Abstract
BACKGROUND
Cruciferous vegetable constituent phenethyl isothiocyanate (PEITC) causes apoptosis in prostate cancer cells through mechanisms not fully understood. The present study was designed to determine the role of inhibitor of apoptosis (IAP) family proteins in PEITC-induced apoptosis induction.
METHODS
Effect of PEITC treatment on protein and mRNA expression of IAP in cells was determined by western blotting and reverse transcription PCR, respectively. Immunohistochemistry was performed to determine the in vivo effect of PEITC administration on X-linked inhibitor of apoptosis (XIAP) and Survivin protein expression. Overexpression of desired protein was achieved by transient transfection. Cell viability was determined by trypan blue dye exclusion assay, whereas apoptosis was quantified by measurement of histone-associated DNA fragment release into the cytosol. Transwell chamber assay was used to determine cell migration.
RESULTS
Exposure of PC-3 and LNCaP human prostate cancer cells to PEITC resulted in downregulation of XIAP and Survivin proteins and Survivin mRNA. PEITC administration to Transgenic Adenocarcinoma of Mouse Prostate mice caused modest but significant downregulation of XIAP and Survivin proteins in the dorsolateral prostate. Proapoptotic response to PEITC was significantly attenuated by ectopic expression of XIAP and Survivin proteins. Survivin overexpression also conferred modest but significant protection against PEITC-mediated inhibition of PC-3 cell migration.
CONCLUSIONS
The present study demonstrates that cellular responses to PEITC, including apoptosis induction and inhibition of cell migration, in prostate cancer cells are mediated by downregulation of XIAP and/or Survivin, which may serve as valid biomarkers of PEITC response in future clinical investigations.
Keywords: Phenethyl Isothiocyanate, XIAP, Survivin, Chemoprevention
INTRODUCTION
Bioactive food components continue to gain traction for chemoprevention of prostate cancer [1–3], which is a leading cause of cancer-related death among American men [4]. Phenethyl isothiocyanate (PEITC) is one such compound that is under active investigation for chemoprevention of prostate cancer as well as other types of malignancies, including colon, lung, and breast [5,6]. PEITC occurs naturally as a thioglucoside conjugate (gluconasturtiin) in a variety of edible cruciferous vegetables, and watercress is a particularly rich source of gluconasturtiin [7]. PEITC is generated upon cutting or chewing of cruciferous vegetables due to myrosinase-catalyzed breakdown of the gluconasturtiin [7]. Initial evidence supporting cancer protective effect of cruciferous vegetables against prostate cancer emerged from population-based case control studies [8,9]. For example, a multi-ethnic case-control study suggested an inverse association of cruciferous vegetable intake with the risk of prostate cancer [8]. In vivo chemopreventive efficacy of PEITC against prostate cancer has now been demonstrated in a transgenic mouse model (Transgenic Adenocarcinoma of Mouse Prostate mice; hereafter abbreviated as TRAMP mice) [10,11]. Incidence of prostate cancer in TRAMP mice was reduced significantly by dietary administration of 0.05% PEITC in association with decreased cell proliferation and increased apoptotic index [10]. We have also shown recently that PEITC administration through diet significantly decreases the incidence as well as burden (affected area) of poorly-differentiated cancer in the dorsolateral prostate of TRAMP mice [11]. Moreover, PEITC administration has been shown to inhibit growth of subcutaneous PC-3 and TRAMP-C1 prostate cancer xenografts as well as augment proapoptotic response to docetaxel against transplanted PC-3 cells in vivo in athymic mice without any adverse side effects [12–14]. Suppression of prostate cancer xenograft growth in athymic mice by N-acetylcysteine conjugate of PEITC has also been reported [15].
Research thus far indicates that PEITC is capable of suppressing multiple oncogenic signaling pathways that are hyperactive in human prostate cancer [16], including nuclear factor-κB [17], epidermal growth factor receptor [18], Akt [19], and signal transducer and activator of transcription 3 [20]. Because etiology of prostate cancer is complex often involving abnormalities in multiple checkpoints and activation of various oncogenes [16], ability to target multiple signaling pathways is desirable for potential chemopreventive agents.
Apoptosis induction seems critical for post-initiation cancer prevention by PEITC [6,10]. PEITC has been under intense scrutiny for elucidation of the mechanisms underlying its proapoptotic response. More recent studies from our laboratory have demonstrated convincingly that the PEITC-induced apoptosis in human prostate cancer cells is associated with production of reactive oxygen species, resulting from inhibition of the complex III of the mitochondrial respiratory chain, leading to activation of multidomain proapoptotic protein Bax [21]. Despite these advances, however, molecular regulators of PEITC-induced apoptosis downstream of reactive oxygen species-mediated Bax activation remain elusive.
Inhibitor of apoptosis (IAP) family proteins play critical role in apoptosis regulation by inhibiting caspases [22–24]. Elevated expression of IAP proteins, including X-linked inhibitor of apoptosis (XIAP) and Survivin has been reported in human prostate cancers [25–27]. Moreover, XIAP expression was shown to be a strong predictor of human prostate cancer recurrence [27]. The present study was designed to determine the role of IAP family proteins in PEITC-induced apoptosis using PC-3 and LNCaP human prostate cancer cells, and the dorsolateral prostates from control and PEITC-treated TRAMP mice.
MATERIALS AND METHODS
Reagents
PEITC (purity ≥98%) was purchased from LKT Laboratories (St. Paul, MN). Stock solution of PEITC was prepared in dimethyl sulfoxide (DMSO), and diluted with complete media immediately before use. An equal volume of DMSO (final concentration <0.1%) was added to the controls. Cell culture reagents, including F-12K medium, fetal bovine serum, and penicillin/streptomycin antibiotic mixture were purchased from Invitrogen-Life Technologies (Carlsbad, CA). RPMI1640 medium was from Mediatech (Manassas, VA). Stromal cell growth medium and prostate epithelial cell growth medium were purchased from Lonza (Walkersville, MD). Antibodies against cIAP2 and cleaved caspase-3 were purchased from Cell Signaling Technology (Danvers, MA); antibody against XIAP used for western blotting was purchased from BD Biosciences ( San Jose, CA); antibody against XIAP used for immunohistochemistry was purchased from Abcam (Cambridge, MA); anti-Survivin antibody (for immunoblotting and immunohistochemistry) was purchased from Novus Biologicals (Littleton, CO); antibody against poly-(ADP-ribose)-polymerase (PARP) was from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-actin antibody, MG132, Hematoxylin solution, and Eosin Y solution were from Sigma-Aldrich (St. Louis, MO). Fugene6 transfection reagent and a kit for quantitation of histone-associated apoptotic DNA fragment release into the cytosol were purchased from Roche Diagnostics (Indianapolis, IN). Transwell Permeable Support (8 μm polycarbonate membrane) chambers used for cell migration assay were purchased from Corning (Corning, NY).
