Abstract
When defining allergic outcomes in epidemiology studies results of the skin prick test (SPT) panel is often dichotomized as positive/negative or categorized based on the number of positive responses. Item Response Theory (IRT) models, however, may prove to be a better alternative with the ability to generate scores that account for both type and number of positive SPTs. IRT was applied to SPT responses administered to 537 children at age two in order to determine predictability of allergic disease at age four. The children received SPTs to 15 aeroallergens and two foods. Atopy predisposition scores were obtained from the IRT model using the posterior distribution of the latent trait, atopy. These scores were used to predict persistent wheeze, rhino-conjunctivitis, and eczema at age four. Results were compared to the dichotomized and categorical (positive to ≥ 2, positive to one, versus negative to all allergens) SPT variables. At age two, 39% of children had at least one positive SPT. All three allergic disease outcomes were significantly associated with IRT atopy scores: persistent wheeze odds ratio (OR)=1.7 (95% confidence interval (CI): 1.2, 2.3); rhino-conjunctivitis OR=1.7 (95% CI:1.2, 2.3); eczema OR=1.6 (95% CI: 1.2, 2.3). In contrast, rhino-conjunctivitis was the only outcome significantly associated with the dichotomized SPT variable with an OR=1.9 (95% CI:1.2, 3.0). For the categorical SPT variable, all three allergic symptoms were significantly associated with positive to ≥ 2 allergens compared to negative to all, but no difference was observed between those with positive to one compared to negative to all. The IRT model proved to be an informative methodology to assess the predictability of early SPT responses and identify the allergens most associated with atopy predisposition.
Keywords: Item Response Theory, Skin Prick Test, Allergy, Atopy, Asthma, Wheeze, Rhino-conjunctivitis, Eczema, Predicting allergies
Item response theory (IRT) models are commonly used in analyses of psychometric tests. It can be applied to data in other fields when it is plausible to assume the existence of an underlying variable that explains individual responses on test items (1–4). Atopy, a complex genetic or familial state of hypersensitivity to allergens is not directly measured. It is assumed, however, to be the underlying trait that predicts individual SPT responses and allergic disease outcomes. In psychometric terms, the predisposition for atopy is considered a “latent” trait, a variable that can not be directly measured but exhibits symptoms that may be observed through the SPT test responses. In the medical literature, IRT methods have been used in the development and assessment of questionnaires for health outcomes and exposures, including quality of life, pain assessment, depression after injury, alcohol and drug depedences, and physical activity levels (5–9). The purpose of this study was to evaluate the application of applying IRT methods to skin prick test (SPT) data.
Skin prick tests are commonly used in clinical practice to diagnose specific allergen sensitization (10). Positive SPTs are highly sensitive indicators of heightened risk for development of clinical allergic respiratory disorders and eczema (11). In epidemiologic studies, a positive SPT to one or more allergens is commonly used to define allergic sensitization or atopy, where the number and type of allergens included in the SPT panel can vary greatly. With this method of defining atopy, all allergens included in the panel are assumed to contribute equally. For example, if tree pollen and dust mite are both included in the panel, being positive to tree pollen is the same as being positive to dust mite and is the same as being positive to both tree pollen and dust mite. This commonly used method fails to account for the type or number of allergens to which the person is positive. Using a categorical variable of positive SPT to two or more, positive to one, and negative to all, can somewhat account for the number of allergens to which a person is positive, but still fails to account for the type of allergen. The ability to generate latent variable scores is a feature of IRT models and has advantages in the application to SPT data, as these scores consider the type of allergen in addition to the number of positive allergen SPTs. The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a prospective high risk birth cohort study, provides an ideal dataset to illustrate the application of IRT to SPT results.
METHODS
Study population
To illustrate this approach, SPT data from a birth cohort study, CCAAPS, is used. The study methods have been described (12). Briefly, infants were identified through birth records between 2001 and 2003 in the greater Cincinnati, Ohio metropolitan area. Eligibility criteria for enrollment included parental atopy, defined as a parent with at least one symptom of allergy or asthma and being SPT positive (SPT+) to at least one of 15 aeroallergens administered at the time of enrollment. Parents signed an informed consent and the study was approved by the Institutional Review Board of the University of Cincinnati. This birth cohort is a high-risk group for the latent trait of predisposition for atopy.
