo, 2009; Paine et al., 2003; Richards et al., 1999). It has also been suggested that with humans, effects of chronic drug exposure on delay discounting may dissipate following periods of abstinence, such that, currently abstinent nicotine (Bickel et al., 1999), alcohol (Petry, 2001), and heroin (Kirby and Petry, 2004) users discount delayed rewards less steeply (i.e., make fewer impulsive choices) than current users. Other research, however, suggests no differences between ex- and current alcohol and cocaine users (Heil et al., 2006; Kirby and Petry, 2004). These studies used a between-groups design, making it impossible to compare discounting functions in currently abstinent individuals across each phase of their drug exposure. It is also impossible to know whether they became abstinent because they were more self-controlled than their non-abstinent counterparts or whether becoming abstinent resulted in more self-controlled choices. Within-subject studies conducted with animals, like the one presented here, are able to determine impulsive choice across all phases of drug exposure, including chronic exposure and withdrawal (e.g., Dallery and Locey, 2005; Diller et al., 2008; Gipson and Bardo, 2009).
By using different rat strains known to vary in DA and 5-HT systems and in baseline rates of delay discounting, the present study may contribute novel information about biological determinants of impulsive choice. Delay discounting was examined in LEW and F344 rats under a non-drug baseline condition, followed by acute and chronic exposure to experimenter administered d-AMP and further withdrawal from d-AMP. As LEW rats, in general, have lower levels of DA and 5-HT and make more impulsive choices, it was hypothesized that LEW rats would have greater rates of impulsive choice relative to F344 rats during baseline conditions in the current study. Given that LEW rats generally have greater baseline rates of impulsive choice, it was expected that, consistent with Perry et al. (2008), acute and chronic d-AMP administration would result in increases in self-controlled choice for LEW rats and decreases in self-controlled choice for F344 rats. Additionally, increases in self-controlled choice were also expected for LEW rats as administration of other psychomotor stimulants result in larger increases in extracellular levels of DA in LEW relative to F344 rats (Camp et al., 1994).
2. Methods
2.1. Subjects
Eight experimentally naïve male LEW rats and eight experimentally naïve male F344 rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) served as subjects. Rats were approximately 2-3 months old at the start of experimentation, and consistent with known strain differences, LEW rats (M=335 g, SEM=5.7) weighed significantly more than F344 rats (M=280 g, SEM=4.7), [t(7)=58.86, p<.01]. Subjects were individually housed with controlled environmental conditions (temperature, 20°C; 12-hour reversed light-dark cycle) and continuous access to water. All sessions were conducted during the dark phase of the light-dark cycle at about the same time each day. Subjects were fed 9-12 g of food one-half hour after each experimental session, which resulted in approximately 22 hours of food restriction prior to the start of experimental sessions. All procedures were conducted in accordance with West Virginia University’s Animal Care and Use Committee.
2.2. Apparatus
Experimental sessions were conducted in eight standard operant-conditioning chambers for rats, each enclosed in a melamine sound-attenuating cubicle (Med Associates, VT). Each chamber contained a working area of 30.5 cm by 24.5 cm by 21.0 cm, a grid floor, and a 45-mg pellet dispenser with a pellet receptacle that was centered between two retractable response levers. The levers were 11.5 cm apart from each other and required a force of 0.25 N for a response to be recorded. The levers were 4.8 cm wide, elevated 8 cm from the grid floor, and protruded 1.9 cm into the chamber. Two 28-V stimulus lights, 2.5 cm in diameter, were located 7 cm above each lever. Each chamber contained a 28-V houselight on the wall opposite the wall containing the operanda. A ventilation fan circulated air and masked extraneous noise. Data collection and programmed consequences were controlled by a personal computer equipped with Med-PC software (Med Associates, VT).
2.3. Procedure
2.3.1. Initial training
During lever-press training, both levers were extended into the chamber, and food was delivered according to a conjoint variable-time (VT) 1-min, fixed-ratio (FR) 1 schedule. Sessions lasted until 60 food pellets were delivered. If lever pressing was not established after five sessions under the conjoint VT 1-min, FR 1 schedule, lever pressing was shaped using reinforcement of successive approximations. After lever-press acquisition, an alternating FR 1 schedule of reinforcement was in effect. Both levers were extended into the chamber and a cue light above one (randomly determined) lever was illuminated. Each response on that lever resulted in delivery of one food pellet. Responses on the other lever were recorded but had no other programmed consequences. After five food pellets were earned on one lever, the position of the illuminated cue light and the FR 1 contingency alternated to the other lever. This alternating procedure occurred within the session until 40 food pellets were delivered. Sessions continued until subjects were reliably earning pellets associated with both levers.
2.3.2. Delay-discounting procedure
After initial training, a discrete-trials choice procedure similar to Evenden and Ryan (1996; 1999) began. All sessions started with a 10-min blackout followed by five blocks of eight trials each. Each block consisted of two forced-exposure and six free-choice trials that started every 100 s, resulting in inter-trial-intervals (ITIs) of varying durations. All trials began with illumination of the houselight. The first two trials in each block were forced-exposure trials with one, randomly determined, lever extended into the chamber, and the cue light above it illuminated. Following a single response on the extended lever, the lever was retracted, the cue light darkened, and a single food pellet was delivered immediately or three food pellets were delivered after a delay. For the second forced-exposure trial, the other lever was extended, the cue light above it was illuminated, and the other outcome was available dependent on a single lever press.
After exposure to both outcomes during forced-exposure trials, the remaining six trials in each block were free-choice trials. During these trials, both levers were extended into the chamber, the cue lights above them were illuminated, and subjects chose between both alternatives. The lever correlated with the larger reinforcer remained constant within and across sessions and free-choice trial contingencies were the same as those programmed during the forced-exposure trials in that block. Following a response on either lever, both levers retracted, both cue lights darkened, and one immediate or three delayed food pellets were delivered depending on which lever was pressed. When a smaller reinforcer was delivered on either forced-exposure or free-choice trials, the houselight flashed as one food pellet was dispensed into the food trough and remained off for the remainder of the trial. When a larger reinforcer was delivered on either forced-exposure or free-choice trials, the houselight remained on during the delay, flashed three times as the three food pellets were dispensed into the food trough, and remained off for the remainder of the trial.
If a lever press was not made within 30 s of a trial onset, that trial was recorded as an omission, the lever(s) retracted, cue light(s) and houselight darkened, and a 70-s ITI began. If six or more free-choice omissions occurred during a session, data from that session were not included in any analyses. Omissions could occur on forced-exposure trials, however, this rarely occurred during baseline and following administration of small-to-moderate doses of d-AMP. For both rat strains, forced-exposure omissions sometimes occurred following administration of the largest dose. Whether forced-exposure omissions occurred for the smaller or larger alternative and whether they occurred earlier or later in the session was not systematic across subjects.
After completion of the six free-choice trials in the first block, the delay to the larger reinforcer increased across subsequent blocks, while the delay to the smaller reinforcer remained constant (0 s). Subjects were initially exposed to a delay series in which both outcomes were delivered immediately across all blocks. Once choice for the larger reinforcer in each block was at least 80%, the delay to the larger reinforcer increased across blocks in the following order: 0, 1, 2, 4, and 6 s, and then 0, 2, 4, 8, and 16 s. All subjects were exposed to each of these delay series until choice for the larger reinforcer during the first free-choice block was at least 80% for three successive sessions. Once this criterion was met, the delay series for each subject was adjusted (increased or decreased) if necessary to obtain delay-discounting functions without floor (near exclusive choice for the smaller reinforcer) or ceiling (near exclusive choice for the larger reinforcer) effects. If ceiling effects occurred during the 16-s delay series, delays were increased to 0, 5, 10, 20, and 40 s, and if necessary, to 0, 10, 20, 40, and 60 s. Sessions ended after 40 total (10 forced-exposure and 30 free-choice) trials and were conducted five days per week (Monday-Friday). Levers associated with the larger reinforcer were counterbalanced across subjects so that half of each group had the left lever associated with the larger reinforcer and half of each group had the right lever associated with the larger reinforcer.