Cell Lines, Cell Viability Assay, and Determination of Apoptosis
PC-3 and LNCaP human prostate cancer cells were obtained from the American Type Culture Collection(Manassas, VA). Cell line authentication was done by Research Animal Diagnostic Laboratory (University of Missouri, Columbia, MO) to test for interspecies contamination and alleles for short tandem repeats identifiable in the American Type Culture Collection database. The cells were last tested in February 2011. Each cell line was found to be of human origin. The genetic profiles for PC-3 and LNCaP cells were consistent with the genetic profiles in the American Type Culture Collection database. The LNCaP cells were maintained in RPMI 1640 medium supplemented with 1 mM sodium pyruvate, 10 mM HEPES, 0.24% glucose, 10% (v/v) fetal bovine serum, and 1%penicillin/streptomyci n antibiotic mixtures. PC-3 cells were cultured in F-12K Nutrient Mixture supplemented with 10% (v/v) fetal bovine serum and 1% penicillin/streptomycin antibiotic mixture. A normal human prostate stromal cell line (PrSC) and a normal human prostate epithelial cell line (PrEC) were purchased from Lonza and cultured according to manufacturer’s recommendations. Each cell line was maintained in an atmosphere of 95% air and 5% CO2 at 37°C. Viability of PC-3 and LNCaP cells after 6-, 12-, or 24-hour treatment with DMSO (control) or PEITC (2.5 or 5 μM) was determined by trypan blue dye exclusion assay essentially as described by us previously [28]. Histone-associated apoptotic DNA fragment release into the cytosol in PC-3, LNCaP, PrSC, and PrEC cells after 6-, 12-, or 24-hour treatment with DMSO (control) or PEITC (2.5 or 5 μM) was determined using a kit and by following the supplier’s instructions.
Western Blotting
After treatment, cells were collected and lysed as described by us previously [11]. Proteins were resolved by sodium dodecyl sulfate -polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (Perkin Elmer, Boston, MA). Immunoblotting was done as described by us previously [11–13].
Reverse Transcription-PCR
Total RNA from DMSO-treated control or PEITC-treated cells was isolated using RNeasy kit (Qiagen, Valencia, CA). The cDNA was synthesized with the use of SuperScript III reverse transcriptase with oligo(dT)20 primer. PCR was done using specific primers: Survivin forward: 5′-AGAACTGGCCCTTCTTGGAGG-3′ and Survivin reverse:
5′-CTTTTTATGTTCCTCTATGGGGTC-3′; XIAP forward:
5′-AAGAGAAGATGACTTTTAACAG-3′ and XIAP reverse:
5′-TGCTGAGTCTCCATATTGCC-3′ with the following amplification conditions: Survivin 94°C for 2 minutes, 28 cycles at 94°C for 15 seconds, 60°C for 20 seconds, 72°C for 15 seconds, and XIAP 94°Cfor 3 minutes, 30 cycles at 94°C for 45 seconds, 48°C for 45 seconds, 72°C for 45 seconds. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control as in our previous study [29]. All primers were purchased from Invitrogen-Life Technologies.
Immunohistochemical Analysis for XIAP and Survivin Protein Expression
Immunohistochemistry for XIAP and Survivin was performed essentially as described by us previously for other proteins [11]. Briefly, dorsolateral prostate sections (3–4 μm) from control and PEITC-treated TRAMP mice were quenched with 3% hydrogen peroxide and blocked with normal serum. The sections were then probed with the desired primary antibody (anti-XIAP or anti-Survivin), washed with Tris-buffered saline (25 mM Tris containing 150 mM NaCl and 2 mM KCl, pH 7.4), and incubated with an appropriate secondary antibody. Characteristic brown color was developed by incubation with 3,3′-diaminobenzidine. The sections were counterstained with Mayer’s Hematoxylin, and examined under a Leica microscope (Leica Microsystems, Bannockburn, IL). The images were captured with Image ProPlus 5.0 software (Media Cybernetics, Bethesda, MD) as previously described [11]. Atleast seven non-overlapping and non-necrotic images were captured from each section and analyzed with the use of Red-Yellow-Blue color histogram tool from Positive Pixel Count V9 algorithm of Aperio Image Scope software (Aperio Technologies, Vista, CA). This software automatically counts blue-negative and Red-Orange-Yellow-positive pixels and categorizes them according to intensity scale of weak positive, positive, and strong positive. Results are automatically computed as percent positive pixel intensity and area.
Transient Transfection
PC-3 and LNCaP cells (1.5–2×105cells/6 -well plate) were transiently transfected at about 50–60% confluency with empty pcDNA3.1 vector or pcDNA3.1 vector encoding for XIAP or empty pCMV6-AC-GFP vector or pCMV6-AC-GFP vector encoding for Survivin using Fugene6 transfection reagent. Twenty-four (PC-3) to thirty-six hours (LNCaP) after transfection, cells were treated with DMSO (control) or PEITC for 24 hours. Cells were then collected and processed for immunoblotting, cell viability assay, cell migration assay, and determination of histone-associated DNA fragment release into the cytosol.
Cell Migration Assay
Empty vector -transfected control and Survivin overexpressing PC-3 cells (2×105) were suspended in serum-free medium and placed in the upper compartment of Transwell chamber containing 8 μm polycarbonate membrane. After 24 hours of incubation with the indicated concentrations of PEITC, non-motile cells from the upper surface of the filter were removed using a cotton swab. The motile cells from the bottom face of the filter were fixed with methanol and stained with hematoxylin and eosin.
