Abstract
Two different strategies for investigating the likely fate, after ingestion, of natural, bioactive berry constituents (anthocyanins and other non-nutritive flavonoids) are compared. A model of the human gastrointestinal tract (TIM-1) which mimicked the biological environment from the point of swallowing and ingestion through the duodenum, jejunum, and ileum (but not the colon) was used to monitor the stability and bioaccessibility of anthocyanins from both maqui berry and wild blueberry. TIM-1 revealed that most anthocyanins were bioaccessible between the second and third hour after intake. Alternatively, biolabeled anthocyanins and other flavonoids generated in vitro from berry and grape cell cultures were administered to in vivo (rodent) models, allowing measurement and tracking of the absorption and transport of berry constituents and clearance through the urinary tract and colon. The advantages and limitations of the alternative strategies are considered.
Keywords: bioavailability, bioaccessibility, radiolabeling, artificial gastrointestinal tract
Introduction
A mounting body of in vitro, in vivo, and clinical evidence strongly supports that bioactive compounds naturally present in berry fruits (phytochemicals) are uniquely able to interface with human therapeutic targets in order to combat chronic disease, or improve metabolic performance1,2. Paradoxically, the key bioactive compounds that are usually linked to health benefits often appear to be only poorly absorbed and to have very limited bioavailability in animals. For example, ample evidence suggests that consumption of plant foods rich in anthocyanins can confer health protection for humans (anticancer, cardioprotective, neuroprotective and antidiabetic), yet anthocyanins are gauged, by all usual laboratory measurements, to have extremely poor bioavailability3,4. Anthocyanins, unlike synthetic drugs, are quite difficult to track in situ, thus questions have not been resolved, and recommendations for human intake remain nebulous.
In addition, because bioactive plant compounds ingested by an animal (or human) are, at varying rates, subject to metabolic breakdown into modified metabolites, and because these compounds are superimposed upon the complex milieu of other nutrients and phytochemicals in the diet, it has been exceedingly difficult to definitively determine bioavailability, pharmacokinetics, tissue distribution, or the mechanisms by which health protective phytochemicals are effective5,6. In vivo experiments have sometimes indicated rapid absorption and clearance of anthocyanins but cellular mechanisms of action remain largely unknown7,8. Bioavailability of anthocyanins in general has generally been gauged to be extremely low4, and may be beyond the limits of detection for all but the most sophisticated laboratory instruments.
Previous studies have suggested that certain anthocyanin moieties may be more available, and may more actively interact with particular therapeutic targets in health-related interactions, than other anthocyanin species. For example, we have shown that gavage with an anthocyanin-enriched extract from blueberry (containing primarily delphinidin and malvidin) effectively lowered blood glucose levels within six hours in diabetic C57b1/6J mice9. When the component anthocyanin moieties from the natural blueberry extract were administered separately, only pure malvidin-3-O-glucoside was significantly hypoglycemic, whereas delphinidin-3-O-glucoside failed to significantly lower blood glucose levels. Robust determinations of which berry constituents are most biologically-active will be essential to furthering the effective use of dietary berries in human health maintenance regimes.
Our collaborating laboratory teams have investigated two alternative approaches for assessing the bioavailability and biodistribution of anthocyanin pigments after ingestion and during metabolism. The TNO gastro Intestinal Model (TIM-1) is a dynamic model of the upper gastrointestinal tract of humans specifically designed to measure bioaccessibility of compounds or nutrients during digestion. Bioaccessibility can be defined as the potential for a substance to interact with or be absorbed by an organism; that is, the potential to become bioavailable. Bioaccessible compounds may or may not be bioavailable, depending upon biological processes that are present in vivo (i.e. specific transporters), but compounds that are not bioaccessible are generally not bioavailable10,11. Using a completely different approach, bioavailability can be tracked in vivo by administering (to rodents) dietary levels of anthocyanins and other berry polyphenols synthesized, and biolabeled, in controlled berry plant cell cultures. The cell cultures are provoked by physicochemical elicitation, thus induced to switch from primary metabolism (growth and development) to accumulation of bioactive metabolites. Anthocyanins and related flavonoids are rapidly, predictably, and reliably generated by in vitro cell cultures and, once they accumulate in these homogenous cells, the isolation and purification process is streamlined because the cultures lack many of the interfering compounds (pectins, sugars, certain enzymes) that complicate isolation and purification from fruits in nature. Most pertinent to bioavailability research, these biologicially-active constituents of the berry cells can be isotopically-labeled to allow pharmacokinetic interpretation of uptake, kinetics, and metabolic fate, after ingestion by animals. Isotopic labeling of the biologically-active berry constituents with 14C (for use in animal studies) or with 13C (for human clinical trials) offers an opportunity to track the distribution of these compounds during subsequent metabolism, determine whole body partitioning of specific compounds, and gauge bioavailability of a diverse array of bioflavonoids that can only be synthesized in planta 5,12–14.
Materials & Methods
Chemicals
Pepsin A from porcine stomach mucosa (2500–3500 units/mg, P-7012), trypsin from bovine pancreas (7500 N-α-benzoyl-L-arginine ethyl ester (BAEE) units/mg, T9201), and α-Amylase Type II-A: from Bacillus species (1333 units/mg A-6380) were obtained from Sigma-Aldrich (Stockholm, Sweden); fresh pig bile from TNO (Zeist, Netherlands), and Rhizopus lipase (150,000 units/mg F-AP-15) from Amano Enzyme Inc. (Nagoya, Japan). Uniformly labeled 14C sucrose or 13C glucose were obtained from ICN Biomedicals Inc. (Irvine, CA) and Cambridge Isotope Laboratories (Andover, MA), respectively. Human insulin (Humulin) was purchased from Eli Lily (Indianapolis, IN). Cyanidin, delphinidin, petunidin, and malvidin glycosides were obtained from Polyphenols Laboratories (Sandnes, Norway). All other chemicals, including cell culture media were obtained from Invitrogen (Carlsbad, CA) or as previously described12,15. All chemicals used were of analytical grade quality.