Measurements
SPT data
Skin prick testing was performed using a panel of allergens including: four mold extracts: Alternaria alternata, Aspergillus fumigatus, Cladosporium herbarum, and Penicillium mix; four indoor aeroallergens: house dust mite mixture of Dermaphagoids (D.) farinae and D. pterynissinus, German cockroach, cat, and dog; short ragweed; two grass pollens: meadow fescue and Timothy; four tree pollens: red cedar, American elm, maple mix, and white oak; and two foods: milk and egg (12). The 15 aeroallergens incorporated in the SPT panel were chosen because they are clinically relevant indoor and outdoor aeroallergens in the Greater Cincinnati area. Milk and egg were chosen because these are the most common food allergen sensitizers during the first two years of life and known to predict childhood and adult asthma in high risk children (13). Histamine dihydrochloride (10 mg/ml) was used as a positive control and normal saline (0.9%) was used as a negative control. SPT+ was defined by a wheal diameter greater than or equal to three millimeters over that of the saline control measured after 15 minutes. All SPTs were performed using Accusets® (ALK-Abello, Round Rock, Tx). For this illustrative example, SPT responses at age two were used both in the IRT model and to predict health outcomes at age four.
Health outcomes
Allergic symptoms were evaluated at age four and were persistent wheeze, rhino-conjunctivitis, or eczema. Persistent wheeze was defined as parent report of two or more wheezing episodes in the previous 12 months at both their child’s age three and four clinical exam or parent report of the child diagnosed with asthma by their private physician at the age four visit. Rhino-conjunctivitis was defined by parental report at the age four exam of sneezing, or a runny nose or blocked nose without a cold or flu and accompanied by itchy-watery eyes in the past 12 months. Eczema was defined by parental report of scratching, and redness, “raised bumps,” or dry skin/scaling for at least six out of the last 12 months at the age four exam.
Statistical analysis
IRT Model
In the testing framework, the observed variables are exam questions or items, such that yij is 1 if examinee i answered item j correctly and 0 otherwise, and the unobserved ability of the examinee is representaed by θ (14). In medical research, θ may be a latent trait, such as physical functioning, or for SPT data, θ can be used to represent the unobserved predisposition for atopy. In this study, atopy is defined as a familial or genetic predisposition for being hypersensitive to environmental allergens.
The IRT model offers an attractive method for the examination of correlated binary SPT response patterns because conditioning on the latent trait accounts for the correlation among allergic responses to allergens included in the test panel. Further, the model parameters are weighting factors, identifying specific allergens that are most associated with the underlying condition, i.e. predisposition for atopy. IRT involves estimating the parameters of the item characteristic curve (ICC). The focus of this study is on the two-parameter logistic (2PL) model for dichotomous items, expressed as:
| (equation 1) |
where i represents the allergens (i = 1, …, I), m the subject (m = 1, …, M), θm is the subject’s score on the latent trait (predisposition for atopy), and Yim the response variable for the ith allergen/item for the mth subject. The parameter δi is the location or difficulty of the item in IRT terms, defined as the level of the latent trait necessary to have a 50% probability of SPT+. The parameter αi determines the slope of the ICC and is called the item discrimination parameter. High slope values, or larger αi, indicate allergens that are more highly associated with a higher atopy predisposition.
The latent trait is assumed to have a standard normal distribution. It is the common thread that underlies and explains all the items, or allergens and represents predisposition for atopy. Thus, each child from this high-risk cohort has an atopy score reflecting their degree (or level) of allergen sensitivity. High atopy scores indicate a high predictive tendency to develop allergic disease. Atopy scores for the observed response patterns were calculated as the mode of the posterior distribution, such as:
| (equation 2) |
where the true parameter values for α and δ are replaced by their maximum likelihood estimates in equation 2 (15).