RESULTS
Kinetics of PEITC-Mediated Inhibition of Cell Viability and Apoptosis Induction
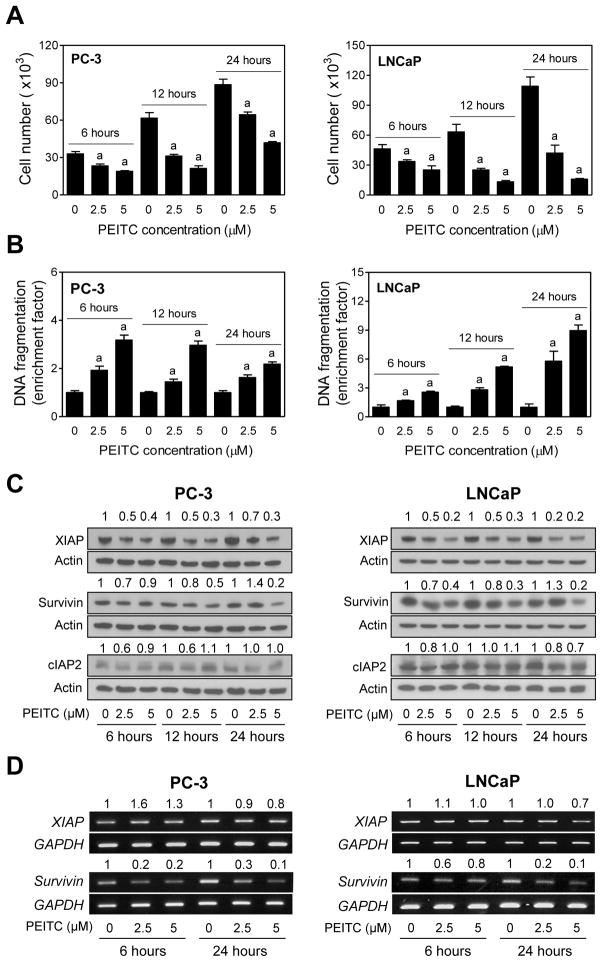
Initially we determined the dose-response and time course-kinetic effect of PEITC treatment on cell viability and apoptosis induction using a pair of well-characterized human prostate cancer cells (PC-3 and LNCaP) differing in their hormone responsiveness and the p53 status. The PC-3 cells are androgen-independent and lack functional p53, whereas the LNCaP cell line is androgen -responsive and expresses wild-type p53. PEITC treatment reduced viability of PC-3 and LNCaP cells in a dose-dependent manner, and this effect was evident as early as 6 hours after treatment at both 2.5 and 5 μM concentrations (Fig. 1A). The LNCaP cell line was relatively more sensitive to PEITC-mediated reduction in cell viability compared with PC-3 cells (Fig. 1A). PEITC-mediated reduction in cell viability was accompanied by dose-dependent apoptosis in both cell lines (Fig. 1B). In addition, the PEITC-induced apoptosis was also evident as early as 6 hours after treatment in both cells (Fig. 1B). In agreement with cell viability data, the LNCaP cell line was relatively more sensitive to PEITC-induced apoptosis compared with PC-3 cells. These results indicated that inhibition of cell viability by PEITC treatment was associated with apoptosis induction.
Fig. 1.
PEITC treatment inhibits cell growth and induces apoptosis through down -regulation of XIAP and Survivin proteins in cultured human prostate cancer cells. A: Effect of PEITC treatment on viability of PC -3 and LNCaP. The cells were treated for specified time periods with DMSO (control)or the indicated concentrations of PEITC and then number of viable cells w as determined by trypan blue dye exclusion assay. Results shown are mean ±SD ( n=3). Significantly different (P< 0.05) compared with acorresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test. B: Effect of PEITC treatment on histone-associated apoptotic DNA fragment release into the cytosol in PC-3 and LNCaP cells. The cells were treated for specified time points with DMSO (control) or the indicated concentrations of PEITC. Apoptosis enrichment relative to corresponding DMSO-treated control is shown. Results shown are mean ±SD ( n=3). Significantly different (P <0.05) compared with acorresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test. C: Western blotting for XIAP, Survivin, and cIAP2 proteins using lysates from PC-3 and LNCaP cells treated with DMSO (control) or the indicated concentrations of PEITC for specified time periods. Blots were stripped and reprobed with anti-actin antibody to correct for differences in protein loading. Numbers on top of bands are fold changes in expression relative to corresponding DMSO-treated control. D: RT -PCR for XIAP and Survivin mRNA expression in PC-3 and LNCaP cells treated with DMSO (control) or the indicated concentrations of PEITC for specified time periods. Numbers above bands represent change in mRNA levels relative to corresponding DMSO-treated control. Each experiment was repeated at least twice.
PEITC Treatment Downregulated Expression of XIAP and Survivin proteins in Cultured PC-3 and LNCaP Cells
The IAP family proteins including XIAP, Survivin, and cIAP2 have emerged as critical regulators of apoptotic cell death by different stimuli [22–24]. We sought to test the possible role of these proteins in regulation of PEITC-induced apoptosis. As can be seen in Fig. 1C, treatment of PC-3 and LNCaP cells with PEITC resulted in a dose-dependent downregulation of XIAP protein expression that was evident as early as 6 hours after treatment and persisted for the duration of the study (24 hours). Effect of PEITC treatment on Survivin protein expression was relatively less pronounced compared with XIAP. Nonetheless, PEITC-mediated downregulation of Survivin was clearly discernible after treatment of PC-3 and LNCaP cells with 5 μM especially at the 24-hour time point (Fig. 1C). Expression of cIAP2 protein was either marginally reduced or not affected at all by PEITC treatment (Fig. 1C). Even though the kinetics of PEITC-mediated reduction in cell viability and apoptosis induction mirrored suppression of XIAP protein expression, the PEITC treatment does not universally decrease protein expression. For example, we have shown previously that the expression Bax or p38 protein is not affected in either PC-3 or LNCaP cells treated for 4-, 16-, or 24-hourswith even 10 μM PEITC [30,31]. We therefore conclude that the downregulation of XIAP and Survivin proteins by PEITC exposure is not a consequence of protein degradation occurring during the cell death process.
Reverse transcription-PCR was performed to test whether PEITC-mediated downregulation of XIAP and/or Survivin proteins was due to their transcriptional repression. PEITC treatment only marginally decreased the levels of XIAP mRNA in PC-3 as well as LNCaP cells (Fig. 1D). To the contrary, levels of Survivin mRNA were markedly suppressed by PEITC treatment in both PC-3 and LNCaP cells (Fig. 1D). These results indicated that the PEITC-mediated downregulation of Survivin, but not XIAP, protein expression was due to suppression of its mRNA level.