The gastrointestinal model
The TNO gastrointestinal model (TIM-1) is a computer-controlled in vitro model that simulates the successive in vivo conditions and kinetic events of the stomach, and small intestine (duodenum, jejunum and ileum) of humans. The physical and biochemical parameters of the model were determined from extensive in vivo data collected from human and animal trials as well from published in vivo studies16,17. Prior TIM experiments have examined the stability of phytochemicals10, digestion of food and bioaccessibility of nutrients11, and bioaccessibility of drugs from formulations under human fasting and fed state conditions 18–20. In short, the model is comprised of four compartments that represent the stomach, duodenum, jejunum and ileum. Each of these compartments is contained by a glass capsule with flexible inner silicone jackets, connected to each other by peristaltic valve pumps that control the transport of the passing chyme from one compartment to the next. To imitate peristalsis, the tubes are squeezed periodically by a pump action on the surrounding water, which also keeps a physiological temperature (37 ± 1°C). The composition of digestive juices used in the model was previously described18. A computer protocol regulates the gastric emptying and intestinal transit time, pH values, and amounts of secretion fluids to simulate fasting state conditions when the extract is provided with water. For bioaccesibility experiments using the TIM-1 model, individually quick-frozen, whole wild (lowbush) blueberries (WBB) (Vaccinium angustifolium Aiton) were obtained from the Wild Blueberry Association of North America (Old Town, ME, USA). The blueberries were a composite of fruits from all major growing sites including Prince Edward Island, New Brunswick, Nova Scotia, and Maine. The composite was made in the fall 2008, individually quick frozen by Cherryfield Foods, Inc. at −15 °C (Cherryfield, ME, USA), and subsequently stored at −80 °C until use. Freeze-dried maqui berries (MB) (Aristotelia chilensis) were obtained from Fundación Chile, Santiago, Chile.
The whole frozen WBB fruits were blended (Waring, Inc., Torrington, CT, USA) with methanol, acidified with 0.3% TFA (fruit to solvent ratio 1:2), and filtered first through multiple layers of muslin sheets, and then Whatman’s filter paper #4 (Florham Park, NJ, USA) with the aid of suction. The collected hydro-alcoholic extract was evaporated to remove organic solvent using a rotary evaporator at a temperature not exceeding 40 °C. The aqueous concentrated extract was loaded on Amberlite XAD-7 (preconditioned with acidified water, 0.3% TFA). The resin was washed thoroughly with acidified water (0.3% TFA) to remove free sugars and organic acids. The polyphenolic mixture was then eluted with methanol (0.3% TFA), and the eluate was evaporated and freeze-dried to afford the polyphenolic-rich extract. An anthocyanin enriched formulation from MB was prepared as described elsewhere9,15. Prepared extracts were subjected to HPLC and LC-MS for both qualitative and quantitative analysis of anthocyanins using external reference standards9,15. Before the start of each experiment 0.5 g of WBB extract or MB extract, alone or with 100 g of meal matrix (standardized High Fat Meal that fulfills the requirements of the U.S. Food and Drug Administration (FDA) and Center for Drug Evaluation and Research (CDER) (December 2002) for experiments with drugs during intake with a high fat meal), was mixed with artificial saliva, which consisted of 100 mL electrolyte solution, 30 mL citrate buffer and 11.5 mg amylase. Deionized water was added to the mixture up to a final volume of 300 mL. This final mixture was introduced in the gastric compartment of the TIM-1 system as described below and the digestion was started. The high fat meal is expected to have the greatest effect on the bioavailability and bioequivalence of drugs, relative to the fasted state, and therefore any bioactive phytochemical compounds of interest.
Absorption of potentially-available anthocyanins was simulated by collection of dialysate fluids passing through semi-permeable capillary membranes (Spectrum Milikros modules M80S-300-01P 0.05 μm pore size) in both the jejunal and ileal compartments. Each experiment was terminated at 240 min where approximately 80% of the stomach contents had passed the ileoceacal valve of the model reaching the ileal efflux. Bioaccessibility (defined as the amount of a compound that is released from a food matrix and is able to pass through membranes with a cutoff of 5 kDa during transit through the stomach and small intestine, thus reflecting availability for absorption in vivo) of the WBB or MB extract was evaluated in the model in three separate digestion experiments under fed and fasted state conditions.
2-Deoxy-[3H]-D-glucose uptake in cell cultures
H4IIE hepatoma cells were incubated in 24-well plates (Greiner Bio Monroe, NC) and grown to near confluency in Dubecco’s Modified Eagle Medium (DMEM), containing fetal bovine serum (FBS) 10% (v/v). Penicillin and gentamicin were always added to the media at concentrations of 100 IU/mL and 50 μg/mL, respectively. For analyses of TIM-1 samples, the H4IIE cells were grown to confluence in 24-well plates, incubated in FBS-free DMEM for 6 h and then exposed for 1 h to fractions collected from the TIM system (MB-TIM fractions) and insulin was included as positive control. MB-TIM fractions were used at 100 μg/mL and insulin at 50 nM. The cells were then rinsed with KRPH (HEPES-buffered Krebs-Ringer phosphate) at 37 °C, consisting of 118 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 30 mM HEPES (pH 7.4). The uptake of the 22 nM 2-Deoxy-[1–3H] glucose (2-DG) at 0.2 μCi/mL, was measured over 15 min. All the uptake measurements were made in triplicate. The uptake of 2-DG was terminated after 15 min by rapidly aspirating off the radioactive incubation medium and washing the cells three times in ice-cold phosphate-buffered saline. The radioactivity associated with the cells was determined by cell lysis in 0.05% Triton X-100, followed by the addition of 3 mL of liquid scintillation (Liquid Scintillation Cocktail, Beckman Coulter, Inc. CA, USA). The mixture of cell lysate and scintillation cocktail was vortexed for twenty seconds, incubated in darkness for 16 h and radioactivity was measured in a LS6500 Multipurpose Scintillation Counter (Beckman Coulter, Brea, CA). Nonspecific glucose uptake was 10% of the total uptake. For all the experiments, corrections for cell number were made on the basis of the protein concentration measured using the BCA Protein assay kit (Pierce Biotechnology, Rockford, IL) according to manufacturer instruction. Glucose uptake results were expressed as fold negative control. The data from in vitro glucose uptake experiments tests was analyzed using ANOVA, Bonferroni’s Multiple Comparison Test.