There were 17 allergens from the two year clinical testing included in the IRT model. Parameter estimates were obtained using marginal maximum likelihood estimation (MMLE) and the Gauss-Hermite quadrature rule (16). Model fit was checked using Pearson’s chi-squared statistic, based on response patterns with a frequency of five or more. A likelihood ratio test was used to compare the fit of the two parameter logistic model to the one parameter logistic model (called the unconstrained Rasch model), which assumes equal slopes for all the items (3). Logistic regression was used to assess the association between the atopy scores from the IRT model and the observed allergic symptoms at age four. Logistic regression was also used to assess the association between allergic symptoms at age four and the binary SPT variable (positive to at least one allergen versus negative to all) and the categorical SPT variable (positive to at least two allergens, positive to one allergen, versus negative to all). To confirm that the IRT model can identify allergens that are more highly associated with predisposition for atopy, the allergen with the highest and the lowest IRT slope parameter were used to predict allergic symptoms at age four using logistic regression models. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and R statistical software (http://www.r-project.org). Both software programs can be used to specify an IRT model and the code is presented in the appendix. SAS was used to assess associations using logistic regression models and R was used to generate figures showing the item characteristic curves.
RESULTS
For this application of IRT, SPT results at age two from 537 children enrolled in the CCAAPS cohort were utilized. Most (81%) of the children were Caucasian. Few mothers reported smoking (12%) and a majority of the mothers breastfed (71%). Maternal and paternal asthma were common in this cohort, 24% and 13%, respectively, as this was a high risk cohort. The frequency of sensitization to specific allergens at age two is presented in Table 1. The prevalence of sensitization to any allergen was 39.5%, and the most common was Timothy grass (7.8%) followed by egg (7.6%), while the least common allergen was milk (2%). A majority, 60.5%, were SPT- to all 17 allergens, 19.6% were positive to only one allergen, and 19.9% were positive to two or more allergens. Of those who were sensitive to at least two allergens (n=107), the most common cosensitization occurred between Timothy grass and maple (12%).
Table 1.
Frequency of Sensitization to Individual Allergens at Age Two
| Allergen* | Age 2 (n=537) |
|---|---|
| MOLD | 58 (10.8%) |
| Alternaria | 21 (3.9%) |
| Aspergillus fumigatus | 23 (4.3%) |
| Cladosporium | 14 (2.6%) |
| Penicillium | 15 (2.8%) |
| INDOOR | 95 (17.7%) |
| Dust mite | 32 (6%) |
| Cockroach | 23 (4.3%) |
| PET | |
| Cat | 33 (6.1%) |
| Dog | 25 (4.7%) |
| POLLENS (OUTDOOR) | 120 (22.3%) |
| Short ragweed | 23 (4.3%) |
| GRASSES | |
| Meadow fescue | 18 (3.4%) |
| Timothy | 42 (7.8%) |
| TREES | |
| Red cedar | 37 (6.9%) |
| American elm | 17 (3.2%) |
| Maple mix | 31 (5.8%) |
| White oak | 23 (4.3%) |
| FOOD | 46 (8.6%) |
| Egg | 41 (7.6%) |
| Milk | 11 (2%) |
| Any allergen | 212 (39.5%) |
| >1 allergen | 107 (19.9%) |
| Any aeroallergen | 201 (37.4%) |
| No allergen | 325 (60.5%) |
Some children may be skin prick positive to more than one allergen.
The IRT model was fit using all 17 allergen responses at age two. Difficulty (δ) is indicated by the location on the latent trait scale in which there is a 50% probability of being SPT+ to that allergen. The difficulty parameters (δ) are shown in Table 2. In Figure 1 the results of the ICC curves for Timothy and cladosporium are shown. The difficulty parameter for Timothy is 1.8, indicating an atopy predisposition score of 1.8 is necessary for a child to have a 50% probability of being SPT+ to Timothy. For cladosporium a child needs to have an atopy score of 2.48 to have a 50% probability of being SPT+ to cladosporium.
Table 2.