2.3.3. Baseline
Following determination of a terminal delay series for a subject, a baseline delay-discounting function was established. Baseline sessions were conducted for a minimum of ten sessions and until no increasing or decreasing trends were observed in larger-reinforcer choice during all free-choice trials across the last five sessions. To ensure sensitivity to the variations in reinforcer magnitude and delay, each Wednesday a 0-s probe session was conducted during which all delay values were set equal to zero (Cardinal et al., 2000; Diller et al., 2008; Evenden and Ryan, 1996; 1999). Subsequent probe sessions were conducted (if needed) until larger-reinforcer choice was at least 80% in each block.
2.3.4. Acute d-AMP administration
After a stable baseline was established, acute effects of d-AMP were determined. Saline or d-AMP (0.1, 0.3, 1.0, and 1.7 mg/kg) was administered to all subjects. Intermediate doses of d-AMP (0.42 and 0.56 mg/kg) were administered when intermediate drug effects needed to be determined. Drug administration occurred on Tuesdays and Fridays if larger-reinforcer choice was at least 80% in all blocks during the last 0-s probe session and was at least 80% in the first free-choice block (when both delay values were 0 s) during the session immediately prior to drug administration (control sessions). Effects of saline were determined for at least one session prior to d-AMP determinations to control for any disruptions resulting from the injection procedure. All doses of d-AMP were administered in a descending then ascending sequence, with an additional saline administration occurring between these sequences. All doses were administered at least twice; additional doses were administered when substantial variability in choice occurred at a particular dose. Table 2 shows the number of sessions during the acute phase and each subsequent condition described below.
Table 2.
Chronic dose selected for individual subjects (Chronic Dose) and number of sessions per condition during acute administration of d-AMP (Acute), chronic administration for 30-50 days (d-AMP 1), redetermination of the dose-effect curve (Chronic), administration of d-AMP for 10-15 days (d-AMP 2), repeated saline administration (Repeat Saline), re-administration of d-AMP for 10 days (d-AMP 3), return to baseline (RTB), and days to pass 0-s probe sessions at the end of experimentation (0-s Probe). Means and standard error of the means are shown for LEW rats responding in the 16-s delay series (LEW 16s), LEW rats responding in the 40-s delay series (LEW 40s), F344 rats responding in the 40-s delay (F344 40s), and F344 rats responding in the 60-s delay series (F344 60s).
| Subject | Chronic Dose (mg/kg) |
Acute | d-AMP-1 | Chronic | d-AMP-2 | Repeat Saline |
d-AMP-3 | RTB | 0-s Probe |
|---|---|---|---|---|---|---|---|---|---|
| LEW-1 | 1.00 | 41 | 30 | 37 | 12 | 12 | 10 | 5 | 1 |
| LEW-2 | 1.00 | 53 | 40 | 45 | 10 | 10 | 10 | 5 | 2 |
| LEW-4 | 1.70 | 47 | 38 | 48 | 15 | 15 | 10 | 5 | 6 |
| LEW-6 | 1.00 | 53 | 45 | 54 | 10 | 10 | 10 | 5 | 2 |
|
| |||||||||
| LEW 16s M(SEM) |
48.5(2.9) | 38.3(3.1) | 46.6(3.5) | 11.8(1.2) | 11.8(1.2) | 10.0(0.0) | 5.0(0.0) | 2.8(1.1) | |
|
| |||||||||
| LEW-3 | 1.70 | 30 | 49 | 34 | 15 | 15 | 10 | 5 | 1 |
| LEW-5 | 0.56 | 45 | 30 | 40 | 15 | 15 | 10 | 5 | 1 |
| LEW-7 | 0.42 | 108 | 49 | 51 | 15 | 15 | 10 | 5 | 2 |
| LEW-8 | 1.70 | 71 | 56a | 41 | 15 | 15 | 10 | 5 | 5 |
|
| |||||||||
| LEW 40s M(SEM) |
63.5(17.1) | 46.0(5.6) | 41.5(3.5) | 15.0(0.0) | 15.0(0.0) | 10.0(0.0) | 5.0(0.0) | 2.3(1.0) | |
|
| |||||||||
| F344-2 | 1.00 | 45 | 35 | 33 | 12 | 15 | 10 | 5 | 1 |
| F344-4 | 1.00 | 32 | 42 | 21 | 15 | 12 | 10 | 5 | 1 |
| F344-5 | 1.00 | 30 | 42 | 21 | 12 | 10 | 10 | 5 | 1 |
| F344-6 | 1.00 | 28 | 45 | 17 | 10 | 13 | 10 | 5 | 1 |
| F344-8 | 0.56 | 40 | 45 | 29 | 13 | 10 | 10 | 5 | 1 |
|
| |||||||||
| F344 40s M(SEM) |
35.0(3.2) | 41.8(1.8) | 24.2(2.9) | 12.4(0.8) | 12.4(0.8) | 10.0(0.0) | 5.0(0.0) | 1.0(0.0) | |
|
| |||||||||
| F344-1 | 0.30 | 40 | 49 | 27 | 15 | 15 | 10 | 5 | 4 |
| F344-3 | 0.56 | 40 | 49 | 33 | 15 | 15 | 10 | 5 | 1 |
| F344-7 | 1.00 | 38 | 42 | 27 | 15 | 15 | 10 | 5 | 6 |
|
| |||||||||
| F344 60s M(SEM) |
39.3(0.7) | 46.7(2.3) | 29.0(2.0) | 15.0(0.0) | 15.0(0.0) | 10.0(0.0) | 5.0(0.0) | 3.6(1.5) | |
LEW-8 received chronic injections during d-AMP 1 for 56, instead of the 50 maximum, consecutive days.
2.3.5. Chronic d-AMP administration
For each subject, after determination of acute d-AMP effects, the dose of d-AMP that resulted in the greatest change in larger-reinforcer choice relative to saline, and maintained 75% or greater larger-reinforcer choice during the first free-choice block was selected as that subject’s chronic (once daily) dose. Each subject’s chronic dose is shown in Table 2. Administration of the chronic dose (d-AMP 1) occurred prior to each session, seven days per week, without 0-s probe sessions, until choice was stable. Stability was defined as a minimum of 30 sessions with no increasing or decreasing trends in larger-reinforcer choice across the last five sessions, and larger-reinforcer choice across all free-choice blocks during each of the last five sessions could not vary by more than 15% from the grand mean. If stability criteria were not met within 50 sessions, the next condition began regardless of variation in choice with one inadvertent exception (see Table 2; LEW-8).
Once choice was stable, or a maximum of 50 sessions had occurred, a chronic dose-effect curve was established. Chronic doses were administered seven days per week, with the exception of Tuesdays and Fridays when they were substituted with either saline or different doses of d-AMP previously administered during acute determinations. Testing began with saline administration followed by each dose of d-AMP administered in a descending then ascending sequence, with an additional saline substitution between sequences. Each dose was administered at least twice; additional substitutions occurred when substantial variability in larger-reinforcer choice occurred at a particular dose.