Cell Cytotoxicity
To assess alterations of the cell membrane integrity and potential cytotoxic effects of MB anthocyanins on H4II cells, cells were seeded at 40 × 103 cells/well in a 24-well plate and allowed to attach for 24 h. Subsequently, fractions were added to the medium at various concentrations (0 to 100 μg/mL). After 16 h treatment, membrane integrity and cell viability were assessed by Calcein-AM (Ca-AM) incorporation. Ca-AM was added to each well at 3 μM, and incubated for 1 h at 37 °C, and then fluorescence was measured in a plate reader (BIOTEK Synergy II, Biotek Instruments Inc, Winooski, VT) with excitation wavelength at 485 nm and emission wavelength of 525 nm. Triplicates were used for each experimental condition.
Biolabeling flavonoids with radio- or stable isotope and in vivo experiments
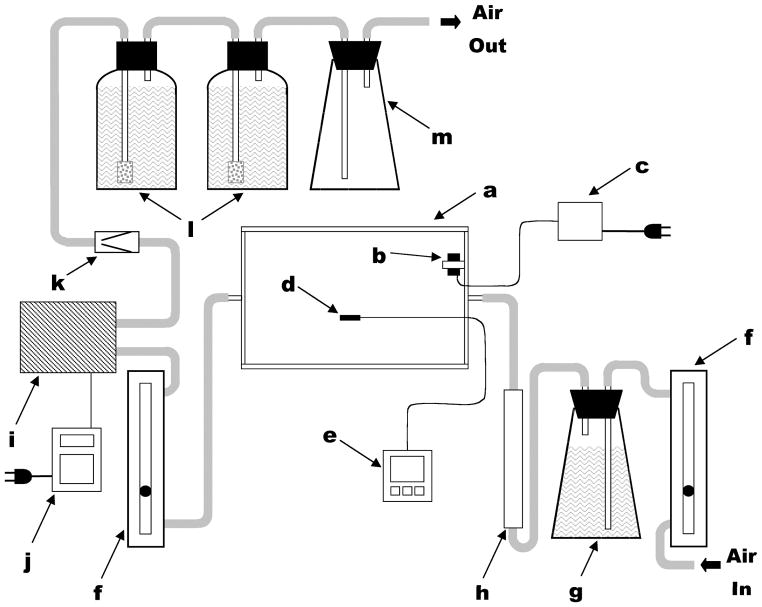
In order to isotopically-label flavonoids from plant cell cultures, 13C or 14C-labeled carbohydrate source (e.g. sucrose or glucose) was introduced in tandem with deliberate elicitation of culture microenvironment (altered irradiance, cold temperatures, change in nitrogen profile, or osmotic stress) to provoke a shift to secondary metabolite production12,13. Specifically for radiolabeling work using living cell cultures, 14C-labeled sugars in the culture broth are oxidized for metabolic energy, leading to the release of 14CO2 from cellular respiration. Therefore, a custom-built growing chamber was constructed in our work, to sequester labeled CO2 which may be expelled by these living metabolizing plant cells5. The transparent plexiglass chamber (polyacrylic walls and base; glass top) was affixed to the platform of a rotary shaker, and harbored 12 culture flasks. The chamber provided a sealed system which contained and trapped any respired carbon dioxide, yet provided for gas exchange and allowed access into the interior (Figure 1).
Figure 1.
Schematic representation of complete enclosed-chamber labeling system. Components (not drawn to scale) include: (a) polyacrylic chamber; (b) 12 V DC circulating fan; (c) 12 V DC power supply; (d) temperature probe; (e) data logger; (f) adjustable gas flow meters; (g) flask with NaOH and air tubes; (h) water vapor absorbent column; (i) air pump; (j) programmable timer; (k) one-way safety valve; (l) screw-capped bottles with NaOH and fritted-glass air bubblers; (m) aerosol trap. Components used in air handling were connected using flexible plastic tubing (indicated in gray). Reprinted from Grusak et al. In Vitro Cell Devel Biol Plant 2004, 40:80–85 by the Society for In Vitro Biology. Reproduced with permission of the copyright owner.
For preparation of biolabeled polyphenolic extracts from plant cell cultures, grape or ohelo berry cell cultures were harvested, and cells were extracted using 70% acetone, which was subsequently removed. The concentrate was analyzed on HPLC-LCQ Deca XP mass spectrometer (Thermo Finnigan Corp., San Jose, CA) electrospray ionization (ESI) in the positive ion mode (m/z 150–2000), with a photodiode array (PDA) detector (200–600 nm). The HPLC separations were carried out on a C18 reversed-phase column (150 × 2.1 mm i.d., particle size 5 μm,) (VYDAC, Western Analytical, Murrieta, CA). The presence of proanthocyanidins and anthocyanins in the extract was verified based on the molecular mass and published data12. Biolabeled fractions with similar composition were combined into major fractions, enrichment level (μCi if 14C per unit mass, or concentration of 13C label) and administered via gavage to fasted rats 14. Rats were surgically implanted with jugular catheters, subcutaneous UF probes, and MD brain probes to sample blood, interstitial fluid, and brain micrdialysate, respectively, and placed in Culex automated blood sampling and caging systems for up to 12 days14.
Phytochemical analyses
Samples obtained from the TIM-1 model representing different compartments (40 mL) were freeze-dried at −51 °C. The dried samples were extracted with 2× 20 mL MeOH (0.3% TFA) with sonication and centrifuged at 4 °C, 4000 rpm for 20 minutes. The supernatant was evaporated to dryness and weight was recorded. The dry extract was then dissolved in 10 mL MeOH (0.3 % TFA), filtered (0.2 μm PTFE filter) and submitted to HPLC analysis for quantification of anthocyanin content. HPLC analyses of both TIM-1 and biolabeling sample analyses were conducted using a 1200 HPLC (Agilent Technologies, Santa Clara, CA) with a photodiode array (PDA) detector, and an autosampler with Chemstation software as a controller and for data processing. Anthocyanin separation was performed using a reversed phase Supelcosil-LC-18 column, 250 mm × 4.6 mm × 5 mm (Supelco, Bellefonte, PA). The mobile phase consisted of 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was constant during HPLC analysis at 1 mL/min with a step gradient of 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively. Samples were injected (10 μL) onto the HPLC column with a constant temperature of 20 °C. Three concentrations of cyanidin-3-O-glucoside were prepared at 1.0, 0.5, and 0.25 mg/mL where 5 μL was injected as an external standard. Quantification of anthocyanins was performed from the peak areas recorded at 520 nm with reference to the calibration curve obtained with cyanidin-3-O-glycoside. Similar HPLC analysis was performed on samples obtained after biolabeling of cell culture extracts and from tissues and serum of rats as previously described 12.