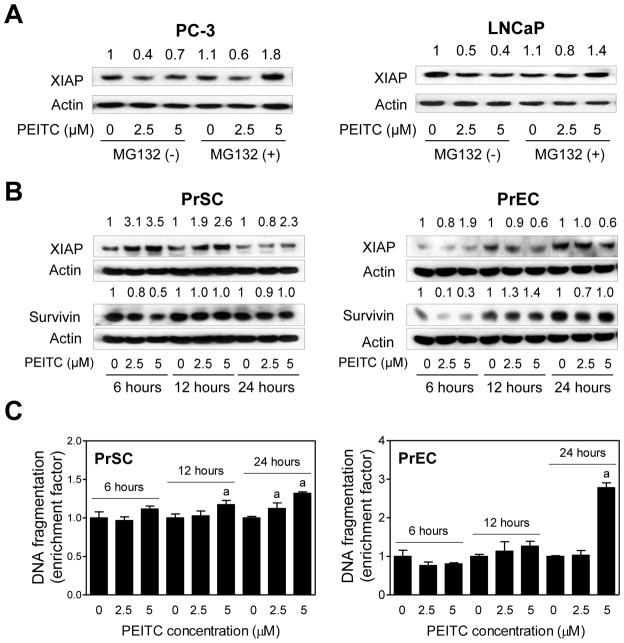
We raised the question of whether PEITC-mediated downregulation of XIAP protein was due to its proteasomal degradation. We explored this possibility using a proteasomal inhibitor (MG132). For these experiments, PC-3 and LNCaP cells were first treated with MG132 (1 μM for PC-3 and 5 μM for LNCaP) for 2 hours and then exposed to DMSO (control) or PEITC (2.5 and 5 μM)in the absence or presence of MG132 for an additional 6 hours. MG132 partially prevented XIAP degradation in both PC-3 and LNCaP cells( Fig. 2A). Based on these results, it is reasonable to conclude that PEITC treatment promotes proteasomal degradation of XIAP in PC -3 and LNCaP cells.
Fig. 2.
Normal prostate stromal and epithelial cells are relatively resistant to PEITC-induced apoptosis. A: Immunoblot for XIAP protein using lysates from PC-3 or LNCaP treated for 6 hours with DMSO (control) or the indicated concentrations of PEITC in the absence or presence of MG132 (1 μM for PC-3 and 5 μM for LNCaP). Cells were treated with MG132 for 2 hours and then exposed to DMSO or PEITC for an additional 6 hours with or without MG132. Blots were stripped and reprobed with anti-actin antibody to correct for differences in protein loading. Numbers on top of band represent change in XIAP protein level reletaive to DMSO-treated cells in the absence of MG132. B: Immunoblot for XIAP and Survivin proteins using lysates from PrSC and PrEC treated for specified time periods with DMSO (control) or the indicated concentrations of PEITC. Blots were stripped and reprobed with anti-actin antibody to correct for differences in protein loading. Numbers on top of bands are fold changes in expression level relative to the corresponding control. C: Quantitation of histone-associated DNA fragment release into the cytosol in PrSC and PrEC treated for specified time points with DMSO or the indicated concentrations of PEITC. Results shown are enrichment relative to corresponding DMSO-treated control. Results are mean ±SD (n=3). Significantly different (P< 0.05) compared with acorresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test. Similar results were observed in replicate experiments.
Effect of PEITC Treatment on Apoptosis Induction, and XIAP and Survivin Protein Expression in PrSC and PrEC Cells
We proceeded to test whether PEITC-mediated alterations in IAP family protein expression were selective for cancer cells. As shown in Fig. 2B, unlike PC-3 and LNCaP cells, PEITC treatment caused a marked increase in levels of XIAP protein in PrSC, which was sustained for the duration of the experiment. On the other hand, 5 μM PEITC treatment caused about 40% decrease in XIAP protein level in PrEC relative to DMSO-treated control at the 12- and 24-hour time points (Fig. 2B). Interestingly, PEITC-mediated decline in Survivin protein level was observed only at 6-hour time point in both PrSC and PrEC, and this effect was not sustainable at 12-or 24 -hour time point (Fig. 2B). The PrSC cell line was somewhat resistant to PEITC-induced apoptosis as evidenced by <50% increase in histone-associated DNA fragment release into the cytosol over DMSO-treated control (Fig. 2C). On the other hand, statistically significant increase in apoptotic DNA fragmentation in PrEC cells was clearly evident after 24-hour treatment with 5 μM PEITC. However, the PEITC-induced apoptosis in PrEC was not observed at 6-or 12 -hour time points at both concentrations (Fig. 2C). Collectively, these results indicated that PrSC and PrEC cells were significantly more resistant to PEITC-induced apoptosis (Fig. 2C) compared with prostate cancer cells (Fig. 1B).
PEITC Administration Suppressed Expression of XIAP and Survivin Proteins in the Dorsolateral Prostate of TRAMP Mice
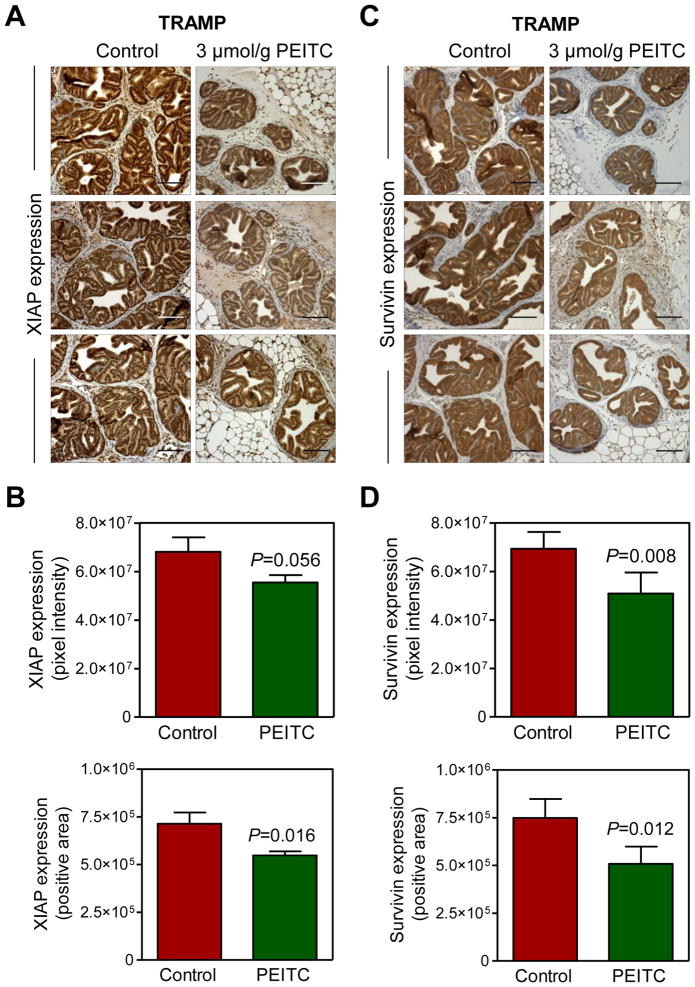
We have shown previously that dietary administration of PEITC (3 μmol PEITC/g diet) significantly inhibits prostate cancer development in TRAMP mice [11]. We used archived dorsolateral prostate sections from control and PEITC-treated TRAMP mice (n=4–5 mice in each group)to determine the in vivo relevance of the cellular findings shown in Fig. 1C. Representative immunohistochemical images for XIAP protein expression in the dorsolateral prostate sections from 3 mice of each group are shown in Fig. 3A. XIAP protein expression was quite high in epithelial cells of the dorsolateral prostates from control mice, although some positivity was discernible in the stroma. XIAP protein expression intensity and positive area in epithelial cells of the dorsolateral prostate was modestly but statistically significantly lower in the PEITC treatment group compared with control (Fig. 3B).