Model Parameter Estimates of Skin Prick Test Positive for Each Allergen Conditional on Predisposition for Atopy
| Log odds αi* | 95% CI | Difficulty (δi) | |
|---|---|---|---|
| Meadow fescue | 2.76 | 1.35, 4.17 | 2.17 |
| Timothy | 2.27 | 1.33, 3.21 | 1.80 |
| Maple mix | 2.25 | 1.27, 3.23 | 2.01 |
| Cladosporium | 2.24 | 1.02, 3.46 | 2.48 |
| White oak | 1.88 | 1.00, 2.76 | 2.37 |
| American elm | 1.79 | 0.87, 2.71 | 2.63 |
| Milk | 1.51 | 0.53, 2.49 | 3.22 |
| Penicillium | 1.41 | 0.55, 2.27 | 3.13 |
| Dog | 1.22 | 0.57, 1.87 | 2.98 |
| Cockroach | 1.12 | 0.47, 1.77 | 3.25 |
| Egg | 1.11 | 0.58, 1.64 | 2.67 |
| Cat | 1.07 | 0.50, 1.64 | 2.98 |
| Red cedar | 1.03 | 0.48, 1.58 | 2.93 |
| Aspergillus fumigatus | 0.79 | 0.18, 1.40 | 4.29 |
| Dust mite | 0.77 | 0.24, 1.30 | 3.93 |
| Alternaria | 0.69 | 0.06, 1.32 | 4.95 |
| Short ragweed | 0.69 | 0.04, 1.34 | 5.04 |
The parameter estimates (αi) can be interpreted as the increase in log odds of SPT positivity to the allergen for a unit increase in predisposition for atopy.
Figure 1.
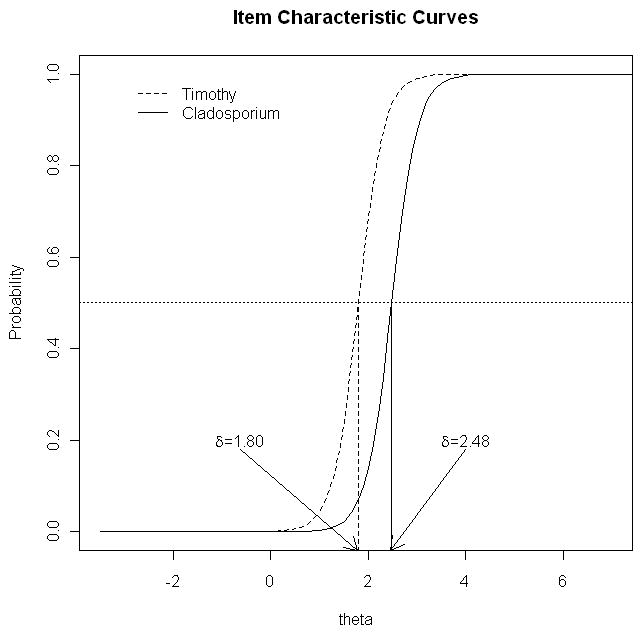
The slope of the ICC indicates how well each allergen is able to discriminate or differentiate individuals with high versus low atopy predisposition scores. The discrimination parameters (α) are shown in Table 2. Meadow fescue was the most discriminating allergen, indicating that this allergen was highly associated with predisposition for atopy with a slope of 2.76 which translates into a log odds of 2.76 (i.e., for each unit increase in the atopy score, the odds of testing SPT+ to meadow fescue increases by 15.8). Timothy allergen and maple mix were the second and third most discriminating allergens for predisposition for atopy with log odds of 2.27 and 2.25, respectively. Short ragweed and Alternaria allergens had the lowest association between testing SPT positive and predisposition for atopy (log odds 0.69). Model fit was assessed using Pearson’s chi-square statistic and indicated that the two parameter logistic model fit the data well (p-value=0.13). In addition, the likelihood ratio test comparing the two parameter logistic model with the one parameter logistic model verified that the two parameter logistic model provided a better fit (p-value = 0.001).
Each child’s atopy predisposition score was estimated by the model using the posterior distribution of the latent trait. Atopy scores are built on allergen characteristics as determined by the IRT model parameters and the child’s SPT response pattern. The atopy score incorporates the complete panel of 17 SPT responses, and is unique for that panel. The SPT response patterns for the 18 children with the three highest and three lowest scores are shown in Table 3. As indicated there were 325 (60.5%) of children who were negative for all SPTs and their atopy predisposition score was −0.31. For two subjects with eight SPTs+ the score ranged from 2.23 to 2.45 depending on which allergens were positive as they only shared positivity on four of the eight allergens (Table 3).