After determination of chronic d-AMP dose-effect curves, the chronic dose was administered for a minimum of 10 days without interruption (d-AMP 2) until larger-reinforcer choice was stable. Stability was defined as a minimum of 10 sessions with no more than 15% variation in total larger-reinforcer choice from the mean during the last five sessions and no increasing or decreasing trends. If stability criteria were not met within a maximum of 15 sessions, the next condition began regardless of variation in larger-reinforcer choice. Following the d-AMP 2 condition, repeated saline administration began. The number of repeated saline administrations matched the number of d-AMP administrations that occurred during d-AMP 2. For example, if there were 13 administrations during the d-AMP 2 condition then there were 13 administrations during the repeated saline condition. Following repeated saline exposure, subjects were re-exposed to their chronic dose of d-AMP for ten days (d-AMP 3).
2.3.6. Return to (non-drug) baseline
Once the above assessments were complete, all injections were terminated and subjects were tested in their delay series for five days. This condition was identical to the baseline condition except 0-s probe sessions were not conducted until the end of the five-day return to baseline (RTB). At this point, 0-s probe sessions were conducted and remained in effect until larger-reinforcer choice was 80% or higher across all blocks within a single session.
2.4. Drugs
d-AMP was obtained from Sigma-Aldrich (St. Louis, MO, USA), dissolved in 0.9% sodium chloride and injected in a volume of 1.0 mg/ml. Saline or d-AMP (0.1, 0.3, 1.0, and 1.7 mg/kg) was administered via intraperitoneal (i.p.) injections immediately prior to the session.
2.5. Data Analysis
Percent larger-reinforcer choice was the primary dependent measure. Delay-discounting functions were plotted as percent larger-reinforcer choice across increasing delays. A nonlinear regression was fit to the choice data from the baseline condition, and indifference points (IPs) were calculated by interpolating the delay value when larger-reinforcer choice was 50% (e.g., Anderson and Woolverton, 2005). The R2 values for nonlinear regressions that were fitted to individual-subject data are presented in the Appendix. For all conditions, area under the curve (AUC) was calculated according to the formula provided by Myerson et al. (2001) by summing the area of the trapezoids formed when vertical lines were drawn from each normalized delay value to the obtained percent choice at each delay. The areas of these trapezoids were summed and divided by the entire possible area of the graph. Steeper discounting functions with shorter IPs and lower AUCs indicate a higher rate of impulsive choice (e.g., Myerson et al., 2001; Richards et al., 1997). Oneway ANOVAs were conducted to assess baseline differences in IPs and AUC between rat strains. Repeated measures ANOVAs were conducted to assess drug effects and group differences for each condition. Rat strain (LEW vs. F344) served as a between-subject variable and mean AUC obtained for each dose served as a within-subject variable. Planned comparisons were conducted to compare differences in AUC within conditions and between strains. Results were considered significant when p<.05.
3. Results
3.1. Baseline
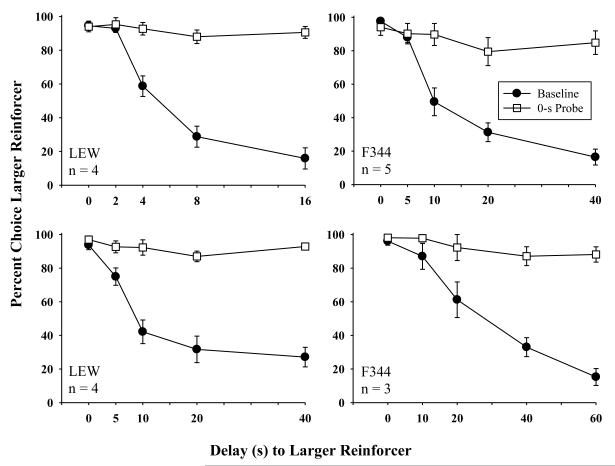
LEW rats required a mean of 94.6 (SEM=15.8) sessions to complete the baseline condition (all delay-discounting sessions prior to the start of the acute condition), and F344 rats required an average of 60.5 (SEM=8.0) sessions. Although LEW rats, on average, required more sessions to reach stability, this difference was not significant. Figure 1 shows mean percent larger-reinforcer choice from the last ten baseline sessions for LEW and F344 rats at their respective delay series. Left panels present larger-reinforcer choice for LEW rats at the 16-s and 40-s delay series. Right panels present larger-reinforcer choice for F344 rats at the 40-s and 60-s delay series. For all rats, regardless of delay series, larger-reinforcer choice decreased as delay to its presentation increased. Data from 0-s probe sessions that occurred prior to drug administration are also shown in each panel of Figure 1 (square symbols). Mean percent larger-reinforcer choice for all 0-s probe sessions, across all blocks, was 92.7 (SEM=1.8) for LEW rats and 89.5 (SEM=3.5) for F344 rats and was not significantly different.
Fig. 1.
Mean percent larger-reinforcer choice as a function of delay for the last 10 baseline sessions (circles) and for 0-sec probe sessions (squares) for LEW (left panels) and F344 (right panels) rats prior to drug exposure. Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
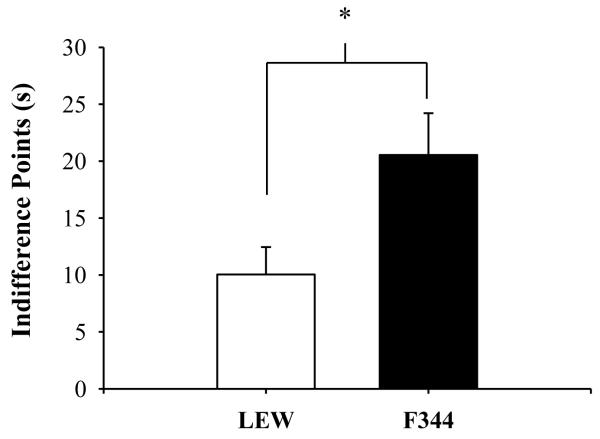
Figure 2 shows mean IPs for LEW and F344 rats for the last ten sessions of baseline. LEW rats made more impulsive choices as the mean IP at baseline was significantly shorter for LEW (M=10.0 s, SEM=2.4) compared to F344 rats (M=20.5 s, SEM=3.7) [F(1,14)=5.73, p<.05, ηG2=.29]. Discounting functions obtained at various delay series were functionally equivalent across rat strains as mean AUC at baseline was not statistically different for LEW (M=0.43, SEM=0.3) compared to F344 rats (M=0.46, SEM=0.4) (see Appendix for individual-subject data for this and subsequent conditions).
Fig. 2.
Mean indifference points (s) for LEW (open bar) and F344 (closed bar) rats for the last 10 baseline sessions. Single asterisks represent significance levels of p<.05, and error bars represent standard error of the mean.
3.2. Acute d-AMP Effects
During acute d-AMP administration, it was not possible to interpolate IPs for all doses as larger-reinforcer choice had increased above 50% across all delays for some doses, therefore, only AUC is reported. For one LEW and two F344 rats, full discounting functions could not be obtained at the 1.7 mg/kg dose because more than six response omissions occurred. Data for these subjects were not included in the overall analysis or subsequent analyses including this particular dose.
