Abstract
Previous studies have shown that large increases in food intake in nondeprived animals can be induced by injections of both the GABAA agonist muscimol and the μ-opioid agonist DAMGO into the nucleus accumbens shell (AcbSh), while injections of the catecholamine agonist amphetamine have little effect. In the current study we examined whether injections of these drugs are able to increase food-reinforced lever pressing in nondeprived rats. Twelve subjects were trained to lever press on a continuous reinforcement schedule while food deprived and were then tested after being placed back on ad libitum feeding. Under these conditions, responding was markedly increased by injections of either muscimol or DAMGO, although the onset of the effects of the latter drug were delayed by 30–40 min In contrast, amphetamine injections failed to increase reinforced lever pressing, although they did enhance responding on a non-reinforced lever, presumably reflecting alterations in behavioral activation. These results demonstrate that stimulation of GABAA and μ-opioid receptors within the AcbSh is able to promote not only food intake, but also food-directed operant behavior. In contrast, stimulation of AcbSh dopamine receptors may enhance behavioral arousal, but does not appear to specifically potentiate behaviors directed towards food procurement.
Keywords: Ventral striatum, feeding, ingestion, appetitive behavior, motivation, reward
1. Introduction
A large number of studies have shown that pronounced alterations in food intake can be induced by experimental manipulations of the medial shell region of the nucleus accumbens (AcbSh) (Stratford, 2007). Activation of the AcbSh, produced either by local injections of excitatory amino acids (Stratford et al,. 1998) or by electrical stimulation (Krause et al. 2010), inhibits ingestive behavior. Conversely, inactivation of the AcbSh produced by injections of inhibitory GABAA receptor agonists (Basso and Kelley, 1999; Reynolds and Berridge, 2002; Soderpalm and Berridge, 2000; Stratford and Kelley, 1997; Stratford and Wirtshafter, 2011), or of non-NMDA ionotropic excitatory amino acid antagonists (Faure et al,. 2010; Maldonado-Irizarry et al,. 1995; Stratford et al., 1998), induces pronounced ingestion of both solid and liquid diets. Water intake and gnawing behavior, however, are not affected by these treatments (Basso and Kelley, 1999; Stratford and Kelley, 1997; Stratford et al., 1998; Stratford and Wirtshafter, 2004), suggesting that they may have specific effects on feeding mechanisms. Ingestive behavior can also be produced by intra-AcbSh injections of μ-opioid agonists (Hanlon et al., 2004; Pecina and Berridge, 2005; Taha et al., 2009; Zhang and Kelley, 1997), although the effects of these drugs are not identical to those of muscimol (Basso and Kelley, 1999; Stratford and Wirtshafter, 2007; Wirtshafter and Stratford, 2012; Zhang and Kelley, 2002).
Very little is known about the functional mechanisms through which drug injections in the AcbSh act to produce feeding. Kelley and her colleagues have suggested that muscimol in the AcbSh may directly activate motor patterns involved in ingestion, and thus promote food intake without actually inducing a motivational state aimed at procuring food (Baldo and Kelley, 2007; Kelley et al,. 2005; Meredith et al., 2008). If this were the case, one would expect that intra-AcbSh muscimol would not potentiate food-reinforced operant behavior. We have found, however, that muscimol injections are able to enhance food-reinforced progressive ratio performance by mildly deprived rats (Wirtshafter and Stratford, 2010). Although these findings suggest that inactivation of the AcbSh enhances the motivation to obtain food, it should be remembered that a variety of “nonspecific” and motor-related factors might also influence performance on progressive ratio schedules (Aberman et al., 1998; Skjoldager et al., 1993). Since performance on these schedules is believed to reflect a balance between the attractive properties of the reinforcer and the amount of time and effort which must be expended in obtaining it, it seems likely that manipulations which alter perceived effort, or willingness to exert effort, would also influence progressive ratio performance. Salamone has suggested, in fact, that the nucleus accumbens may play a major role controlling effort expenditure (Salamone et al., 2007), so it is not implausible that muscimol injections into this structure might alter performance through effects not specific to feeding. It is striking in this regard that although amphetamine in the AcbSh has little or no effect on food intake (Hanlon et al., 2004), injections of this compound are able to enhance food-reinforced progressive ratio responding (Wirtshafter and Stratford, 2010; Zhang et al., 2003).