Table 3.
SPT Response Patterns for High and Low Atopy Scores at Age Two
| SPT Response Patterns at Age Two
|
||||||
|---|---|---|---|---|---|---|
| High | Low | |||||
| Alternaria | − | − | + | + | − | − |
| Aspergillus fumigatus | − | − | − | − | − | − |
| Cladosporium | − | + | + | − | − | − |
| Penicillium | − | − | + | − | − | − |
| Dust mite | − | + | − | − | − | − |
| Cockroach | − | − | + | − | − | − |
| Cat | − | − | − | − | − | − |
| Dog | + | − | + | − | − | − |
| Short ragweed | − | − | − | − | + | − |
| Meadow fescue | + | + | − | − | − | − |
| Timothy | + | + | − | − | − | − |
| Red cedar | + | − | + | − | − | − |
| American elm | + | − | + | − | − | − |
| Maple mix | + | + | + | − | − | − |
| White oak | − | + | − | − | − | − |
| Egg | + | − | − | − | − | − |
| Milk | + | − | − | − | − | − |
|
| ||||||
| Atopy score | 2.45 | 2.27 | 2.23 | 0.144 | 0.143 | −0.31 |
| Number SPT positive | 8 | 6 | 8 | 1 | 1 | 0 |
| Number of children with this score | 1 | 1 | 1 | 8 | 7 | 325 |
+ = positive response, − = negative response
In order to determine if an atopy score truly reflects a child’s tendency to develop allergic symptoms at age four, unadjusted odds ratios were calculated. At age four, 194 (36%) children had at least one allergic symptom reported. All three allergic disease outcomes were significantly associated with IRT atopy scores (Table 4). To compare IRT model atopy scores with the binary SPT variable and with the categorical SPT variable, unadjusted associations between the binary and categorical SPT variables and age four allergic symptoms were also calculated. For the binary SPT variable, rhino-conjunctivitis was the only significantly associated allergic symptom. Whereas, all three allergic symptoms, persistent wheeze, rhino-conjunctivitis, and eczema were significantly associated with the IRT atopy predisposition score. For the categorical SPT variable, all three allergic symptoms were significantly associated with the SPT+ to two or more allergens compared to negative to all, but no difference was observed between those with just one positive compared to negative to all (Table 4). This finding supports the need to incorporate the number of SPT+.
Table 4.
Unadjusted Associations between Age Four Allergic Symptoms and Measures of Allergic Sensitization at Age Two
| Age Four Allergic Symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Persistent Wheeze (events = 77) | Rhino-conjunctivitis (events = 97) | Eczema (events = 69) | |||||||
| Age Two | OR (95% CI) | SE | p-value | OR (95% CI) | SE | p-value | OR (95% CI) | SE | p-value |
| Atopy score (per unit increase) | |||||||||
| 1.65 (1.19, 2.29) | 0.17 | 0.003 | 1.66 (1.22, 2.25) | 0.16 | 0.001 | 1.64 (1.17, 2.31) | 0.18 | 0.005 | |
| Any SPT+ (binary) | |||||||||
| ≥1 positive | 1.50 (0.92, 2.44) | 0.25 | 0.101 | 1.91 (1.23, 2.98) | 0.23 | 0.004 | 1.58 (0.95, 2.62) | 0.26 | 0.078 |
| None | ref | - | - | - | - | - | - | - | - |
| Any SPT+ (3 categories) | |||||||||
| ≥2 positive | 2.05 (1.17, 3.60) | 0.29 | 0.012 | 2.14 (1.26, 3.65) | 0.27 | 0.005 | 2.14 (1.19, 3.84) | 0.30 | 0.011 |
| 1 positive | 1.00 (0.51, 1.96) | 0.34 | 0.992 | 1.70 (0.97, 2.96) | 0.28 | 0.064 | 1.07 (0.53, 2.14) | 0.36 | 0.858 |
| None | ref | - | - | - | - | - | - | - | - |
| Ragweed (binary) | |||||||||
| Positive | 1.52 (0.49, 4.67) | 0.57 | 0.47 | 1.14 (0.37, 3.48) | 0.57 | 0.82 | 0.35 (0.05, 2.63) | 1.03 | 0.31 |
| Negative | ref | - | - | - | - | - | - | - | - |
| Meadow fescue (binary) | |||||||||
| Positive | 3.15 (1.15, 8.66) | 0.52 | 0.03 | 1.31 (0.42, 4.06) | 0.58 | 0.64 | 1.37 (0.39, 4.86) | 0.65 | 0.63 |
| Negative | ref | - | - | - | - | - | - | - | - |
To further illustrate the importance of the type of allergen and how this is accounted for in the IRT model, unadjusted associations between SPT+ to short ragweed (lowest discriminating allergen) and SPT+ to meadow fescue (highest discriminating allergen) with age four allergic symptoms were computed. No significant associations were observed between being SPT+ to short ragweed at age two and allergic symptoms at age four. Whereas, SPT + to meadow fescue was very discriminating with over a three fold increase in persistent wheezing at age four (OR = 3.15, 95% CI: 1.15, 8.66). This finding supports that the IRT model is accurately identifying the types of allergens that are most associated with the underlying trait, predisposition for atopy. The IRT model incorporates both the number of SPT+ as well as the type of allergen that is positive.