Fig. 3.
PEITC administration decreases expression of XIAP and Survivin proteins in the dorsolateral prostate of TRAMP mice. A: Representative immunohistochemical images depicting XIAP protein expression in the dorsolateral prostates of control and PEITC-treated TRAMP mice (magnification, ×200). Scale bar=100 μm. B: Quantitative analysis of XIAP protein expression in the dorsolateral prostates of control and PEITC-treated TRAMP mice. At least seven randomly selected fields from each section were analyzed. Results shown are mean ± SD (n=4 for both control and PEITC treatment groups). Statistical significance was determined by two-sided Student’s t-test. C: Representative immunohistochemical images depicting Survivin protein expression in the dorsolateral prostates of control and PEITC-treated TRAMP mice (magnification, ×200). Scale bar=100 μm. D: Quantitative analysis of Survivin protein expression in the dorsolateral prostates of control and PEITC-treated TRAMP mice. At least 7 randomly selected fields from each section were analyzed. Results shown are mean ±SD (n=5 for both control and PEITC treatment groups). Statistical significance was determined by two-sided Student’s t-test.
Fig. 3C depicts representative immunohistochemical images for Survivin protein expression in the dorsolateral prostate sections of control and PEITC treatment groups. Similar to XIAP, PEITC treatment resulted in a modest but significant decrease in the protein levels of Survivin compared with control (Fig. 3D).
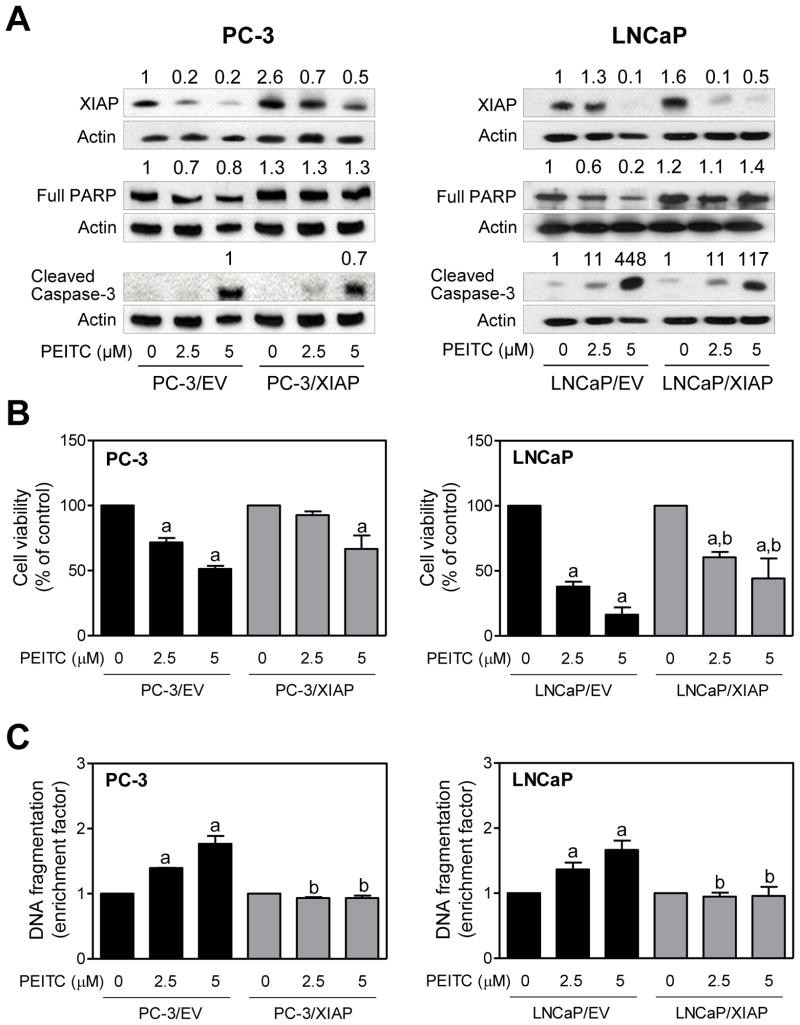
Ectopic Expression of XIAP and Survivin Proteins Conferred Significant Protection Against PEITC-Induced Apoptosis in PC -3 and LNCaP Cells
Next, we designed experiments to determine the functional significance of PEITC-mediated downregulation of XIAP protein in apoptosis regulation. Transient transfection of PC-3 and LNCaP cells with XIAP plasmid resulted in 2.6-and 1.6-fold increase in its protein level, respectively, compared with corresponding empty vector -transfected control cells (Fig. 4A). PEITC treatment caused downregulation of overexpressed XIAP protein in both PC-3 and LNCaP cells especially at the 5 μM concentration (Fig. 4A). In LNCaP cells, XIAP overexpression conferred partial but statistically significant protection against PEITC-mediated reduction in cell viability (Fig. 4B). Partial protection against reduction in cell viability is expected because PEITC treatment also causes autophagic cell death in PC-3 and LNCaP cells [32], and this response may not be sensitive to XIAP overexpression. A trend of a partial protection against PEITC-mediated reduction in cell viability by ectopic expression of XIAP was also observed in PC-3 cells, although the difference did not reach statistical significance (Fig. 4B). On the other hand, histone-associated apoptotic DNA fragment release into the cytosol resulting from PEITC exposure was significantly blocked by overexpression of XIAP in both PC-3 and LNCaP cells(Fig. 4C). Consistent with these results, PEITC -mediated decrease in levels of full-length PARP protein and cleavage of caspase-3 was markedly higher in empty vector-transfected PC-3 and LNCaP cells than in XIAP overexpressing cells (Fig. 4A).