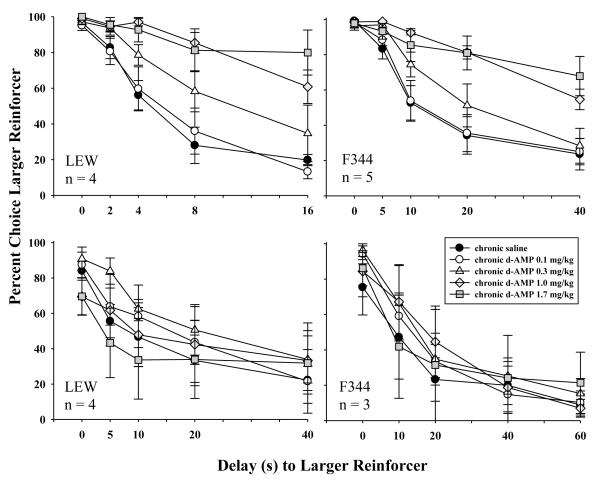
Figure 3 shows mean percent larger-reinforcer choice as a function of delay for each acute dose of d-AMP administration for LEW (left panels) and F344 (right panels) rats. For LEW rats, d-AMP dose dependently increased percent larger-reinforcer choice without disrupting choice in the first block of trials when the smaller and larger alternatives were both delivered immediately. For F344 rats responding in the 40-s delay series (top right panel), d-AMP did not have clear effects on percent larger-reinforcer choice, and the largest dose decreased choice below 75% in the first block of trials when both alternatives were delivered immediately. For F344 rats responding in the 60-s delay series (bottom right panel), d-AMP appeared to decrease percent larger-reinforcer choice. This was accompanied, however, by a disruption in larger-reinforcer choice in the first block of trials following administration of larger doses of d-AMP.
Fig. 3.
Mean percent larger-reinforcer choice as a function of delay for acute saline and each acute dose of d-AMP for LEW (left panels) and F344 (right panels) rats. Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
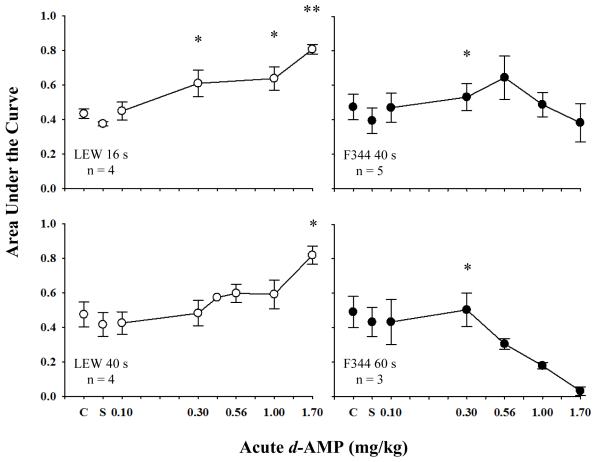
Figure 4 shows mean AUC following acute d-AMP administration for LEW rats responding in the 16-s and 40-s (left panels) delay series and for F344 rats responding in the 40-s and 60-s (right panels) delay series. Acute d-AMP resulted in differential effects for each delay condition indicated by a dose-by-delay series interaction [F(12,36)=5.60, p<.01, ηG2 =.50]. For LEW rats responding in the 16-s and 40-s delay series, d-AMP dose dependently increased AUCs (i.e., increased larger-reinforcer choice) relative to saline. This occurred at the 0.3, 1.0, and 1.7 mg/kg doses for LEW rats responding in the 16-s delay series [F(1,3)=11.20, p<.05, ηG2 =.60; F(1,3)=19.38, p<.05, ηG2 =.70; F(1,2)=646.88, p<.01, ηG2 =.98, respectively], and at the 1.7 mg/kg dose for LEW rats responding in the 40-s delay series [F(1,3)=31.24, p<.05, ηG2 =.78]. For F344 rats responding in the 40-s and 60-s delay series, d-AMP increased AUC at the 0.3 mg/kg dose relative to saline [F(1,4)=21.02, p<.05, ηG2 =.17; F(1,2)=19.87, p<.05, ηG2 =.07, respectively]. AUC appeared to decrease at larger doses, however, effects of other doses did not differ significantly from those of saline.
Fig. 4.
Mean normalized AUC values are plotted as a function of acute doses of d-AMP for LEW (open symbols) and F344 (closed symbols) rats. Error bars represent standard error of the mean. AUC is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. Asterisks represent significant effects of each dose relative to saline. Single asterisks represent significance levels of p<.05 and double represent p<.01.
3.3. Chronic d-AMP Effects
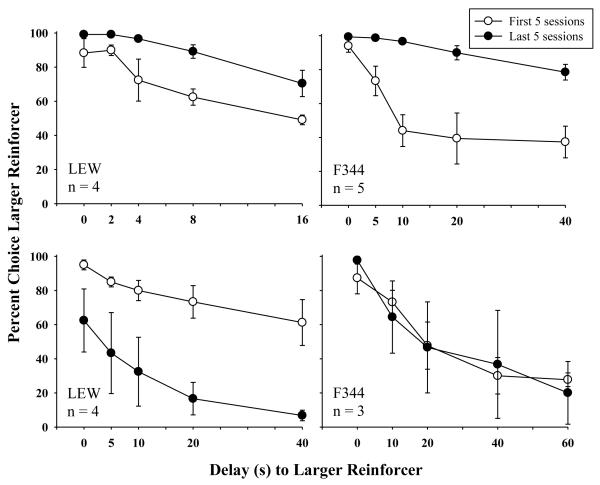
During the chronic d-AMP 1 condition, there were no significant differences in the number of days to reach stability between LEW and F344 rats (see Table 2). Figure 5 shows mean percent larger-reinforcer choice from the first and last five sessions of chronic d-AMP 1 for LEW and F344 rats at their respective delay series. Left panels present larger-reinforcer choice for LEW rats at the 16-s and 40-s delay series. Right panels present larger-reinforcer choice for F344 rats at the 40-s and 60-s delay series. Effects of chronic d-AMP depended on the delay series. Total larger-reinforcer choice increased from the first five to the last five sessions of chronic d-AMP 1 for LEW and F344 rats responding in shorter delay series (16 s and 40 s) [F(1,3)=18.93, p<.05, ηG2 =.66; F(1,4)=17.88, p<.05, ηG2 =.66, respectively]. For LEW rats responding in longer delay series, larger-reinforcer choice appeared to decrease from the first five to the last five sessions of d-AMP 1. This decrease did not reach statistical significance, perhaps as a result of variability between subjects and insufficient power. There was no significant change associated with F344 rats responding in the longer delay series (60 s). It should be noted, however, that for these F344 rats, larger-reinforcer choice decreased from the first to the last five sessions of d-AMP 1 for two of the three subjects (F344-1 and F344-3) and increased from the first to the last five sessions for one subject (F344-7).
Fig. 5.
Mean percent larger-reinforcer choice as a function of delay for the first (open symbols) and last (closed symbols) five sessions of d-AMP 1 for LEW (left panels) and F344 (right panels) rats. Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
Strain differences were observed for LEW and F344 rats responding in the same (40 s) delay series (see Figure 5, top right and bottom left panels). There were no significant differences in larger-reinforcer choice during the first and last five sessions of d-AMP 1 for these LEW rats, but there was a significance increase in larger-reinforcer choice for these F344 rats. For two of these four LEW rats, larger-reinforcer choice in the first block, when both alternatives were delivered immediately, decreased below 75%. A decrease in larger-reinforcer choice in the first block was not observed for these F344 rats.
Figure 6 shows mean percent larger-reinforcer choice as a function of delay for each chronic dose of d-AMP administration for LEW (left panels) and F344 (right panels) rats. For LEW and F344 rats responding in shorter delay series (top panels), percent larger-reinforcer choice dose dependently increased relative to saline. For LEW and F344 rats responding in longer delay series (bottom panels), percent larger-reinforcer choice following chronic d-AMP administration was similar to saline. For three of four LEW rats responding in the 40-s delay series, choice in the first block of trials, when both alternatives were delivered immediately, decreased below 75% following administration of 1.0 and 1.7 mg/kg of chronic d-AMP.