DISCUSSION
This study represents a novel application of IRT methodology to SPT data. Though predisposition for atopy is not directly measured, it is indicated by the SPT response patterns, and in the IRT framework, predisposition for atopy fits the definition of a latent variable (17).
Common IRT models assume that the distribution of the latent variable is normally distributed. In addition, three key assumptions underlie IRT modeling: local independence, monotonicity, and unidimensionality. Since the initial development of IRT models, many researchers have expanded upon these models to allow for relaxation of the assumptions. Nonparametric IRT models have been introduced that do not assume a single function of the item response curve (18,19). Multidimensional IRT models have been introduced that do not require unidimensionality of the underlying latent variable (20–22). However, these models are more complex and have not been as well developed as the one and two parameter logistic models.
The findings from the illustrated example have some limitations including the potential for misclassification of SPT responses, defined to be positive if the wheal size was greater than or equal to three millimeters over that for the saline control. The predictive value of the SPT can depend on the clinician administering the test, as well as the diameter chosen as the cutoff for defining a positive result (23,24). However, this criterion has been shown to have a positive predictive value of 0.79 for milk and 0.92 for egg in children under age two, using food challenge as the standard and is commonly used in clinical practice to indicate clinical allergy (25–28). The relatively short follow-up period between age two SPT and age four allergic symptoms may not be sufficient for capturing the full development of allergic symptoms and precludes the inclusion of all allergic disease outcomes, in particular asthma. Limitations of the model include the assumption of the latent trait measuring predisposition for atopy. The model estimates of atopy scores were shown to be associated with allergic symptoms providing evidence that the assumption of the latent trait is valid. In addition, the latent trait was assumed to follow a standard normal distribution in the population which may not be valid, since these children were at high risk for allergic symptoms and likely have higher predisposition for atopy than in the general population. Since 54% of the population has allergic sensitization, however, this limitation may be minimal (29). Allergic sensitization can change with age (30) and this study only includes age two SPT, so our future work includes the assessment of atopy scores over time using IRT modeling techniques.
The IRT model proved to be a novel and informative methodology to assess SPT responses during early childhood. By identifying allergens with high associations between SPT positive and predisposition for atopy, the model can be used to determine the best allergens to include in the SPT panel for the age of the children to be tested and the geographical area of residence. In this study, meadow fescue, timothy, and maple mix were found to be the top three most important allergens to include in our SPT panel for children of age two who reside in the greater Cincinnati region. Short ragweed, Alternaria alternata, and dust mite were the three least important allergens and could be considered for exclusion from the panel. In addition, with the IRT model, atopy scores can be obtained using the posterior distribution of the latent trait. Atopy scores depend on allergen characteristics, as determined by the IRT model parameters, and the child’s response pattern. Unlike the categorical SPT variable, the atopy score is not merely dependent on the number of positive test responses, but incorporates a higher weighting for better discriminating allergens (i.e., meadow fescue in this study). These atopy scores can be used to predict health outcomes. Although for this study panel, the categorical SPT variable and the atopy scores both predicted age four allergic symptoms this may not always be the case. For example, if the SPT panel included two or more allergens that do not discriminate well, are not associated with atopy predisposition, then the categorical variable may not do as well as the IRT atopy scores which accounts for the discriminating ability of the type of allergen.