Typically, animals working on a progressive ratio schedule consume only a small amount of food before responding terminates; performance on these schedules is thus limited primarily by the amount of effort that has to be expended, not by the amount of food that the animal consumes. One might thus refer to a progressive ratio schedule as an “effort-limited” task, and it is clear that performance on it might be affected by anything which alters the “activation level” or “effort tolerance” of the animal. Consider, in contrast, a paradigm in which an animal has to make only a simple response, such as a single lever press, to obtain food; it seems unlikely that effort-limitation would play a major role in this situation, especially if animals were tested in the absence of deprivation when baseline response rates would be low. If under these “non-effort limited” conditions, inactivation of the accumbens shell were to increase the motivation to obtain food, one would expect that lever pressing would be promoted. On the other hand, if muscimol worked only to activate feeding reflexes, or primarily affected some effort related variable, one would not predict that responding would be increased.
In view of these considerations, we here examined the effects of intra-AcbSh injections of muscimol and amphetamine on continuously reinforced operant responding by nondeprived rats. In order to assess the extent to which changes in responding might reflect alterations in locomotor activity, which might result in animals “inadvertently” depressing the lever, testing was conducted in two-lever operant boxes in which responding on only one lever was reinforced. Based on the conception that muscimol injections increase feeding motivation whereas amphetamine injections have a more generalized effect not directed at a particular goal object, we predicted that muscimol, but not amphetamine, would selectively enhance lever pressing on the reinforced lever. We also examined the effects of intra-AcbSh injections of DAMGO to determine the extent to which they resembled those of muscimol.
2. Methods
2.1. Animals
Subjects were 12 male Sprague-Dawley rats obtained from Charles-River Inc. (Chicago, IL) weighing approximately 300 g at the time of surgery. Animals were individually housed in plastic cages with food (Harlan 2018 Rodent Diet) and water available ad libitum, except as noted below.
2.2 Surgery
Rats were anesthetized with sodium pentobarbital (60 mg/kg) and bilateral 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted using standard, flat-skull stereotaxic techniques. The guide cannulae were aimed so as to terminate 2.0 mm dorsal to the AcbSh using the following coordinates: anteroposterior: 1.6, mediolateral: ±0.8, dorsoventral: −6.1 (mm from bregma). The guide cannulae were held in place using denture lining material and stainless steel screws and stainless steel obturators were inserted into the lumen of each cannula to help maintain patency. Each rat was allowed to recover for at least seven days before being placed on deprivation and beginning operant training.
2.3 Apparatus
Animals were trained in one of six identical standard twin lever operant chambers (Med-Associates, St. Albans, VT) housed within sound attenuating chambers with ventilation and masking noise provided by an exhaust fan. The chambers were equipped with a click generator to provide audible feedback of food delivery, and an infrared photobeam was placed across the entrance to the food hopper to allow for the recording of times of head entries.
2.4 Operant Training
After recovering from surgery, rats were placed on a restricted feeding schedule in which they were given 17–18g of lab chow to eat each day. After one week on this schedule, animals were given two daily, 30 min magazine training sessions in the operant boxes during which reinforcers (F0021 45 mg Precision Dustless pellets, BioServe, Frenchtown, NJ) were presented at one min intervals, with a “click” being generated at the same time as food delivery. Animals were then manually shaped to lever press over one or two days, and then each day for the next five days were run for one-hour on a continuous reinforcement schedule. At the end of this training period, food was returned to the rats ad libitum, but they continued to receive daily sessions in the operant boxes near the middle of their light period. After at least 6 runs under nondeprived conditions, drug injections began, as described below.