Fig. 6.
Mean percent larger-reinforcer choice as a function of delay for chronic saline and each chronic dose of d-AMP for LEW (left panels) and F344 (right panels) rats. Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
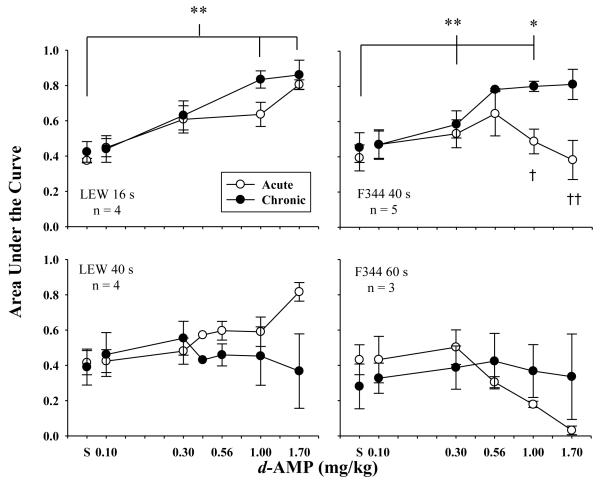
Figure 7 shows mean AUC following acute and chronic d-AMP administration for LEW rats responding in the 16-s and 40-s (left panels) delay series and for F344 rats responding in the 40-s and 60-s (right panels) delay series. Effects d-AMP during redetermination of the chronic dose-effect curve depended on the delay series in effect such that AUC increased relative to saline for LEW and F344 rats responding in shorter delay series (16 s and 40 s, respectively) [F(4,12)=26.71, p<.01, ηG2 =.70; F(4,16)=7.12, p<.01, ηG2 =.53, respectively]. AUC during chronic d-AMP administration was significantly larger following administration of 1.0 and 1.7 mg/kg relative to saline for these LEW rats [F(1,3)=37.28, p<.01, ηG2 =.83; F(1,3)=59.84, p<.01, ηG2 =.75, respectively] and following 0.3 and 1.0 mg/kg relative to saline for these F344 rats [F(1,4)=26.30, p<.01, ηG2 =.14; F(1,4)=19.33, p<.05, ηG2 =.66, respectively]. For F344 rats responding in the 40-s delay series, AUC following chronic administration of 1.0 and 1.7 mg/kg of d-AMP were larger compared to AUC following acute administration of the same doses [F(1,4)=16.85, p<.05, ηG2 =.68; F(1,3)=126.94, p<.01, ηG2 =.66, respectively].
Fig. 7.
Mean normalized AUC values are plotted as a function of acute (open symbols) and chronic (closed symbols) doses of d-AMP for LEW (left panels) and F344 (right panels) rats. Error bars represent standard error of the mean. AUC is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. Asterisks represent significant effects of each chronic dose relative to chronic saline. Crosses represent significant differences between acute and chronic administration of a particular dose. Single asterisks and crosses represent significance levels of p<.05 and double represent p<.01.
For LEW and F344 rats responding in longer delay series (40 s and 60 s, respectively), chronic d-AMP administration did not result in statistically significant changes in AUC. Individual-subject data for these LEW and F344 rats were variable. For these LEW rats, AUC increased for individual subjects following administration of at least one dose of d-AMP compared to saline. For LEW-3, LEW-7, and LEW-8, the dose that increased AUC was 0.3 mg/kg of d-AMP. Following administration of larger doses of d-AMP, AUC decreased and was accompanied by a decrease in larger-reinforcer choice below 75% in the control block. For LEW-5, AUC increased to a maximum of 1.0 following administration of 1.7 mg/kg of d-AMP. A similar effect occurred for the F344 rats responding in longer delay series. At least one dose of d-AMP increased AUC for individual subjects. For F344-1 and F344-3, the doses the increased AUC were 0.1 and 0.3 mg/kg of d-AMP, and for F344-7, the 1.7 mg/kg dose of d-AMP resulted in the greatest increase in AUC.
Figure 8 shows mean percent larger-reinforcer choice during the last 10 sessions of d-AMP 2 (closed circles), repeated saline (open circles), and d-AMP 3 (closed triangles) for LEW and F344 rats in their respective delay series. For LEW and F344 rats responding in shorter delay series (top panels), AUC was largest during d-AMP 2 and d-AMP 3, indicating more larger-reinforcer choices relative to repeated saline. For LEW rats responding in the 16-s delay series, AUC obtained during d-AMP 2 was significantly larger relative to repeated saline [F(1,3)=23.12, p<.05, ηG2 =.85] and trended toward an increase from repeated saline to d-AMP 3 [F(1,3)=8.46, p=.06, ηG2 =.72]. For F344 rats responding in the 40-s delay series, AUC during d-AMP 2 and d-AMP 3 were significantly larger relative to repeated saline [F(1,4)=29.27, p<.01, ηG2 =.86; F(1,4)=33.99, p<.01, ηG2 =.84, respectively]. These changes in AUC occurred within 10-15 session for both strains.
Fig. 8.
Mean percent larger-reinforcer choice as a function of delay for the last 10 sessions of d-AMP 2 (closed circles), repeated saline (open circles), and d-AMP 3 (closed triangles) for LEW (left panels) and F344 (right panels) rats. Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
For LEW and F344 rats responding in longer delay series (bottom panels), no significant changes in AUC from d-AMP 2 to repeated saline and back to d-AMP 3 were observed. As with effects reported above for d-AMP 1 and for chronic determination of the dose-effect function, individual-subject data for these LEW and F344 rats were variable. AUC was generally largest during repeated saline compared to d-AMP 2 and d-AMP 3. For LEW-5 and F344-7, however, AUC was generally largest during d-AMP 2 and d-AMP 3 compared to repeated saline. For two of the four LEW rats (LEW-3 and LEW-8) responding in the 40-s delay series, choice in the first block of trials decreased below 75%.
3.4. Return to Baseline
Figure 9 shows mean percent larger-reinforcer from the last five sessions of baseline (closed circles), RTB (open circles), and 0-s probe sessions (open squares) for LEW and F344 rats in their respective delay series. Regardless of delay series, larger-reinforcer choice decreased as delay to its presentation increased for both baseline and RTB conditions. AUC from the last five sessions of baseline compared to RTB sessions were not significantly different for either strain in any delay series. During 0-s probe sessions, mean percent larger-reinforcer choice was generally above or near 80% across all blocks.
Fig. 9.
Mean percent larger-reinforcer choice as a function of delay for the last five sessions of baseline (closed circles), return to baseline (RTB) (open circles), and all 0-s probe sessions conducted at the end of the RTB condition (open squares). Error bars represent standard error of the mean. Larger-reinforcer choice is shown in the top left panel for LEW (n=4) responding in the 16-s delay series, bottom left panel for LEW (n=4) responding in the 40-s delay series, top right panel for F344 (n=5) responding in the 40-s delay series, and bottom right panel for F344 rats (n=3) responding in the 60-s delay series. The delay series for each group is displayed along the x-axis for respective panels.