In conclusion, SPTs are commonly used in clinical practice and often used for research purposes. The IRT modeling approach to the SPT panel was successful in identifying allergens associated with atopy predisposition and estimating atopy scores that can be used to predict later allergic symptoms. Additional research with varying SPT panels in different geographical regions will provide further evidence of the usefulness of this methodology.
Acknowledgments
Funding This work was supported by the National Institute of Environmental Health Sciences [T32 ES10957 and ES11170].
Appendix. IRT models in SAS and R
One parameter logistic IRT model
SAS code
Sample data structure for SAS IRT model
Each subject has a row for each allergen (17 rows) with the response (resp) for testing positive or negative to that allergen.
| longSPTdata | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | alt | asp | cla | pen | ced | elm | oak | map | fes | tim | rag | cat | dog | dus | roa | mil | egg | resp |
| 100027 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100027 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100027 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100027 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
proc nlmixed data=longSPTdata qpoints=21 method=GAUSS technique=NEWRAP NOAD MAXFUNC=500; parms b1–b17=0; b=b1*alt + b2*asp + b3*cla + b4*pen + b5*ced + b6*elm + b7*oak + b8*map + b9*fes + b10*tim + b11*rag + b12*cat + b13*dog + b14*dus + b15*roa + b16*mil + b17*egg; eta=theta-b; p=1/(1+exp(−eta)); model resp~binary(p); random theta~normal(0,1) subject=id; run;
R code
Sample data structure for R IRT model
Each row of data contains the SPT response pattern for one subject. The data for the first four subjects is printed below.
| SPTdata | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alt | asp | cla | pen | ced | elm | oak | map | fes | tim | rag | cat | dog | dus | roa | mil | egg | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
library(ltm) fit2<-rasch(SPTdata)
Two parameter logistic IRT model
SAS code
proc nlmixed data=longSPTdata qpoints=21 method=GAUSS technique=NEWRAP NOAD MAXFUNC=500; parms b1–b17=0.1 a1–a17=0.1; b=b1*alt + b2*asp + b3*cla + b4*pen + b5*ced + b6*elm + b7*oak + b8*map + b9*fes + b10*tim + b11*rag + b12*cat + b13*dog + b14*dus + b15*roa + b16*mil + b17*egg; a=a1*alt + a2*asp + a3*cla + a4*pen + a5*ced + a6*elm + a7*oak + a8*map + a9*fes + a10*tim + a11*rag + a12*cat + a13*dog + a14*dus + a15*roa + a16*mil + a17*egg; eta=a*(theta-b); p=1/(1+exp(−eta)); model resp~binary (p); random theta~normal (0,1) subject=id; run;
R code
ltm(SPTdata~z1)
References
- 1.Baker F, Kim SH. Item Response Theory. 2. New York: Marcel Dekker, Inc; 2004. [Google Scholar]
- 2.van der Linden W, Hambleton R. Handbook of modern Item Response Theory. New York: Springer-Verlag; 1997. [Google Scholar]
- 3.Rasch G. Probabilistic models for some intelligence and attainment tests. Chicago: Mesa Press; 1960. [Google Scholar]
- 4.Lord FN, Novick MR. Statistical theories of mental test scores. Reading: Addison- Wesley; 1968. [Google Scholar]
- 5.Langer MM, Hill CD, Thissen D, Burwinkle TM, Varni JW, DeWalt DA. Item response theory detected differential item functioning between healthy and ill children in quality-of-life measures. J Clin Epidemiol. 2008;61:268–276. doi: 10.1016/j.jclinepi.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jette AM, McDonough CM, Ni P, et al. A functional difficulty and functional pain instrument for hip and knee osteoarthritis. Arthritis Res Ther. 2009;11:R107. doi: 10.1186/ar2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graves DE, Bombardier CH. Improving the efficiency of screening for major depression in people with spinal cord injury. J Spinal Cord Med. 2008;31:177–184. doi: 10.1080/10790268.2008.11760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu LT, Pan JJ, Blazer DG, et al. An item response theory modeling of alcohol and marijuana dependences: a National Drug Abuse Treatment Clinical Trials Network study. J Stud Alcohol Drugs. 