Results & Discussion
Bioaccessibility determinations in the TIM-1 model
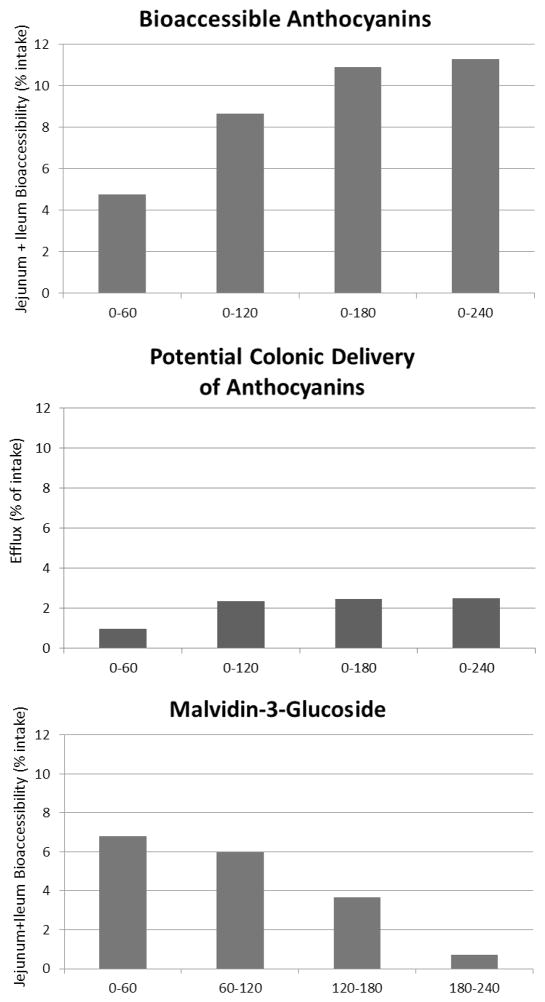
In a preliminary study using TIM-1 in the fasted state, the bioaccessibility of WBB (wild blueberry) anthocyanins was measured over a 4 h period as shown in Figure 2. The greatest increase in anthocyanins bioaccessibility occurred during the second hour, and there were clear differences between anthocyanin species in relative bioaccessibility. Over the 4 hour period, the total bioaccessiblity of the anthocyanins as a group was less than 10% of that administered to TIM-1. In current experiments bioaccessibility of individual anthocyanins from WBB versus the mixture at different feeding levels, under both fed and fasted conditions, is under evaluation.
Figure 2.
Determination of the bioaccessibility of wild blueberry anthocyanins using TIM-1. Based on the percentage of anthocyanins provided to TIM (intake), bioaccessibility of anthocyanins was measured over 4 hours in the fasted state and presented as the cumulative anthocyanins secreted into the combined jejunum and ileum compartments (top panel), anthocyanins collected during each hour as efflux from TIM-1 (middle panel) or amounts of malvidin-3-glucoside collected during each hour (bottom panel).
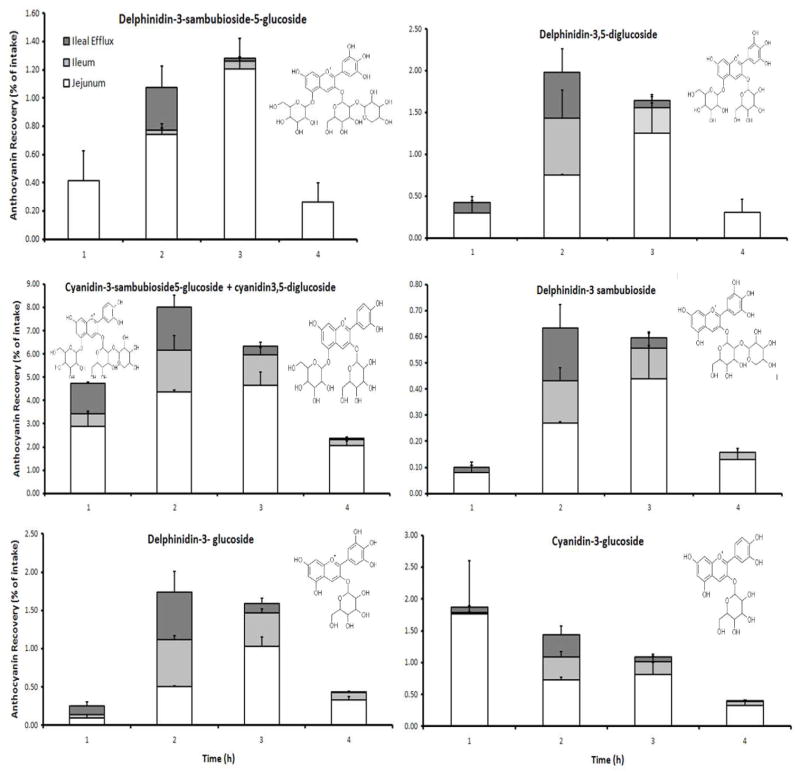
Results for MB (maqui berry) indicated that small-intestinal absorption (bioaccessibility) of the anthocyanins varied considerably depending on the individual anthocyanin species (Table 1). The concentrations of the individual anthocyanins were measured separately from samples of the jejunal, ileal and ileal efflux compartments collected each 1 h for 4 h. The jejunal and ileal absorption determinations estimate the amounts of the compounds that are bioaccessible for jejunal and ileal absorption in humans. The ileal efflux determinations represent the amount of the anthocyanins that would reach the colon in humans and then be exposed to the high density microbiota of the large intestine that are responsible for further digestion and bioconversion. Most of the anthocyanins from MB had a relatively low total bioaccessibility (0.75–4.28% of the intake) with the exception of the mixed sample consisting of cyanidin-3-sambubioside-5-glucoside + cyanidin-3,5-diglucoside which was higher (17.84% of the intake). It is possible that the combination of the cyanidin with 2 sugars attached creates a relatively more stable anthocyanin structure, but this effect has not been previously addressed.