2.5 Intracerebral injections
In order to make injections, rats were gently restrained, the obturators removed, and a 28-gauge stainless steel injection cannula, extending 2.0 mm beyond the ventral tip of the guide, inserted into each guide cannula. Rats then received simultaneous bilateral 0.50 μl infusions at a rate of 0.33 μl/min. using a motor-driven microsyringe connected to the injection cannulae through a length of fluid filled polyethylene tubing. After the infusions, the injection cannulae were left in place for an additional 60 seconds in order to minimize leakage up the tracks after which they were removed and replaced with the obturators. Animals were then immediately placed in the operant chambers. Animals were given one injection of saline several days before the start of drug testing in order to acclimate them to the procedure. Animals were tested following injections of either muscimol (molecular weight=114.1, 50 ng/side), d-amphetamine (molecular weight =135.2, 10 μg/side) or [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO, molecular weight =513.6, 0.25 μg/side). The dose chosen have all been shown to produce effects in previous studies of operant behavior following injections in the AcbSh (Covelo et al, 2011; Wirtshafter and Stratford, 2010; Zhang et al., 2003).
2.6 Procedure
Rats received six test sessions in the operant chambers following intracranial drug injections separated from each other by at least two days during which animals continued to be run. These six sessions were divided into three groups of two injections, each consisting of a drug injection and a paired saline treatment; i.e., a separate saline control session was run in proximity to each drug test. The order of drug and saline injections was randomized within each drug condition, and the order of drug testing was randomized between subjects. Test sessions following injections of DAMGO, or its paired vehicle treatment, were 240 min. in duration, based on data suggesting that the effects of this are drug frequently delayed (Bakshi and Kelley, 1993; Taha et al., 2009; Zhang et al., 2003; Zhang and Kelley, 1997), whereas other test sessions were of 60 min duration.
2.7. Perfusion and Histology
At the completion of behavioral studies, animals were deeply anesthetized with sodium pentobarbital and perfused transcardially with saline followed by 10% formalin. The brains were then removed and stored in formalin for several days after which cryostat sections were prepared through the injection site at a thickness of 50 μm and subsequently stained with cresyl violet.
3. Results
3.1 Histology
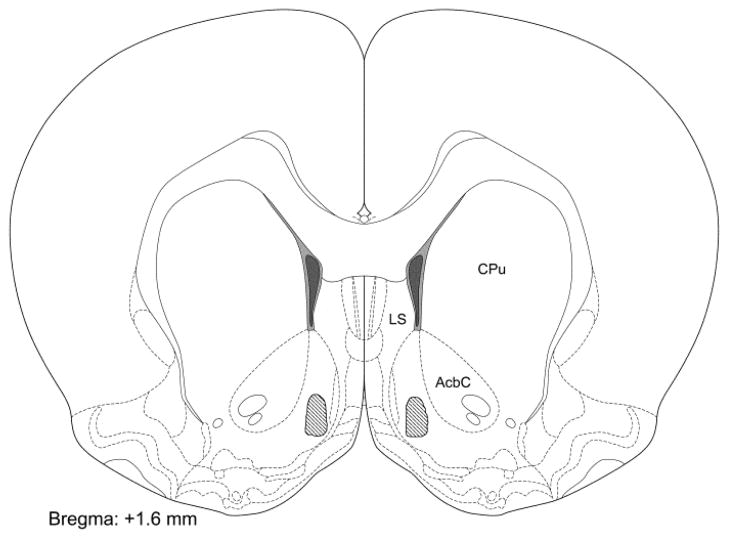
As can be seen in Fig 1, all of the cannulae in the current study terminated within the medial portion of the AcbSh in a region similar to that we have targeted in previous studies (Stratford and Wirtshafter, 2004, 2011, 2012; Wirtshafter and Stratford, 2010).
Fig. 1.
Schematic representation of the location of cannula tips within the AcbSh; all tips terminated within the shaded region. LS: lateral septum, AcbC: nucleus accumbens core, CPu: caudate-putamen. Section modified from Paxinos and Watson (2007).