2009;70:414–25. doi: 10.15288/jsad.2009.70.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jago R, Baranowski T, Watson K, et al. Development of new physical activity and sedentary behavior change self-efficacy questionnaires using item response modeling. Int J Behav Nutr Phys Act. 2009;6:20. doi: 10.1186/1479-5868-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint task force on practice parameters for the diagnosis and treatment of asthma. The American academy of Allergy, Asthma and immunology and the American college of allergy, asthma and immunology. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 11.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999;402:B5–11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 12.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotaniemi-Syrjanen A, Reijonen TM, Romppanen J, Korhonen K, Savolainen K, Korppi M. Allergen-specific immunoglobulin E antibodies in wheezing infants: the risk for asthma in later childhood. Pediatrics. 2003;111:e255–261. doi: 10.1542/peds.111.3.e255. [DOI] [PubMed] [Google Scholar]
- 14.Embretson SE, Reise SP. Item Response Theory for Psychologists. Mahwah: Lawrence Erlbaum Associates, Inc; 2000. [Google Scholar]
- 15.Rizopoulos D, Moustaki I. Generalized latent variable models with non-linear effects. British Journal of Mathematical and Statistical Psychology. 2008;61:415–438. doi: 10.1348/000711007X213963. [DOI] [PubMed] [Google Scholar]
- 16.Rizopoulos D. ltm: an R package for latent variable modeling and item response theory analyses. Journal of statistical software. 2006;17:1–25. [Google Scholar]
- 17.Bartholomew D, Steele F, Moustaki I, Galbraith J. The analysis and interpretation of multivariate data for social scientists. London: Chapman & Hall; 2002. [Google Scholar]
- 18.Cliff N, Donoghue JR. Ordinal test fidelity estimated by an item sampling model. Psychometrika. 1992;57:217–236. [Google Scholar]
- 19.Ramsay JO. Kernel smoothing approaches to nonparametric item characteristic curve estimation. Psychometrika. 1991;56:611–630. [Google Scholar]
- 20.Bock RD, Gibbons R, Muraki EJ. Full information item factor analysis. Applied Psychological Measurement. 1988;12:261–280. [Google Scholar]
- 21.Adams RA, Wilson M, Wang WC. The multidimensional random coefficients multinomial logit model. Applied Psychological Measurement. 1997;21:1–23. [Google Scholar]
- 22.Embretson SE. A multidimensional latent trait model for measuring learning and change. Psychometrika. 1991;56:495–516. [Google Scholar]
- 23.Chinn S, Jarvis D, Luczynska CM, Lai E, Burney PG. Measuring atopy in a multi- centre epidemiological study. Eur J Epidemiol. 1996;12:155–162. doi: 10.1007/BF00145501. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet PJ, Chatzi L, Jarvis D, Burney P. Assessing skin prick tests reliability in ECRHS-I. Allergy. 2008;63:341–346. doi: 10.1111/j.1398-9995.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- 25.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 26.Dreborg S. Skin tests used in type I allergy skin testing. Position paper prepared by the Subcommittee on Skin Tests of the European Academy of Allergology and Clinical Immunology. Allergy. 1989;44 (suppl):1–59. [PubMed] [Google Scholar]
- 27.Dreborg S. Skin testing in allergen standardization and research. Immunol Allergy Clinics N Am. 2001;21:329–354. [Google Scholar]
- 28.Host A, Andrae S, Charkin S, et al. Allergy testing in children: why, who, when and how? Allergy. 2003;58:559–569. doi: 10.1034/j.1398-9995.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 29.Arbes SJ, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Kjaer HF, Eller E, Andersen KE, Host A, Bindslev-Jensen C. The association between early sensitization patterns and subsequent allergic disease. The DARC birth cohort study. Pediatr Allergy Immunol. 2009;20:726–734. doi: 10.1111/j.1399-3038.2009.00862.x. [DOI] [PubMed] [Google Scholar]