Fig. 4.
XIAP overexpression confers protection against PEITC-induced apoptosis in human prostate cancer cells. A: Immunoblotting for XIAP, full-length PARP, and cleaved caspase-3 using lysates from PC-3 and LNCaP cells transiently transfected with empty vector (abbreviated as PC-3/EV and LNCaP/EV) or vector encoding for XIAP (abbreviated as PC-3/XIAP and LNCaP/XIAP) and treated for 24 hours with DMSO (control) or the indicated concentrations of PEITC. Numbers above bands represent change in protein levels relative to empty vector-transfected cells treated with DMSO(except for cleaved caspase-3 in PC-3 cells). Effect of PEITC treatments on (B)cell viability and (C) histone-associated DNA fragment release into the cytosol in PC-3 and LNCaP cells transiently transfected with empty vector or vector encoding for XIAP and treated for 24 hours with DMSO or the indicated concentrations of PEITC. Results shown are mean ± SD (n=3) normalized to corresponding DMSO-treated control. Significantly different (P< 0.05) compared with acorresponding DMSO-treated control and bbetween empty vector -transfected cells and XIAP overexpressing cells at each dose (0, 2.5 and 5 μM PEITC) by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
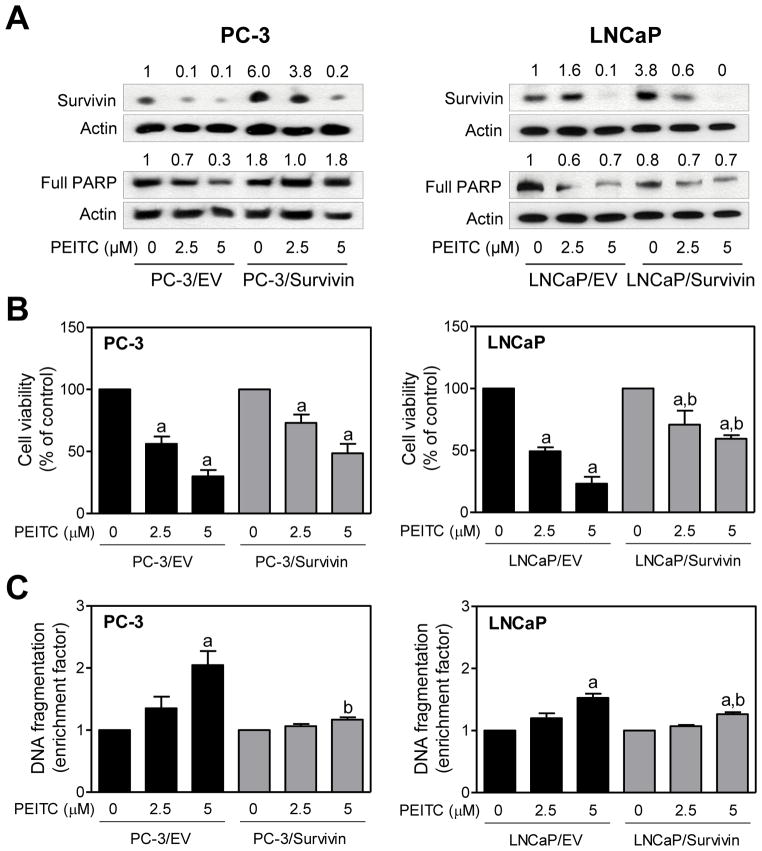
Similar experiments were carried out using empty vector -transfected PC-3 and LNCaP cells and those transfected with Survivin plasmid. Expression of Survivin protein was 6- and 3.8-fold higher in PC-3 and LNCaP cells transiently transfected with Survivin plasmid, respectively, compared with corresponding empty vector-transfected control cells (Fig. 5A). Similar to XIAP, overexpression of Survivin partially attenuated PEITC-mediated reduction in PC-3 and LNCaP cell viability (Fig. 5B). Moreover, Survivin overexpressing PC-3 and LNCaP cells were significantly more resistant to PEITC-induced apoptosis compared with empty vector-transfected control cells especially at the 5 μM concentration (Fig. 5C). Collectively, these results indicated that downregulation of XIAP and Survivin proteins contributed to PEITC-induced apoptosis in both PC -3 and LNCaP cells.
Fig. 5.
Survivin overexpression confers protection against PEITC-induced apoptosis in human prostate cancer cells. A: Immunoblotting for Survivin and full-length PARP using lysates from PC-3 and LNCaP cells transiently transfected with empty vector (abbreviated as PC-3/EV and LNCaP/EV) or vector encoding for Survivin (abbreviated as PC-3/Survivin and LNCaP/Survivin) and treated for 24 hours with DMSO (control) or the indicated concentrations of PEITC. Numbers above bands represent change in protein levels relative to empty vector-transfected cells treated with DMSO. Effect of PEITC treatments on (B)cell viability and (C)histone -associated DNA fragment release into the cytosol in PC-3 and LNCaP cells transiently transfected with empty vector or vector encoding for Survivin and treated for 24 hours with DMSO or the indicated concentrations of PEITC. Results shown are mean ±SD (n=3)and normalized to corresponding DMSO-treated control. Significantly different (P< 0.05) compared with acorresponding DMSO-treated control and bbetween empty vector-transfected cells and Survivin overexpressing cells at each dose (0, 2.5, and 5 μM PEITC) by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
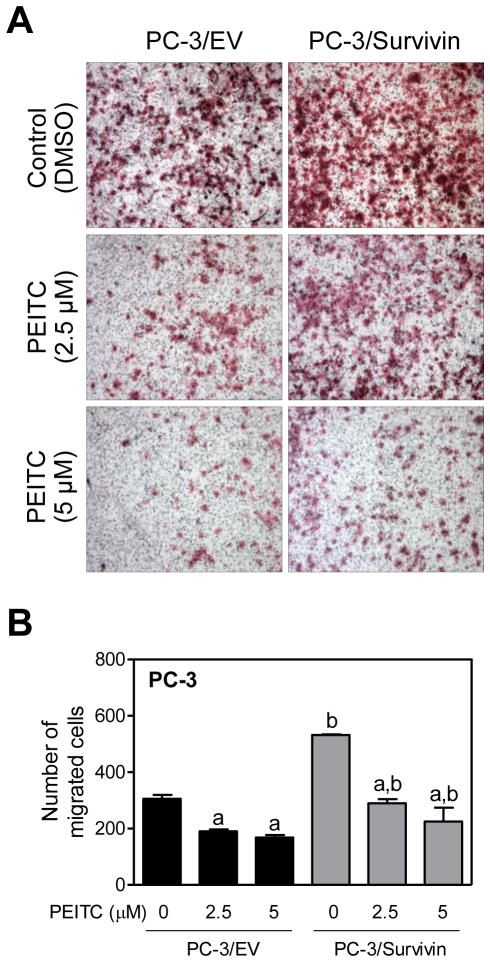
Effect of Survivin Overexpression on Inhibition of PC-3 Cell Migration by PEITC
Knockdown of Survivin protein expression has been shown to attenuate motility of prostate cancer cells[ 33]. We designed experiments to determine if PEITC inhibited migration of PC-3 cells and whether this effect was affected by Survivin overexpression. Because LNCaP cells are not invasive, this experiment was restricted to the PC-3 cell line. Transwell chamber assay revealed increased PC-3 cell migration by overexpression of Survivin consistent with literature data [33] (Fig. 6A). Moreover, Survivin overexpression conferred modest but significant protection against PEITC-mediated inhibition of PC-3 cell migration (Fig. 6B).