Table 1.
Bioaccessibility of maqui berry anthocyanins using TIM System.
| Chemical Name | Total MB amount fed to TIM (mg) | Total Bioaccessible Anthocyanin Amount (mg) | Total Bioaccessibility as % of intake | Recovery in Ileal Efflux as a % of Intake |
|---|---|---|---|---|
| Delphinidin-3-sambubioside-5-glucoside | 33.80 | 0.92 +/− 0.06 | 2.72 +/− 0.17 | 0.32+/− 0.32 |
| Delphinidin-3,5-diglucoside | 32.50 | 1.17 +/− 0.57 | 3.61 +/− 1.74 | 0.77 +/− 0.77 |
| Cyanidin-3-sambubioside-5-glucoside + cyanidin3,5-diglucoside | 22.40 | 4.00 +/− 0.59 | 17.84 +/− 2.61 | 3.55 +/− 1.54 |
| Delphinidin-3-sambubioside | 63.50 | 0.78 +/− 0.31 | 1.23 +/− 0.49 | 0.26 +/− 0.24 |
| Delphinidin-3-glucoside | 96.05 | 3.02 +/− 1.83 | 3.15 +/− 1.91 | 0.87 +/− 0.78 |
| Cyanidin-3-sambubioside | 2.15 | 0.02 +/− 0.02 | 0.75 +/− 0.75 | 0.33 +/− 0.33 |
| Cyanidin-3-glucoside | 56.75 | 2.42 +/− 0.38 | 4.28 +/− 0.67 | 0.52 +/− 0.40 |
| Total | 307.15 | 12.34 +/− 3.83 | 4.03 +/− 1.25 | 0.81 +/− 0.59 |
Delphinidin-3-sambubioside-5-glucoside (D3S5G) had a lower bioaccessibility (2.7%) relative to the intake, than delphinidin-3,5-diglucoside (D3G5G; 3.6%). Likewise, a greater bioaccessibility of delphinidin and cyanidin glucosides was observed than for their corresponding sambubioside derivatives (Table 1 and Figure 3). Time course data demonstrate that most of the anthocyanins showed the greatest bioaccessibility during the second and third hour after intake, with the exception of cyanidin-3-glucoside (Figure 3).
Figure 3.
Bioaccessibility of six abundant maqui berry anthocyanins expressed as percentage of intake using TIM. Recoveries were calculated as a percentage of the ingested amount of anthocyanin that was administered to TIM. The availability for intestinal absorption (bioaccessibility) of each anthocyanin was separately measured in 1 h time periods for 4 h. The amount of jejunal absorption (white bars), ileal absorption (light gray bars) and ileal efflux (dark grey bars) are shown separately for each time period as determined by HPLC/MS and presented as stacked bar graphs, which represent the total bioaccessibility of each anthocyanin for each time period. The chemical structure of each anthocyanin is shown. The results are the mean of two independent bioaccessibility experiments +/− the dispersion range.
The highest amounts of absorption for each of the anthocyanins occurred in the jejunum compartment. Over the 4 h duration of this study, the amount of total anthocyanins contained in the ileal efflux from TIM was very low (0.81%) suggesting that only low amounts of intact maqui berry anthocyanins would reach the colon in humans (Table 1).
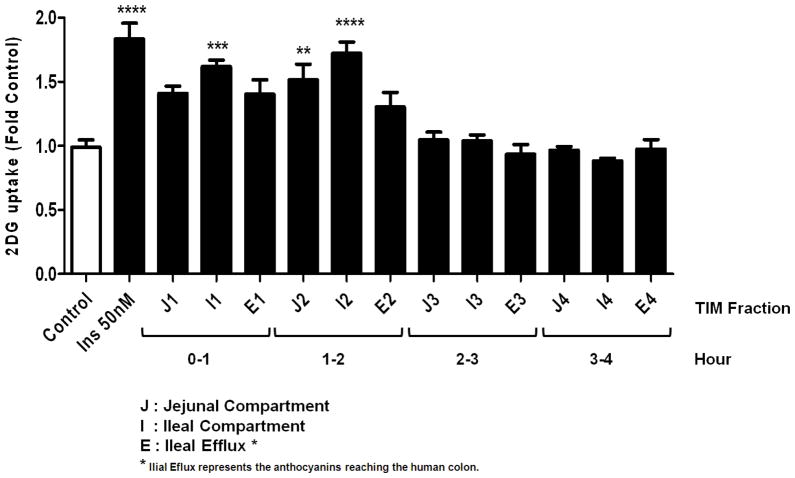
We have previously reported the anti-diabetic effect of anthocyanins from maqui berry15 and here we used rat H4IIE cells to study the effect of maqui berry-fed TIM-1 fractions on hepatic glucose uptake. No toxic effects were observed in any of the applied treatments. We observed that the most active fractions were collected from jejunal (J) and ileal (I) compartments during first two hours after feeding in the TIM-1 system (Figure 4).
Figure 4.