4. Discussion
Delay discounting was observed for all subjects, in that choice for the larger reinforcer decreased as delay to its presentation increased, a finding consistent with previous research (e.g., Anderson and Woolverton, 2005). The delay series used in the present study were determined individually for each subject to avoid ceiling or floor effects at baseline. Similar AUC values obtained between LEW and F344 rats at baseline support the claim that delay series were functionally equivalent across rat strains. LEW rats made more impulsive choices at baseline compared to F344 rats as indicated by shorter IPs for LEW rats, and this finding is consistent with previous research (Anderson and Diller, 2010; Anderson and Woolverton, 2005; Garcia-Lecumberri et al., 2010; Madden et al., 2008; but see Wilhelm and Mitchell, 2009). One possible explanation for greater decreases in larger-reinforcer choice in LEW rats may relate to neurochemical differences between these strains. Lower levels of DA and 5-HT are related to impulsive choice (e.g., Kheramin et al., 2004; Mobini et al., 2000; Wogar et al., 1993), and LEW rats have lower levels of DA and 5-HT in various brain regions compared to F344 rats (Burnet et al., 1992; Flores et al., 1998; Selim and Bradberry, 1996). Although there were significant baseline differences in IPs in the current study, some overlap occurred between strains. LEW rats with the longest IPs were similar to F344 rats with the shortest IPs. Future research should correlate specific neurotransmitter levels with delay discounting to identify individual differences in impulsive choice, regardless of rat strain.
Acute d-AMP administration dose dependently increased AUC for LEW rats responding in both delay series without disrupting choice in the first block of trials when the delay to both alternatives was 0 s. Acute d-AMP administration increased AUC following administration of the 0.3 mg/kg dose for F344 rats responding in both delay series. AUC appeared to decrease following administration of relatively large doses of d-AMP for F344 rats, however, the effect was not significant, and larger-reinforcer choice in the first block of trials was below 75%. This suggests that larger doses of d-AMP may have disrupted discrimination of reinforcer amount and makes these data difficult to interpret. Differential effects of acute d-AMP administration on larger-reinforcer choice in LEW and F344 rats is consistent with neurochemical differences found in these strains following acute administration of stimulant drugs. Cocaine, methamphetamine, and amphetamine increase extracellular levels of DA in the ventral striatum and increased basal levels of DA in the nucleus accumbens shell and core to a greater extent in LEW compared to F344 rats (Cadoni and Di Chiara, 2007; Camp et al., 1994). LEW rats also cleared locally applied DA at a slower rate than F344 rats (Gulley et al., 2007). Higher levels of DA and longer activity following stimulant-drug administration may have contributed to increases in AUC observed in LEW relative to F344 rats during acute d-AMP administration in the present study.
Acute effects of d-AMP also depended on baseline rates of choice. On average, impulsive choice during baseline was greatest for LEW rats, and subsequent administration of d-AMP increased self-controlled choice for these rats. Perry et al. (2008) found that d-AMP’s effects depended on differences in baseline rates of impulsive choice with rats raised in either isolated or enriched environments. Choice was more impulsive for rats raised in isolated environments during baseline conditions, and d-AMP administration increased self-controlled choice. For rats raised in enriched environments, choice was more self-controlled during baseline conditions and d-AMP administration increased impulsive choice. Results from Perry et al. (2008) and the present study show that stimulant drug effects may depend on initial rates of impulsive choice. Thus, baseline rates of choice may, in part, account for the discrepant findings in the literature regarding effects of stimulant drugs on impulsive choice.
Chronic d-AMP administration for 30 to 50 days differentially affected larger-reinforcer choice depending on the delay series in effect. For both strains responding in shorter delay series (16 s for LEW and 40 s for F344), AUC increased from the first five to the last five sessions of chronic administration. This is inconsistent with Gipson and Bardo’s (2009) finding that daily, long-access (6 h) d-AMP self-administration increased impulsive choice in rats. However, in their study, d-AMP was self-administered after daily delay-discounting sessions. In the present study, d-AMP was administered by the experimenter prior to daily delay-discounting sessions. Consistent with Gipson and Bardo’s findings, Richards et al. (1999) found that repeated post-session administration of methamphetamine increased impulsive choice. Both of these studies examined post-session administration of a stimulant drug, and Richards and colleagues examined experimenter-administered drug. It seems that post-session administration versus pre-session administration may account for the discrepant results between these studies and the present one. Certainly, there are known differences in effects on behavior due to mere exposure to the drug (post-session administration) compared to completion of a behavioral, i.e., experimental, task under the repeated influence of the drug (pre-session administration) (e.g., Carlton and Wolgin, 1971; Chen, 1968). The present results are consistent with Diller et al. (2008) and Slezak and Anderson (2011) who found that repeated pre-session caffeine and methylphenidate administration, respectively, increased larger-reinforcer choice in rats.
For rats responding in longer delay series (40 s for LEW and 60 s for F344), mean AUC did not change from the first five to the last five sessions of the 30 to 50 days of chronic administration and trended toward a decrease in AUC for LEW rats, however, some variability between subjects was noted. For two of the four LEW rats responding in a longer delay series and receiving the largest dose of d-AMP (1.7 mg/kg) as their chronic dose, larger-reinforcer choice in the first block of trials was below 75%. This suggests that chronic d-AMP administration may have disrupted discrimination of reinforcer amount for these subjects and makes interpretation of these data difficult. It seems that baseline rates of impulsive choice, or the absolute delay series maintaining choice, may also underlie effects of chronic d-AMP on delay discounting (cf. Perry et al., 2008) as AUC increased following chronic d-AMP administration in LEW and F344 rats responding in shorter delay series. Strain differences also contributed to effects of chronic d-AMP on impulsive choice as drug effects were different for the LEW and F344 rats responding in the same (40 s) delay series, and this was consistent throughout the chronic phases of d-AMP administration.
After 30 to 50 days of exposure to d-AMP, dose-effect functions were re-established. For LEW rats responding in shorter delay series, AUCs for each dose tested during chronic d-AMP administration were similar to those obtained during acute d-AMP administration in that d-AMP dose dependently increased AUC relative to those obtained during saline. For F344 rats responding in shorter delay series, AUC increased relative to saline and were also larger following chronic administration of the 0.3 and 1.0 mg/kg doses relative to the acute determinations of those doses. This finding is consistent with research showing that less neuroadaptation within the mesolimbic DA system following chronic cocaine exposure occurs for LEW compared to F344 rats (Haile et al., 2001). It is possible that this resilience to neuroadaptation in the mesolimbic DA system during chronic stimulant administration contributed to the similar patterns in choice following acute and chronic d-AMP administration in LEW rats responding in shorter delay series in the present study. The finding that F344 rats had significant changes in the mesolimbic DA system during chronic cocaine administration (Haile et al., 2001) is consistent with behavioral changes that occurred in the present study with F344 rats responding in shorter delay series following repeated administration of d-AMP, namely, that there was a significant increase in larger-reinforcer choice from acute to chronic d-AMP administration.
During d-AMP 2, when the chronic dose was administered for 10 to 15 days without substitution of other doses, larger-reinforcer choice was similar to that obtained during the last five sessions of chronic d-AMP 1. For LEW and F344 rats responding in shorter delays, mean larger-reinforcer choice was greater than repeated saline. For LEW and F344 rats responding in longer delays, mean larger-reinforcer choice was similar to repeated saline. During repeated saline administration, larger-reinforcer choice returned to levels obtained during baseline within 10 to 15 sessions for LEW and F344 rats responding in shorter delay series. This suggests that increases in larger-reinforcer choice during d-AMP 2 relative to repeated saline were a result of drug effects and not maturation or experimental history. At this point in the experiment, subjects had been exposed to d-AMP for approximately three months. The decrease in larger-reinforcer choice during repeated saline relative to d-AMP 2 indicates that drug effects may not be long lasting. This is consistent with previous research examining chronic stimulant exposure on impulsive choice (e.g., Dallery and Locey, 2005; Diller et al., 2008; Gipson and Bardo, 2009). After repeated saline exposure, the chronic dose was administered during the last condition (d-AMP 3) for 10 days. Again, increases in larger-reinforcer choice during d-AMP 3 relative to repeated saline occurred for LEW and F344 rats responding in shorter delay series. This effect occurred rapidly, and provides further support that changes observed in larger-reinforcer choice were a result of drug administration and not other variables.