Fig. 6.
Survivin overexpression confers modest protection against PEITC-mediated inhibition of PC-3 cell migration. A: Representative images from Transwell chamber assay depicting migration by PC-3 cells transiently transfected with empty vector (abbreviated as PC -3/EV) or vector encoding for Survivin (abbreviated as PC-3/Survivin) and treated for 24 hours with DMSO (control) or the indicated concentrations of PEITC. B: Quantitation of migrated PC-3 cells from experiment shown in panel A. Experiment was repeated twice in duplicate and combined results are shown as mean ±SD ( n=4). Significantly different (P< 0.05) compared with acorresponding DMSO-treated control and bbetween empty vector -transfected cells and Survivin overexpressing cells at each dose (0, 2.5, and 5 μM PEITC) by one-way ANOVA followed by Bonferroni’s multiple comparison test.
DISCUSSION
PEITC is a promising cancer chemopreventive constituent of edible cruciferous vegetables with inhibitory effect in various chemically-induced rodent cancer models and transgenic mice prone to spontaneous cancer development [5,10,11,34–36]. Even though apoptosis induction is now regarded as an important mechanism for PEITC-mediated inhibition of post-initiation cancer development, molecular regulators of its proapoptotic response are not fully known. The primary objective of the present study was to determine if proapoptotic response to PEITC in prostate cancer cells is associated with alterations in expression of IAP family proteins. The IAP proteins are critical regulators of apoptosis by different stimuli, including death receptor activation, growth factor withdrawal, radiation exposure, and genotoxic insults [22–24]. In addition, IAP proteins are functionally implicated in adaptive response to cellular stress, differentiation, motility, and immune response [37]. This family of proteins is structurally characterized by baculovirus IAP repeat (BIR) domains [37,38]. Of the eight IAP protein members identified to date, XIAP is the best characterized and most potent inhibitor of caspase-3 and −7 [39]. Anti-caspase activity of XIAP is attributed to its BIR domains; BIR3 domain inhibits caspase-9 whereas the BIR2 linker region is implicated in inhibition of caspase-3 and −7 [39]. XIAP overexpression correlates with poor prognosis in childhood acute myelogenous leukemia and bladder cancer[39–41]. The present study reveals that PEITC exposure decreases protein expression of XIAP and Survivin in prostate cancer cells. In addition, PEITC-mediated inhibition of prostate cancer development in TRAMP mice in vivo [11] is associated with modest but significant suppression of both these proteins in the dorsolateral prostate. Overexpression of XIAP and Survivin confers statistically significant protection against PEITC-mediated DNA fragmentation in PC-3 and LNCaP cells. These observations lead us to conclude that PEITC-induced apoptosis in prostate cancer cells is mediated by suppression of XIAP and Survivin.
The present study reveals that the mechanisms underlying PEITC -mediated downregulation of XIAP and Survivin are different. PEITC treatment suppresses mRNA levels of Survivin but not the XIAP. Even though Survivin and XIAP proteins have functional resemblance of caspase inhibitory activity, their structures are strikingly different. The XIAP protein is structurally characterized by 3 BIR domains (BIR1, BIR2, and BIR3) and a RING-finger domain. The RING-finger domain functions as an E3 ubiquitin ligase [37,39]. On the other hand, Survivin contains a single BIR domain (BIR1) and an extended C-terminal helical coiled-coil domain, but lacks the RING-finger domain [37,39]. Consequently, XIAP is susceptible to auto-ubiquitination and proteasomal degradation [42,43]. Accordingly, PEITC-mediated suppression of XIAP protein is partially reversible in the presence of a proteasomal inhibitor (MG132). Previous studies have also shown that MDM2 physically interacts with the internal ribosome entry segment of the 5′-untranslated region of XIAP, and positively regulates XIAP internal ribosome entry segment activity [44]. It is possible that PEITC treatment affects translational of XIAP, but further studies are needed to experimentally validate this possibility.
Recent studies have pointed towards important roles of Survivin in both cell cycle regulation and apoptosis control [45]. Survivin expression is absent or very low in most terminally differentiated normal tissues, but this protein is overexpressed in different tumor types [45,46]. Survivin overexpression in tumors correlates with aggressive disease and treatment resistance [45,47]. We found that Survivin expression is also very high in the dorsolateral prostate of TRAMP mice and PEITC treatment significantly downregulates its protein level in TRAMP mice. Moreover, the PEITC-induced apoptosis is significantly attenuated by Survivin overexpression in prostate cancer cells. Survivin has been shown to regulate cell migration [33]. Our data are consistent with these findings because overexpression of Survivin results in modest but significant increase in PC-3 cell migration. In addition, Survivin overexpressing PC-3 cells are modestly but significantly more resistant to inhibition of cell migration by PEITC compared with empty vector -transfected cells.