3.2 Operant Behavior
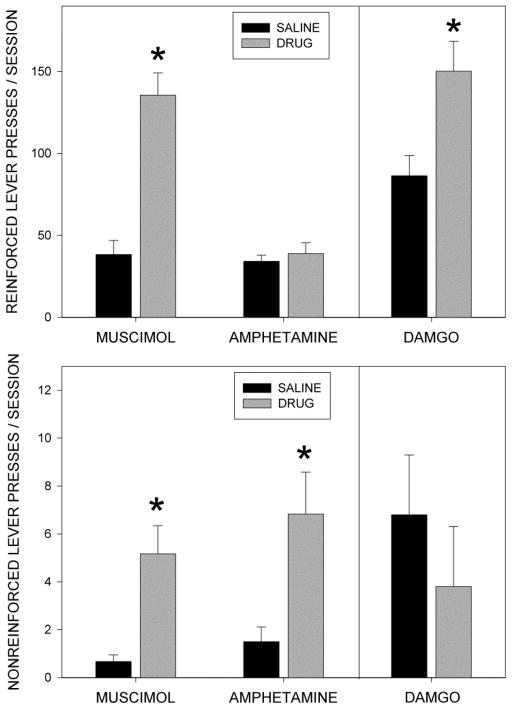
The upper panel of Fig 2 displays the mean numbers of reinforced lever presses made in the test periods following injection of muscimol, DAMGO and amphetamine, as well as responses following paired saline injections. Note that the test sessions were 60 min in duration for rats treated with muscimol and amphetamine, and their matched saline injections, and 240 min following DAMGO and its matched saline injection. It can be seen that muscimol and DAMGO induced large increase in responding as compared to the paired saline condition, whereas amphetamine appeared to have little effect. Animals consumed all of the earned pellets under each of the testing conditions. Repeated measures ANOVAs conduced on total reinforced responses generated after saline and drug conditions indicated significant effects of muscimol (F(1,11)=58.8, p<0.001) and DAMGO (F(1,11)=8.9, p<0.02), but not amphetamine (F<1). At the 60 min time point, DAMGO treated rats tended to press more than their saline controls (86.0±23.5 vs 44.9±6.2), but this difference was not statistically significant (F(1,11)=3.4, p<0.1).
Fig. 2.
Upper Panel: Mean numbers of responses on the reinforced levers across the session following injections of muscimol (50 ng/side), DAMGO (250 ng/side) and amphetamine (10 μg/side), or paired injections of saline into the nucleus accumbens shell. Session duration was 60 min following injections of muscimol or amphetamine, or their paired saline runs, and 240 min following injections of DAMGO, or its paired saline run. Lower Panel: Mean numbers of responses on the non-reinforced lever following injections of muscimol, DAMGO, and amphetamine, or their matched saline injections, into the accumbens shell. Note the difference in scaling on the Y-axis between the upper and lower panels. *=p<0.001
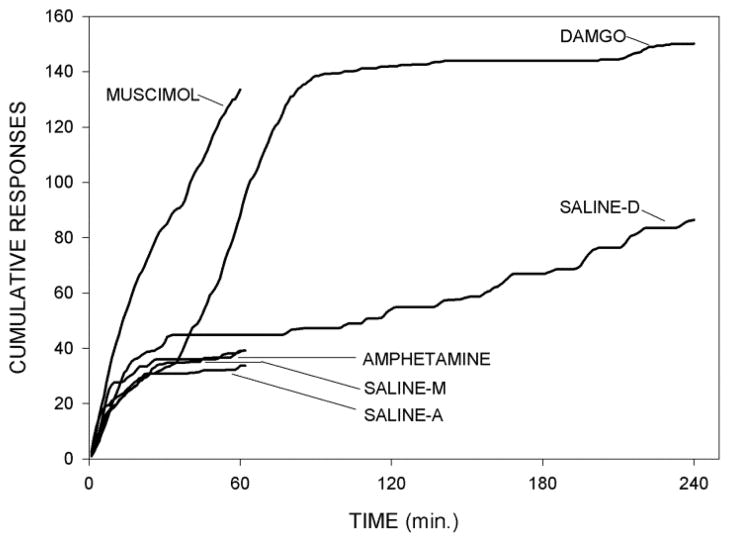
Fig. 3 shows averaged cumulative records for subjects receiving muscimol, DAMGO and amphetamine and their matched saline injections. It can be seen that whereas the enhancement of lever pressing elicited by muscimol was rapid in onset, the response to DAMGO appeared delayed by about 30–40 min., but once lever pressing was stimulated, it continued at about the same rate after injections of either drug
Fig. 3.
Mean cumulative responses, plotted in 1 min time bins, following injections of muscimol, DAMGO and amphetamine, and their matched saline treatments (saline-M, saline-D and saline-A, respectively). Responses were measured for 60 min following muscimol and amphetamine and their matched saline runs and 240 min following injections of DAMGO and its matched saline run.