Across the various conditions of chronic d-AMP administration, the same general effects emerged. For LEW and F344 rats responding in shorter delay series, chronic d-AMP increased larger-reinforcer choice, and for LEW and F344 rats responding in longer delay series, effects of chronic d-AMP were variable. Differences in larger-reinforcer choice following chronic d-AMP administration for rats responding in shorter versus longer delay series depended more on the delay series in effect and less on rat strain. It is interesting that strain differences may have been the most critical variable in determining effects of acute d-AMP on larger-reinforcer choice, but following repeated administration, the relative importance of rat strain in determining effects of chronic d-AMP on larger-reinforcer choice may have been overshadowed by, or interacted with, the delay series maintaining choice. The role of the particular delay series in determining effects of chronic d-AMP on larger-reinforcer choice for LEW and F344 rats, however, was not systematically explored in the present study. In addition, speculation about the potential role of the absolute delay series in the current study is based on data obtained from a small number of subjects responding in each delay series. Future research could systematically manipulate absolute delay series to examine its importance in determining effects of chronic d-AMP administration on larger-reinforcer choice within or between rat strains.
After the last condition of d-AMP administration, subjects no longer received injections for five sessions, constituting the RTB condition. For all subjects, larger-reinforcer choice returned to levels similar to those obtained at baseline. For F344 rats responding in the longest delay series (60 s), there was a trend toward decreases in larger-reinforcer choice. For these subjects, it is possible that extended exposure to the longer delay series resulted in an overall change in larger-reinforcer choice across time or that drug effects persisted across the RTB condition. Extending this condition longer than five days may have provided a clearer picture with this group of subjects.
In sum, the present study has implications for research aimed at isolating and systematically exploring the roles that genetic and environmental variables have on impulsive choice. LEW and F344 rats have behavioral and neurochemical differences, and these differences are correlated with baseline differences in impulsive choice in that LEW rats made more impulsive choices compared to F344 rats. During acute administration of d-AMP, effects depended largely on the rat strain rather than absolute delay series, suggesting a genetic influence in determining acute effects of d-AMP on impulsive choice. Across both delay series, LEW rats made more impulsive choices, suggesting baseline rate of discounting also may have influenced acute effects of d-AMP on impulsive choice. Effects of chronic administration of d-AMP on impulsive choice were largely determined by the delay series (or baseline rate of choice) in effect. Taken together, the differential drug effects in the present study seemed to be influenced by an interaction between genetic (strain) and environmental (delay series) variables.
Highlights.
LEW rats made more impulsive choices than F344 rats under baseline conditions
Acute d-AMP administration increased self-controlled choice for LEW but not F344 rats
Repeated d-AMP administration increased self-controlled choice for F344 but not LEW rats
Differential drug effects may have resulted from biological variables
Acknowledgements
The authors thank Natalie Bruner and Tyson Sears for their help in conducting early portions of the study. This study was supported by R03 DA019842 (to K.G.A.) from the National Institute on Drug Abuse.
Appendix
Indifference points and R2 at baseline and area under the curve (AUC) at baseline and each drug condition, including: acute administration of d-AMP (Acute AUC), chronic administration for the first and last 5 of 30-50 days of administration (d-AMP 1 AUC), redetermination of the dose-effect curve (Chronic AUC), administration of d-AMP for 10-15 days (d-AMP 2), repeated saline administration (Repeat Saline), re-administration of d-AMP for 10 days (d-AMP 3), and return to baseline (RTB). Doses of d-AMP are in mg/kg.
| Baseline (last 10) | Acute AUC | d-AMP 1 AUC | Chronic AUC | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | IP | R2 | AUC | Control | Saline | 0.1 | 0.3 | 0.56 | 1.0 | 1.7 | First 5 | Last 5 | Saline | 0.1 | 0.3 | 0.56 | 1.0 | 1.7 | d-AMP 2 | Repeat Saline | d-AMP 3 | RTB |
| LEW-1 | 4.8 | 0.97 | 0.37 | 0.50 | 0.40 | 0.45 | 0.81 | --/-- | 0.67 | omit | 0.61 | 0.84 | 0.59 | 0.50 | 0.70 | --/-- | 0.84 | 1.00 | 0.78 | 0.59 | 0.59 | 0.61 |
| LEW-2 | 6.6 | 0.91 | 0.47 | 0.44 | 0.38 | 0.58 | 0.65 | --/-- | 0.56 | 0.85 | 0.76 | 084 | 0.40 | 0.62 | 0.78 | --/-- | 0.96 | 1.00 | 0.93 | 0.42 | 0.93 | 0.36 |
| LEW-3 | 9.2 | 0.61 | 0.39 | 0.54 | 0.49 | 0.35 | 0.64 | --/-- | 0.82 | 0.93 | 0.82 | 0.01f | 0.64 | 0.79 | 0.83 | --/-- | 0.48f | 0.13f | 0.03f | 0.78 | 0.02f | 0.76 |
| LEW-4 | 3.9 | 0.94 | 0.35 | 0.42 | 0.34 | 0.33 | 0.48 | --/-- | 0.50 | 0.76 | 0.73 | 0.98 | 0.36 | 0.35 | 0.65 | --/-- | 0.73 | 0.77 | 0.89 | 0.24 | 0.92 | 0.28 |
| LEW-5 | 8.5 | 0.90 | 0.34 | 0.26 | 0.21 | 0.33 | 0.28 | --/-- | 0.42 | 0.75e,f | 0.64 | 0.53 | 0.16 | 0.22 | 0.40 | 0.52 | 0.91 | 1.00 | 0.46 | 0.23 | 0.57 | 0.37 |
| LEW-6 | 9.4 | 0.86 | 0.54 | 0.37 | 0.39 | 0.43 | 0.49 | --/-- | 0.81 | 0.81 | 0.54f | 0.85 | 0.33 | 0.29 | 0.40 | --/-- | 0.80 | 0.67 | 0.95 | 0.40 | 0.94 | 0.34 |
| LEW-7a | 12.5 | 0.79 | 0.39 | 0.53 | 0.51 | 0.61 | 0.51 | 0.54f | 0.53 f | 071 | 0.59 | 0.31 | 0.44f | 0.52 | 0.45 | 0.40 | 0.26f | 0.18f | 0.30 | 0.32 | 0.22 | 0.34 |
| LEW-8b | 25.5 | 0.87 | 0.55 | 0.56 | 0.45 | 0.40 | 0.50 | --/-- | 0.60 f | 0.89 | 0.93 | 0.08f | 0.32 | 0.33f | 0.55f | --/-- | 0.17f | 0.16f | 0.02f | 0.55 | 0.04f | 0.50 |
| Mean | 10.0 | 0.86 | 0.43 | 0.45 | 0.39 | 0.44 | 0.54 | 0.60 | 0.61 | 0.81 | 0.70 | 0.56 | 0.41 | 0.45 | 0.59 | 0.46 | 0.64 | 0.61 | 0.55 | 0.44 | 0.53 | 0.44 |
| SEM | 2.4 | 0.04 | 0.03 | 0.04 | 0.03 | 0.04 | 0.06 | 0.05 | 0.05 | 0.03 | 0.05 | 0.13 | 0.05 | 0.07 | 0.06 | 0.06 | 0.11 | 0.14 | 0.14 | 0.07 | 0.14 | 0.06 |
| F344-1c | 19.7 | 0.96 | 0.38 | 0.49 | 0.37 | 0.39 | 0.45 | 0.36 | 0.19 f | 0.01 f | 0.58 | 0.20 | 0.04f | 0.18 | 0.15 | 0.15 | 0.12f | 0.08f | 0.11 | 0.09 | 0.11 | 0.06 |
| F344-2 | 191 | 0.95 | 0.53 | 0.70 | 0.65 | 0.70 | 0.75 | 0.77 | 0.25 | omit | 0.18 | 0.93 | 0.51 | 0.50 | 0.64 | 0.79 | 0.75 | 0.60 | 0.74 | 0.31 | 0.58 | 0.43 |
| F344-3 c | 35.0 | 0.99 | 0.57 | 0.65 | 0.60 | 0.68 | 0.69 | 0.25 | 0.14 f | omit | 0.31 | 0.26 | 0.46 | 0.48 | 0.55 | 0.43 | 0.35 | 0.10 | 0.24 | 0.58 | 0.16 | 0.48 |