Effect of MB-TIM fractions on the glucose uptake of liver cells. The TIM fractions were collected in 1 h intervals during a 4 h digestion period. Glucose uptake of H4IIE cells was measured after treatment with MB-TIM fractions at 100 μg/mL, insulin at 50 nM or vehicle (Control). Glucose uptake was measured according to the protocols described in Materials and Methods. The experiment is representatitive of a series of experiments and values are the mean of three replicates +/− SD, **: p<0.05 vs control, ***: p<0.01 vs control, ****: p<0.001 vs initial, One-way ANOVA, Bonferroni’s Multiple Comparison Test
The data published on the bioavailability of dietary anthocyanins from berries have not always been consistent or complete with respect to their absorption and metabolism21–23. There seems to be a consensus, however, that nM to μM concentrations of the glycosylated forms of anthocyanins can reach the blood stream and target tissues within minutes and remain detectable up to several hours after the oral administration of anthocyanins from berries or berry extracts23, and the time required to reach the maximum plasma levels (Cmax) of anthocyanins ranges from 0.5 to 2 h. The data obtained from the gastrointestinal tract model (TIM-1) also showed that most of the anthocyanins of MB were bioaccessible within the first 2 h after feeding the TIM system, primarily in the jejunal and ileal compartments (Table 1 and Figure 3), which is also consistent with the bioactivity, as the most active fractions were also collected during the first two hours of digestion (Figure 4). The total bioaccessibility of the MB anthocyanins was relatively low, only 4.0% for combined anthocyanins (Table 1). Interestingly, the sambubiosides in MB were characterized with lower bioaccessibility than the glycosides, regardless of the anthocyanidin aglycone (Figure 3). For example cyanidin-3-sambubioside (C3S) had lower bioaccessibility than cyanidin-3-glucoside (Table 1). The difference in bioaccesibility of MB and WBB anthocyanins observed in the TIM experiments may be explained by the different anthocyanin profile of both extracts, which in turn can result in different degradation and bioconversion reactions occurring in the gastrointestinal tract. Anthocyanins in general are known to be unstable at high pH, and the shift from the acidic pH (pH 2.2) of the stomach to the almost neutral pH in the duodenum (pH 6.5) may be responsible for their specific hydrolysis and/or degradation. The hydrolysis of the anthocyanins with sugar substituents bound to the 3 and 5 positions, to their corresponding anthocyanins with a sugar bound to only the 3 position is possible, but cannot be determined from the data. Low concentrations of the intact anthocyanins were detectable in the ileal efflux from the TIM-1 system, which represents that portion of the extract that would be subject to bioconversion by the colon microflora23. Consistent with these low levels of anthocyanins, the fractions from efflux also lack bioactivity as shown in our glucose uptake experiments (Figure 4).
Bioavailability determinations using the isotope labeling strategy
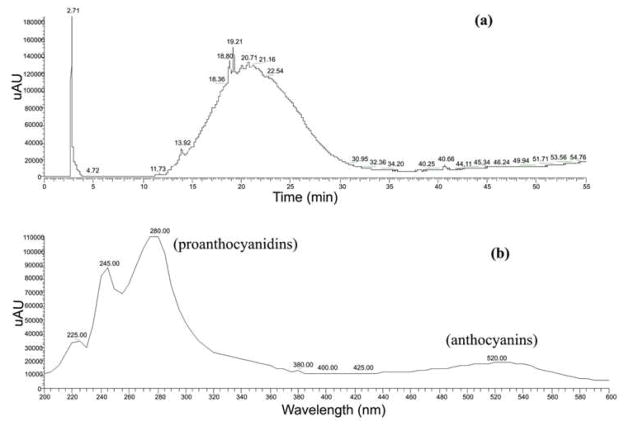
The 14C or 13C-biolabeling of flavonoids from rigorously-engineered continuous berry cell cultures revealed bioavailability distribution and pharmacokinetics in periphery and central nervous system following oral administration. The detected label allowed us to conclusively demonstrate that bioflavonoids or their metabolites are able to penetrate the blood-brain barrier14, a phenomenon which has been subject to some debate, and is particularly relevant in light of the evidence suggesting polyphenolics can aid in prevention of Alzheimers and Parkinsons disease. Grape cell culture extracts were separated into 5 fractions; fractions I, II, and III (largely proanthocyanidin monomers, dimers, and trimers), and fractions IV and V (more highly polar anthocyanin glycosides). The anthocyanin glycosides (mainly cyanidins and peonidins) demonstrated enhanced absorption compared to the less polar, proanthocyanidin fractions barrier12,14. However, anthocyanin glycosides comprised up to 78% of the flavonoid content in the grape cell culture extracts, and proanthocyanidin content was relatively minor. The Vaccinium (ohelo berry) cultures yielded extracts that, unlike the grape cell cultures, were more highly enriched in proanthocyanidins (Figure 5). Proanthocyanidins (catechin/epicatechin) in ohelo ranged from monomers to polymeric forms of up to six molecules of catechin/epicatechin combinations. While the majority of polyphenolics in ohelo berry extract was in the form of proanthocyanidins, anthocyanins were also observed and consisted of mainly cyanidin 3-O-glucosides (Figure 5).
Figure 5.
HPLC output of ohelo berry cell culture enriched with 14C label showing the maximum absorption for proanthocynidins and anthocyanins using photodiode array detector (200–600 nm). (a) The characteristic proanthocyanidin ‘hump’ in Vaccinium berry spectra (b) Anthocyanins also present in ohelo radiolabeled extract.
The radiotracing strategy for tracking the metabolism of grape bioactive constituents demonstrated bioavailability in serum and interstitial fluid, as well as accumulation in organs and deposition into bone. Blood peak 14C concentration from various fractions of grape ranged from 15 min to 4 hours14.
Pros and cons of the two alternative methods
Two gaps in our understanding have hindered both elucidation of how biologically-active agents from plants prevent or provide therapy against human ailments, and translation of these insights into practical utility for human consumers:
A disconnect between epidemiological, laboratory and clinical evidence which suggests that biologically-active constituents from plants prevent or provide therapy against human ailments, versus multiple indicators which alternatively indicate that the key active compounds in dietary foods, after ingestion, have only limited absorption, and
A general lack of solid mechanistic insight as to how the plant phytochemicals trigger and sustain human health improvements, and whether they must be bioavailable in order to do so.