The lower panel of Fig. 2 shows the total numbers of responses made on the non-reinforced levers in the test periods following drug injections and matched control runs. Analysis of this data by means of repeated measures ANOVAs indicated significant effects of muscimol (F(1,11)=13.2, p<0.005) and amphetamine (F(1,11)=11.1, p<0.01), but not DAMGO (p>0.1). Although muscimol significantly increased pressing on the nonreinforced lever, this effect was of trivial magnitude compared to that seen on the reinforced lever, and the ability of muscimol to increase reinforced responding over saline is still significant even if the data are expressed as the difference between presses on the reinforced and non-reinforced levers (F(1,11)=52.3, p<0.001).
In addition to the data reported above, we also analyzed mean latencies to begin lever pressing, mean time intervals from the delivery of reinforcement to head entry into the food receptacle, and mean run rates, that is the mean numbers of responses per min made across the session deleting periods without responding lasting more than either 10 or 30 sec. None of these variables were significantly altered by drug injections.
4. Discussion
In the current study, nondeprived rats emitted means of about 40 lever presses for food reward following injections of saline into the AcbSh. Presumably, the tendency of the animals to respond under these conditions, in the apparent absence of “need,” resulted largely from the novelty of the food available in the operant chambers. The numbers of pellets eaten here were well within the range of those we have observed in nondeprived animals tested in pelletometers (Stratford and Wirtshafter, 2012), in which no operant response had to be made other than removing the food from the hopper, suggesting that the effort necessary to lever press in the operant chambers did not have a large effect on the total amount of responding. Presumably, responding after saline injections was limited primarily by effects secondary to the consumption of the food, such as the development of satiety or habituation to the taste of the pellets. Under these “non-effort limited” conditions, intra-AcbSh injections of muscimol produced large increases in lever pressing, just as they have previously been shown to increase the amount of freely available food consumed. Since it would be difficult to account for this effect in terms of changes in the “effort tolerance” of the animals, these findings strongly support the notion that inactivation of the AcbSh induces a motivational state aimed at the procurement of food, rather than simply activating a motor feeding pattern generator. A similar logic has been used by previous authors to support claims that motivational systems can be affected by stimulation of the lateral hypothalamus (Miller, 1957), systemic injections of benzodiazepines (Wise and Dawson, 1974) and intraventricular injections of NPY (Jewett et al., 1992). Muscimol injections in this experiment did produce a significant increase in responding on the non-reinforced lever, but this effect was very small compared to that on the reinforced lever indicating that the increases in reinforced responding cannot have simply reflected changes in the general activity levels of the subjects. The conclusion that inactivation of the AcbSh results in a specific increase in food-related motivation is consistent with previous studies which found that intra-AcbSh muscimol injections increased responding on a food-reinforced, but not a water-reinforced, progressive ratio schedule (Covelo et al., 2011, Wirtshafter and Stratford, 2010).
We observed in the current study that intra-AcbSh injections of the μ-agonist DAMGO also increased lever pressing by nondeprived rats. This finding is consistent with previous reports of potentiation of food-reinforced progressive ratio responding after DAMGO injections (Zhang et al., 2003) and suggests that this drug, like muscimol, increases some aspect of food motivation. It should be noted that the behavioral effects of muscimol and DAMGO in the AcbSh are not always identical; for example, DAMGO, but not muscimol, increases the intake of nonnutritive saccharine and saline solutions (Basso and Kelley, 1999; Stratford and Wirtshafter, 2007; Zhang and Kelley, 2002). These findings suggest that although there are certain similarities between the behavioral effects of the two drugs, they must produce different patterns of effects within the Acb, a possibility consistent with the complex pharmacological interactions which have been reported between drugs acting at GABA and mu-opioid receptors (Znamensky et al., 2001). It is also interesting in this regard that whereas we found the effects of muscimol to be of rapid onset, DAMGO’s response enhancing effects were delayed by 30–40 min following injections. Similar reports of delayed effects of intra-Acb DAMGO on feeding and operant behavior have been observed in a number of previous studies (Bakshi and Kelley, 1993; Taha et al., 2009; Zhang et al., 2003; Zhang and Kelley, 1997), although they have not always been obtained (Skelly et al,. 2011). The reasons for these delays are not currently clear. Bakshi and Kelley (1993) suggested that diffusion to a distant active site is not likely to provide the explanation, and proposed that delays in onset might be a general property of effects mediated by μ-opioid receptor stimulation, perhaps reflecting the time needed for second messenger activation. In some situations, however, responses to DAMGO injections can occur with very short latencies (Klitenick and Wirtshafter, 1995). An alternative possibility is that the substrate for DAMGO’s effects may be dispersed throughout the ventral striatum and that feeding is only stimulated only when the drug has spread to involve a sufficiently large region. The so-called “cell clusters” (Herkenham et al., 1984) which occur throughout both the shell and core of the accumbens and are enriched in opioid receptors, would be a interesting candidate for such a substrate.