| F344-4 | 19.0 | 0.71 | 0.48 | 0.57 | 0.41 | 0.59 | 0.67 | --/-- | 0.65 | 0.50 ef | 0.77 | 0.93 | 0.60 | 0.55 | 0.75 | --/-- | 0.80 | 0.98 | 0.93 | 0.25 | 0.83 | 0.28 |
| F344-5 | 8.6 | 0.97 | 0.30 | 0.27 | 0.20 | 0.23 | 0.31 | --/-- | 0.60 | 0.52 | 0.65 | 0.94 | 0.20 | 0.25 | 0.31 | --/-- | 0.72 | 0.89 | 0.67 | 0.36 | 0.76 | 0.98 |
| F344-6 | 16.8 | 0.84 | 048 | 0.41 | 0.33 | 0.49 | 0.46 | --/-- | 0.45 | 0.45 | 0.38 | 0.92 | 0.32 | 0.35 | 0.53 | --/-- | 0.87 | 0.98 | 0.90 | 0.23 | 0.84 | 0.27 |
| F344-7 d | 36.7 | 0.95 | 0.59 | 0.34 | 0.33 | 0.23 | 0.37 | 0.30 | 0.20 | 0.06 f | 0.50 | 0.93 | 0.34 | 0.32 | 0.47 | 0.69 | 0.64 | 0.82 | 0.58 | 0.33 | 0.63 | 0.23 |
| F344-8 | 9.4 | 0.92 | 0.32 | 0.42 | 0.37 | 0.34 | 0.47 | 0.52 | 0.49f | 0.05 f | 0.39 | 0.78 | 0.64 | 0.69 | 0.69 | 0.78 | 0.86 | 0.61 | 0.76 | 0.49 | 0.77 | 0.62 |
| Mean | 20.5 | 0.91 | 0.46 | 0.48 | 0.41 | 0.46 | 0.52 | 0.44 | 0.37 | 0.27 | 0.47 | 0.74 | 0.39 | 0.42 | 0.51 | 0.57 | 0.64 | 0.63 | 0.62 | 0.33 | 0.59 | 0.42 |
| SEM | 3.7 | 0.03 | 0.04 | 0.05 | 0.05 | 0.07 | 0.06 | 0.09 | 0.07 | 0.10 | 0.07 | 0.11 | 0.07 | 0.06 | 0.07 | 0.12 | 0.09 | 0.13 | 0.10 | 0.05 | 0.10 | 0.10 |
LEW-7 was administered 0.42 mg/kg d-AMP which also served as its chronic dose.
LEW-8 received chronic injections during d-AMP 1 for 56 consecutive days.
F344-1 and F344-3 did not meet the 15% stability criterion during d-AMP 1; variation in total larger-reinforcer choice across the last 5 sessions was 21% and 23%, respectively. To avoid exceeding the maximum of 50 day exposure criterion, these subjects began the chronic DEC after day 49 and 48, respectively.
F344-7 had 9 baseline sessions in the 60-s delay series.
Based on one determination.
Larger-reinforcer choice was less than 75% in the first delay block (0s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010;21:754–64. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–93. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbelivien A, Billy E, Lazarus C, Kelche C, Majchrzak M. Rats with different profiles of impulsive choice behavior exhibit differences in responses to caffeine and d-amphetamine and in medial prefrontal cortex 5-HT utilization. Behav Brain Res. 2008;187:273–83. doi: 10.1016/j.bbr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Mefford IN, Smith CC, Gold PW, Sternberg EM. Hippocampal 8-[3H]Hydroxy-2-(Di-n-Propylamino) tetralin binding site densities, serotonin receptor (5-HT1A) messenger ribonucleic acid abundance, and serotonin levels parallel the activity of the hypothalamopituitary-adrenal axis in rat. J Neurochem. 1992;59:1062–70. doi: 10.1111/j.1471-4159.1992.tb08348.x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem. 2007;103:487–99. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res. 1994;668:180–93. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signaled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–75. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. Choosing delayed rewards: perspectives from learning theory, neurochemistry, and neuroanatomy. In: Heather N, Vuchinich R, editors. Choice, behavioral economics and addiction; Amsterdam: 2003. pp. 183–213. [Google Scholar]
- Carlton PL, Wolgin DL. Contingent tolerance to the anorexigenic effects of amphetamine. Physiology of Behavior. 1971;7:221–23. doi: 10.1016/0031-9384(71)90287-3. [DOI] [PubMed] [Google Scholar]
- Chen CS. A study of the alcohol-tolerance effect and an introduction of a new behavioral technique. Psychopharmacologia. 1968;12:433–40. doi: 10.1007/BF00401349. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Diller JW, Saunders BT, Anderson KG. Effects of acute and repeated administration of caffeine on temporal discounting in rats. Pharmacol Biochem Behav. 2008;89:546–55. doi: 10.1016/j.pbb.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–21. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava KL. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brian Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Torres I, Martin S, Crespo JA, Miguens M, Nicanor C, et al. Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol. 2011;25:783–91. doi: 10.1177/0269881110367444. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Bardo MT. Extended access to amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology. 2009;207:391–400. doi: 10.1007/s00213-009-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Everett CV, Zahniser NR. Inbred Lewis and Fischer 344 rat strains differ not only in novelty- and amphetamine-induced behaviors, but also in dopamine transporter activity in vivo. Brain Res. 2007;1151:32–45. doi: 10.1016/j.brainres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;43:179–90. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- Hand DJ, Fox AT, Reilly MP. Differential effects of d-amphetamine on impulsive choice in spontaneously hypertensive and Wistar-Kyoto rats. Behav Pharmacol. 2009;20:549–53. doi: 10.1097/FBP.0b013e3283305ee1. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Beh. 2006;31:1290–94. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2004;175:206–14. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav. 2008;90:333–44. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior: the effect of delay and of intervening events on reinforcement value. Lawrence Erlbaum Associates; Hillside, New Jersey: 1987. pp. 55–73. [Google Scholar]
- Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2000;149:313–18. doi: 10.1007/s002130000385. [DOI] [PubMed] [Google Scholar]
- Mocsary C, Bradberry CW. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706:194–98. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–43. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–50. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Parker JL, Le AD. Dexfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption. Behav Pharmacol. 1996;7:395–99. doi: 10.1097/00008877-199608000-00011. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology. 1999;146:432–39. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–64. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–36. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of acute and chronic methylphenidate on delay discounting. Pharmacol Biochem Behav. 2011;99:545–51. doi: 10.1016/j.pbb.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug and Alcohol Dependence. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–34. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–31. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–82. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–43. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: Interactions with basal levels of impulsivity, dopamine signaling, and reward-related cues. Psychopharmacology. 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]