Although the TNO gastrointestinal model (TIM-1) and the radiolabeling strategy provide different kinds of information, both methods offer some insights into anthocyanin and other flavonoid absorption, bioavailability, distribution and kinetics post ingestion. Our laboratory and others have hypothesized that bioactive phytochemicals may not require large doses to be efficacious and maintain homeostasis in a human consumer but instead, 1) are biologically-active even with low density uptake of plant compounds or metabolites, 2) are active only by virtue of potentiating interactions with other ingested phytochemicals, or 3) are capable, upon intake into the gastrointestinal tract, of rapidly triggering or stimulating synthesis and release of endogenous human proteins which are active at the target site. Both TIM and radiolabeling strategies can facilitate investigation of these hypotheses, yet both models have limitations that preclude direct applicability of data to human studies.
A strong advantage to use of the TIM-1 model for tracking biodistribution of plant compounds is that it is understood, accepted, and familiar to the pharma/medical community, since it has been well validated for pharmaceutical and drug delivery evaluations. TIM does not measure bioavailability, but instead gauges the bioaccessibility of compounds in the diet. While the bioaccessibility data is not the same as bioavailability measured in animal models, the model does provide valid insights into relative stability of anthocyanins and other flavonoids, and the potential for different bioflavonoid entities to be bioavailable. The model illustrates differential bioaccessibility of different species of berry anthocyanins. The TIM-1 model is designed to mimic the biological environment of the duodenum, jejunum, and ileum but of course it cannot fully recreate the complex and dynamic biological environment. For example, the dialysis and filtering on which the TIM-1 model depends do not completely mimic the endothelial cells which line the human GI tract.
Other disadvantages include the inability of TIM-1 to assess colonic microconversion. While TIM-1 closely replicates all the dynamic changes that occur inside of the upper gastrointestinal tract (GIT), it does not contain any microorganisms. The upper GIT in humans does contain microorganisms that are thought to have beneficial effects on immune function, but they are believed to have negligible effects on the digestibility of its contents, unlike the microflora of the large colon that are responsible for widespread bioconversion. Since bioconversion in the colon is also significant for health and disease, a dynamic in vitro model of the large intestine that simulates colonic fermentation by intestinal microflora has also been developed and is called TIM-2. Like TIM-1, the TIM-2 is a computer controlled system that mimics all processes of the large intestine by maintaining standardized conditions of the lumen including pH, rate of secretion, delivery of substrate, peristaltic mixing, and transport of intestinal content. TIM-2, however, does contain complex anaerobic microbiota of human origin. The conversion of green tea, black tea, citrus and soy flavonoids into smaller phenolic acids has been examined using TIM-224. Since flavonoids are poorly absorbed from the gastro intestinal tract, the products that they become as a result of colonic conversion may be of greater health significance than the parent molecules. In this particular study, only 3,4-dihydroxyphenylacetic acid was shown to have antiproliferative activity in colon cancer cells (HCT116) out of all of the phenolic acids tested24. The study was conducted, however, with the direct addition of the flavonoid preparation to TIM-2, without consideration to the effects of the upper gastro intestinal tract (or TIM-1) on the composition of the flavonoids. The direct effects of colonic conversion on these flavonoids is a practical method for looking at metabolites and their respective activities but is not strictly relevant to the in vivo situation where particular compounds may not reach the colon in their native form. This consideration is especially important for the anthocyanins from blueberry that are notoriously unstable at neutral pH. Our preliminary data from TIM-1 suggests that only very small amounts of anthocyanins are able to reach the colon under the fasted conditions used for preliminary studies. TIM-1 results in this study were not incongruent with previously reported clinical studies with ileostomy patients25,26 which indicated relatively low recovery (1.5%) of cyanidin-3-glucoside in the first 4 h. All of the other anthocyanins monitored in this study (e.g. delphinidin-3-sambubioside-5-glucoside, delphinidin-3,5-diglucoside, etc), however, were not tracked in previous ileostomy studies. In spite of the limitations of an in vitro intestinal model like TIM, our results for the anthocyanin species in common with the ileostomy trials are similar. TIM bioaccessibility data overestimates actual bioavailability, however TIM provided comparative information on the relative stability of different anthocyanin species, demonstrated that some anthocyanin species are more accessible than others, and confirmed that most anthocyanins would not reach the colon intact. Kalt et al.7 did find intact ANC in all the tissues from blueberry-fed pigs examined, and He et al.6 also found good relative stability of anthocyanins in the GIT. Additional research is needed to validate the use of TIM-1 as a predictive model of human gastrointestinal physiology.
Radio-tracing of natural mixtures as well as isolated semi-purified phytochemicals, when administered via gavage or controlled feeding to rodents fitted with subcutaneous and jugular probes and connected to an automated blood sampling system for blood, interstitial fluid, urine, and feces collection, can definitively demonstrate kinetics of the transport, with serum and tissue distribution tracked via scintillation counting and by accelerator mass spectrometry. Pharmacokinetics and biodistribution can be stringently and precisely quantified, allowing assessment of bioavailability and bioaccessibility, and pinpoint mechanisms of action in localized regions, e.g. anti-inflammatory or anti-androgenic effects, blocking localized enzymes in situ, or membrane fortification. However, all of the administered label has not been recovered in these analyses, as labeled flavonoids can accumulate in organs that are not analyzed in any particular experiment. In addition, although they are similar, flavonoid profiles accumulated in cell cultures may not be identical to profiles found in fruits or vegetative tissues in nature12. Most importantly, only a subset of functional food plants have been successfully adapted to plant cell cultures, which precludes use of this technique to examine the bioactive phytochemicals produced by most plant species.
Unlike TIM-1, the radio-tracing methods allow tracking beyond the ileal efflux and into the colon, as well as clearance via the urinary tract and colon. Because they are poorly absorbed, ingested PACs are likely to remain for an extended time in the colon, available for transformation by gut microflora. Transformation by endogenous microflora is likely to impact on both biological efficacy and bioavailability27. The radiolabeling strategy in animal models can provide a means to metabolically track and investigate proanthocyanidin metabolites after conversion by colonic bacteria, which has implications for gut health and function.
Acknowledgments
We appreciated the technical assistance of Randy B. Rogers in radiolabeling trials. Thanks to the Wild Blueberry Association of North America and Fundación Chile for providing fruit samples for use in the TIM-1 experiments.
This research was supported through the National Center for Complementary and Alternative Medicine sponsored Purdue University of Alabama Birmingham Botanicals Center for Dietary Supplement Research (NIH, 2 P50 AT000477-06) and the NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome (NIH, 1-P50 AT002776-01).