It is possible that drug injections in the AcbSh modify food directed behavior as a result of increases in the perceived palatability of the ingestates. The evidence for this hypothesis, however, is mixed at best, (Faure et al., 2010; Pecina and Berridge, 2005; Reynolds and Berridge, 2002 ;Taha et al., 2009; Wirtshafter and Stratford, 2007). Alternatively, the effects of these injections might be mediated through “pre-reinforcer mechanisms” such as changes in “drive” or in the “expected reward value” of the ingestate. These latter possibilities are consistent with observations that intra-AcbSh muscimol may decrease latencies to initiate feeding (Stratford and Wirtshafter, 2012). Clearly much work will be needed to clarify the processes involved in the effects of intra-AcbSh drug injections, and this task is made much more difficult by the fact that that there is no general agreement on the best way to characterize the functional organization of motivational systems. It is, of course, possible that different behavioral mechanisms might underlie the effects of muscimol and DAMGO.
In contrast to both muscimol and DAMGO, amphetamine was without significant effect on lever pressing in the current experiment. This result is in striking contrast to previous studies which have shown that amphetamine in the accumbens shell can potentiate both food- and water-reinforced responding on progressive ratio schedules (Covelo et al., 2011; Hanlon et al., 2011; Wirtshafter and Stratford, 2010; Zhang et al., 2003). Our results are, however, consistent with reports that amphetamine in the shell has no effect on food intake in nondeprived animals (Hanlon et al., 2004). The most likely explanation of these differences is that dopamine receptor stimulation produces an effect which is most apparent under “effort-limited” conditions, a conclusion that is in concordance with at least one of the major theoretical approaches to the functions of accumbens dopamine (Salamone et al., 2007). Amphetamine did significantly increase responding on the non-reinforced lever, an effect which is likely to be reflective of changes in activity level of the animals throughout the test period. It seems that whereas amphetamine may be able to promote certain behaviors that the animal is disposed to produce, it lacks the capacity of muscimol and DAMGO to direct the animal’s behaviors towards the attainment of food.
The current results highlight the striking differences between the effects of muscimol and amphetamine. Although both drugs increase responding on food-reinforced progressive ratio schedules, they have different effects on food intake, water-reinforced progressive ratio tasks and locomotor activity (Heidbreder and Feldon, 2011; Ikemoto, 2002; Pothuizen et al., 2005; Schildein et al., 1999; Stratford and Kelley, 1997). It seems impossible to account for these two different patterns as resulting from alterations in the value of a single functional parameter, such as, for example, “wanting”, and it seems that a more complex theoretical framework is necessary to adequately describe them. It is also challenging to explain how these two different behavioral syndromes can result from the actions of the two drugs on the same populations of cells. The simplest explanation may be that muscimol and amphetamine target partially non-overlapping sets of cells, although it is also possible that their different behavioral effects may reflect different patterns of activity in the same set of neurons.
In summary, the current findings demonstrate that stimulation of either GABAA or mu-opioid receptors in the AcbSh is able to stimulate food reinforced operant behavior under non-effort-limited conditions, suggesting that these receptors in the AcbSh are components of a food motivation system. In contrast, similar effects were not seen after amphetamine injections, a result consistent with the possibility that dopamine may predominantly affect effort related variables (Salamone et at., 2007).
RESEARCH HIGHLIGHTS.
Injections of muscimol into the AcbSh increase food-reinforced operant responding.