A misperception about naturally-occurring chemopreventive isothiocyanatesis that they have common mechanism of action. While this reasoning is correct to some extent because most isothiocyanates, including PEITC and its close structural analog benzyl isothiocyanate (BITC) are inducers of phase 2 drug metabolizing enzymes [5,6]. However, evidence continues to accumulate to suggest that isothiocyanates with even subtle structural change may exhibit striking mechanistic differences. For example, we have shown previously that, unlike the results of the present study, BITC treatment causes induction of Survivin, albeit in human breast cancer cells [48]. Differences in efficacy between PEITC and BITC against chemically-induced cancers in rodents have also been documented [5]. Accordingly, extrapolation of mechanistic data between isothiocyanates is not advisable.
In conclusion, we provide experimental evidence to indicate that the PEITC-induced apoptosis in prostate cancer cells is associated with suppression of XIAP and Survivin proteins and this response is independent of p53 status and androgen responsiveness. PEITC-mediated suppression of XIAP and Survivin is also discernible in the dorsolateral prostate of TRAMP mice. Based on these observations, we suggest that XIAP as well as Survivin protein expression may be viable biomarkers of PEITC response.
Acknowledgments
This study was supported by the USPHS grant RO1 CA101753-08, awarded by the National Cancer Institute.
Abbreviations
- PEITC
phenethyl isothiocyanate
- TRAMP
transgenic adenocarcinoma of mouse prostate
- IAP
inhibitor of apoptosis
- XIAP
X-linked inhibitor of apoptosis
- DMSO
dimethyl sulfoxide
- PARP
poly-(ADP-ribose)-polymerase
- BIR
baculovirus IAP repeat
Footnotes
Disclosure Statement: None.
References
- 1.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104(1):339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 3.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269(2):305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32(3–4):395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12(1):87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 8.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9(8):795–804. [PubMed] [Google Scholar]
- 9.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92(1):61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 10.Barve A, Khor TO, Hao X, Keum YS, Yang CS, Reddy B, Kong AN. Murine prostate cancer inhibition by dietary phytochemicals-curcumin and phenyethylisothiocyanate. Pharm Res. 2008;25(9):2181–2189. doi: 10.1007/s11095-008-9574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powolny AA, Bommareddy A, Hahm ER, Normolle DP, Beumer JH, Nelson JB, Singh SV. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst. 2011;103(7):571–584. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao D, Lew KL, Zeng Y, Xiao H, Marynowski SW, Dhir R, Singh SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27(11):2223–2234. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 13.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11(7):2670–2679. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 14.Xiao D, Singh SV. Phenethyl isothiocyanate sensitizes androgen-independent human prostate cancer cells to docetaxel-induced apoptosis in vitro and in vivo. Pharm Res. 2010;27(4):722–731. doi: 10.1007/s11095-010-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, Liu D. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25(8):1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- 16.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Shen G, Chen C, Gélinas C, Kong AN. Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through lκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24(28):4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Xu C, Keum YS, Reddy B, Conney A, Kong AN. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with β-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27(3):475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis In vitro and Ex vivo. Cancer Res. 2007;67(5):2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 20.Gong A, He M, Krishna Vanaja D, Yin P, Karnes RJ, Young CY. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol Nutr Food Res. 2009;53(7):878–886. doi: 10.1002/mnfr.200800253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B, Singh SV. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285(34):26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17(25):3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 23.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13(3):239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasula SM, Ashwell JD. IAPs: What’s in a Name? Mol Cell. 2008;30(2):123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A, PeehI D, Gao GJ, Reed JC. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9(13):4914–4925. [PubMed] [Google Scholar]
- 26.Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, Glinsky GV. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65(6):2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 27.Seligson DB, Hongo F, Huerta-Yepez S, Mizutani Y, Miki T, Yu H, Horvath S, Chia D, Goodglick L, Bonavida B. Expression of X-linked inhibitor of apoptosis protein is a strong predictor of human prostate cancer recurrence. Clin Cancer Res. 2007;13(20):6056–6063. doi: 10.1158/1078-0432.CCR-07-0960. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23(33):5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 29.Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res. 2011;4(7):1107–1117. doi: 10.1158/1940-6207.CAPR-10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao D, Johnson CS, Trump DL, Singh SV. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Cancer Ther. 2004;3(5):567–575. [PubMed] [Google Scholar]
- 31.Xiao D, Choi S, Lee YJ, Singh SV. Role of mitogen-activated protein kinases in phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Mol Carcinog. 2005;43(3):130–140. doi: 10.1002/mc.20099. [DOI] [PubMed] [Google Scholar]
- 32.Bommareddy A, Hahm ER, Xiao D, Powolny AA, Fisher AL, Jiang Y, Singh SV. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69(8):3704–3712. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Coen JJ, Suzuki Y, Siedow MR, Niemierko A, Khor LY, Pollack A, Zhang Y, Zietman AL, Shipley WU, Chakravarti A. Survivin is a potential mediator of prostate cancer metastasis. Int J Radiat Oncol Biol Phys. 2010;78(4):1095–1103. doi: 10.1016/j.ijrobp.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, Wagner SA. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51(8):2063–2068. [PubMed] [Google Scholar]
- 35.Jiao D, Eklind KI, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54(16):4327–4333. [PubMed] [Google Scholar]
- 36.Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN. Chemoprevention of familial adenomatous polyposis in Apc Min/+mice by phenethyl isothiocyanate (PEITC) Mol Carcinog. 2008;47(5):321–325. doi: 10.1002/mc.20390. [DOI] [PubMed] [Google Scholar]
- 37.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs: more than just inhibitors of apoptosis proteins. Cell Cycle. 2008;7(8):1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 38.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83(7):1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 39.Dean EJ, Ranson M, Blackhall F, Dive C. X-linked inhibitor of apoptosis protein as a therapeutic target. Expert Opin Ther Targets. 2007;11(11):1459–1471. doi: 10.1517/14728222.11.11.1459. [DOI] [PubMed] [Google Scholar]
- 40.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796–1803. [PubMed] [Google Scholar]
- 41.Li M, Song T, Yin ZF, Na YQ. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J. 2007;120(6):469–473. [PubMed] [Google Scholar]
- 42.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288(5467):874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 43.Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17(1):54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu L, Zhu N, Zhang H, Durden DL, Feng Y, Zhou M. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell. 2009;15(5):363–375. doi: 10.1016/j.ccr.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18(6):609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 47.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28(6):1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 48.Kim SH, Singh SV. p53-Independent apoptosis by benzyl isothiocyanate in human breast cancer cells is mediated by suppression of XIAP expression. Cancer Prev Res. 2010;3(6):718–726. doi: 10.1158/1940-6207.CAPR-10-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]