References
- 1.Stull A, Cash K, Johnson W, Champagne C, Cefalu W. Bioactivities in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140:1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vauzour D, Vafeiadou K, Rendeiro C, Corona G, Spencer J. The inhibitory effects of berry-derived flavonoids against neurodegenerative processes. J Berry Res. 2010;1:45–53. [Google Scholar]
- 3.Charron C, Kurilich A, Clevidence B, Simon P, Harrison D, Britz S, Baer D, Novotny J. Bioavailability of anthocyanins from purple carrot juice: effects of acylation and plant matrix. J Agric Food Chem. 2009;57:1226–1230. doi: 10.1021/jf802988s. [DOI] [PubMed] [Google Scholar]
- 4.Felgines C, Texier O, Besson C, Fraisse D, Lamaison JL, Remesy C. Blackberry anthocyanins are slightly bioavailable in rats. J Nutr. 2002;132:1249–1253. doi: 10.1093/jn/132.6.1249. [DOI] [PubMed] [Google Scholar]
- 5.Grusak MA, Rogers RB, Yousef GG, Erdman JW, Jr, Lila MA. An enclosed-chamber labeling system for the safe 14C-enrichment of phytochemicals in plant cell suspension cultures. In Vitro Cell Devel Biol. 2004;40:30–35. [Google Scholar]
- 6.He J, Wallace TC, Keatley KE, Faila ML, Giusti MM. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J Agr Food Chem. 2009;57:3141–3148. doi: 10.1021/jf900567t. [DOI] [PubMed] [Google Scholar]
- 7.Kalt W, Blumberg JB, McDonald JE, Vinquist-Typchuk MR, Filmore SA, Graf B, O’Leary J, Milbury P. Identification of anthocyanins in the liver, eye and brain of blueberry-fed pigs. J Agr Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agr Food Chem. 2005;53:7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- 9.Grace M, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanquet-Diot S, Soufi M, Rambeau M, Rock E, Alric M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J Nutr. 2009;139:876–883. doi: 10.3945/jn.108.103655. [DOI] [PubMed] [Google Scholar]
- 11.Verwei M, Arkbage K, Havenaar R, Van den Berg H, Witthoft C, Schaafsma G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J Nutr. 2003;133:2377–2383. doi: 10.1093/jn/133.7.2377. [DOI] [PubMed] [Google Scholar]
- 12.Yousef G, Seigler D, Rogers R, Kraft T, Knight C, Grusak M, Erdman J, Lila MA. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. J Agr Food Chem. 2004;52:1138–1145. doi: 10.1021/jf035371o. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann N, Campbell J, Rupassara S, Rogers R, Garlick P, Lila M, Erdman JW., Jr Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [13C]-carotenoid production. Journal of Agricultural and Food Chemistry. 2010;58:9979–9987. doi: 10.1021/jf101942x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janle E, Lila M, Grannan M, Wood L, Higgins A, Yousef G, Rogers R, Kim H, Jackson G, Ho L, Weaver C. Pharmacokinetics and tissue distribution of 14C labeled grape polyphenols in the periphery and in the central nervous system following oral administration. J Med Food. 2010;13:926–933. doi: 10.1089/jmf.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, Dorn R, Grace MH, Lila MA, Raskin I. In vitro and in vivo anti-diabetic effects of anthocyanins from maqui berry (Aristotelia chilensis) Food Chem. 2012;131:387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minekus M, Smeets-Peeters M, Bernalier A, Marol-Bonnin S, Havenaar R, Marteau P, Alric M, Fonty G, Huis in’t Veld JH. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol. 1999;53:108–114. doi: 10.1007/s002530051622. [DOI] [PubMed] [Google Scholar]
- 17.Anson NM, Selinheimo E, Havenaar R, Aura AM, Mattila I, Lehtinen P, Bast A, Poutanen K, Haenen GR. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J Agr Food Chem. 2009;57:6148–6155. doi: 10.1021/jf900492h. [DOI] [PubMed] [Google Scholar]
- 18.Larsson M, Minekus M, Havenaar R. Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in-vitro gastrointestinal model. J Sci Food Agric. 1999;73:99–106. [Google Scholar]
- 19.Naylor TA, Connolly PC, Martini LG, Elder DP, Minekus M, Havenaar R, Zeijdner E. Use of a gastro-intestinal model and GASTROPLUS™ for the prediction of in vivo performance. Ind. Pharm. 2006;12:9–12. [Google Scholar]
- 20.Mateo AN, Van den Berg R, Havenaar R, Bast A, Haenen G. Bioavailability of ferulic acid is determined by its bioaccessibility. J Cereal Sci. 2009;49:296–300. [Google Scholar]
- 21.Prior RL, Gu L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry. 2005;66:2264–2280. doi: 10.1016/j.phytochem.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Pittman HE, 3rd, McKay S, Prior RL. Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs. J Nutr. 2005;135:2417–2424. doi: 10.1093/jn/135.10.2417. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Pittman HE, 3rd, Prior RL. Fate of anthocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption. J Agr Food Chem. 2006;54:583–589. doi: 10.1021/jf052108+. [DOI] [PubMed] [Google Scholar]
- 24.Gao K, Xu A, Krul C, Venema K, Liu Y, Niu Y, Lu J, Bensoussan L, Seeram N, Heber D, Henning S. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J Nutr. 2006;136:52–57. doi: 10.1093/jn/136.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Barrio R, Borges G, Mullen W, Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem. 2010;58:3933–3939. doi: 10.1021/jf100315d. [DOI] [PubMed] [Google Scholar]
- 26.Kahle K, Kraus M, Scheppach W, Ackermann M, Ridder F, Richling E. Studies on apple and blueberry fruit constituents: do the polyphenols reach the colon after ingestion? Mol Nutr Food Res. 2006;50:418–423. doi: 10.1002/mnfr.200500211. [DOI] [PubMed] [Google Scholar]
- 27.Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signaling. 2001;3:957–967. doi: 10.1089/152308601317203503. [DOI] [PubMed] [Google Scholar]