Injections of DAMGO into the AcbSh increase food-reinforced operant responding.
Intra-AcbSh amphetamine does not increase food-reinforced operant responding.
AcbSh GABAA and μ-opioid receptors can mediate food-directed operant behavior.
Acknowledgments
This publication is based upon the work supported by grants DK071738 from the National Institute of Diabetes and Digestive and Kidney Diseases and 0641943 from the National Science Foundation, and DA020802 from the National Institute for Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: Role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: Regional mapping and characterization of macronutrient and taste preference. Behav. Neurosci. 1999;113:324–326. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Covelo IR, Wirtshafter D, Stratford TR. GABAA and dopamine receptors in the nucleus accumbens shell differentially mediate performance of a water-reinforced progressive ratio task. Pharmacol. Biochem. Behav. 2011;101:57–61. doi: 10.1016/j.pbb.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol Reg Integr Comp Physiol. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLOS. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology. 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Benca RM, Baldo BA, Kelley AE. REM sleep deprivation produces a motivational deficit for food reward that is reversed by intra-accumbens amphetamine in rats. Brain Res Bull. 2011;83:245–254. doi: 10.1016/j.brainresbull.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 2011;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Moon Edley S, Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetyhcholinesterase and subcorftical afferent terminations. Neuroscience. 1984;11:561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y on food-reinforced behavior in satiated rats. Pharmacol Biochem Behav. 2011;42:207–212. doi: 10.1016/0091-3057(92)90517-j. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Wirtshafter D. Behavioral and neurochemical effects of opioids in the paramedian midbrain tegmentum including the median raphe nucleus and ventral tegmental area. J Pharmacol Exp Ther. 1995;273:327–336. [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Strut Funct. 2008;231:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE. Experiments on Motivation. Science. 1957;126:1271–1278. doi: 10.1126/science.126.3286.1271. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2007. [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do μ-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Fendon J. The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacology. 2005;30:683–696. doi: 10.1038/sj.npp.1300643. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schildein S, Agmo A, Huston JP, Schwarting RKW. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1999;790:185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Guy EG, Howlett AC, Pratt WE. CB1 receptors modulate the intake of a sweetened-fat diet in response to mu-opioid receptor stimulation of the nucleus accumbens. Pharmacol Biochem Behav. 2011;97:144–151. doi: 10.1016/j.pbb.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjoldager P, Pierre PJ, Mittleman G. Reinforcer magnitude and progressive ratio responding in the rat: Effects of increased effort, prefeeding, and extinction. Learning and Motivation. 1993;24:303–343. [Google Scholar]
- Soderpalm AH, Berridge KC. Food intake after diazepam, morphine or muscimol microinjections in the nucleus accumbens shell. Pharmacol Biochem Behav. 2000;66:429–434. doi: 10.1016/s0091-3057(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Stratford TR. The nucleus accumbens shell as a model of integrative subcortical forebrain systems regulating food intake. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight: Integrative Systems and the Development of Anti-Obesity Drugs. London: Elsevier; 2007. pp. 27–65. [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Swanson CJ, Kelley AE. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. NeuroReport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Society for Neuroscience Meeting Planner. 2007. Activation of GABA-A receptors in the nucleus accumbens shell elicits opposite effects on consumption of sucrose and saccharin solutions. Program No. 630.5. [Google Scholar]
- Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Evidence that the nucleus accumbens shell, ventral pallidum and lateral hypothalamus are components of a lateralized feeding circuit. Behav Brain Res. 2012;226:548–554. doi: 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Katsuura Y, Noorvaash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neurosci. 2009;161:718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR. Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol Biochem Behav. 2010;96:342–346. doi: 10.1016/j.pbb.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Dawson V. Diazepam-induced eating and lever pressing for food in sated rats. J Comp Physiol Psychol. 1974;86:930–941. doi: 10.1037/h0036404. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology. 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Echo JA, Lamonte N, Christian G, Ragnauth A, Bodnar RJ. γ-Aminobutyric acid receptor subtype antagonists differentially alter opioid-induced feeding in the shell region of the nucleus accumbens in rats. Brain Research. 2001;906:84–91. doi: 10.1016/s0006-8993(01)02558-6. [DOI] [PubMed] [Google Scholar]