Abstract
Background
Neuroinflammation is an important pathological process for almost all acquired neurological diseases. Microglial cells play a critical role in neuroinflammation. We determined whether lidocaine, a local anesthetic with antiinflammatory property, protected microglial cells and attenuated cytokine production from activated microglial cells.
Methods
Mouse microglial cultures were incubated with or without 1 µg/ml lipopolysaccharide and 10 U/ml interferon γ (IFNγ) for 24 h in the presence or absence of lidocaine for 1 h started at 2, 3 or 4 h after the onset of lipopolysaccharide and IFNγ stimulation. Lactate dehydrogenase release and cytokine production were determined after the cells were stimulated by lipopolysaccharide and IFNγ for 24 h.
Results
Lidocaine dose-dependently reduced lipopolysaccharide and IFNγ-induced microglial cell injury as measured by lactate dehydrogenase release. This effect was apparent with lidocaine at 2 µg/ml (30.3 ± 5.8 and 23.1 ± 9.7%, respectively, for stimulation alone and the stimulation in the presence of lidocaine, n = 18, P = 0.025). Lidocaine applied at 2, 3 or 4 h after the onset of lipopolysaccharide and IFNγ stimulation reduced the cell injury. This lidocaine effect was not affected by the mitochondrial KATP channel inhibitor 5-hydroxydecanoate. Similar to lidocaine, QX314, a permanently charged lidocaine analog that usually does not permeate through the plasma membrane, reduced lipopolysaccharide and IFNγ-induced microglial cell injury. QX314 also attenuated the stimulation-induced interleukin-1β production.
Conclusions
Delayed treatment with lidocaine protects microglial cells and reduces cytokine production from these cells. These effects may involve action site(s) on the cell surface.
Introduction
Microglial cells participate in host defense and inflammation in the brain.1 They respond to various stimulants including bacterial products to produce cytokines and other factors to modulate/induce host defense and inflammation in the central nervous system.1–3 The end results of this process may include death and dysfunction of brain cells including microglial cells. Although neuroinflammation has been considered a major pathological process for many human neurological diseases, such as Alzheimer disease,1,4 knowledge on how to regulate microglial cell functions and preserve their structural integrity is scare.
Lidocaine is a local anesthetic and has been shown to have significant antiinflammatory property.5,6 It also provides neuroprotection when it is used during brain ischemia in rats.7,8 Lidocaine used immediately at the onset of simulated reperfusion also reduces neuronal death in rat hippocampal slice cultures.9 However, it is not known whether lidocaine can protect other brain cells, such as microglial cells. The effects of lidocaine on the production of neuroinflammation mediators from microglial cells are not known. We hypothesized that lidocaine can protect microglial cells and inhibit cytokine production from activated microglial cells. We tested this hypothesisby using mouse microglial cells stimulated with lipopolysaccharide plus interferon γ (IFNγ). This method of stimulation is commonly used to in vitro simulate endotoxemia and the subsequent inflammation.3,10
Materials and Methods
Materials
C8-B4 cells (CRL-2540™), a microglial clone isolated from mouse cerebellum, were purchased from the American Type Culture Collection (Manassas, VA). Lipopolysaccharide from Escherichia coli 0111:B4 and other chemicals except for those described below were obtained from Sigma-Aldrich (St. Louis, MO). Heat inactivated fetal bovine serum, recombinant rat IFNγ produced from E. coli and Alexa Fluor 488 goat anti-mouse immunoglobulin (Ig)G antibody were obtained from Invitrogen Corporation (Carlsbad, CA). Lactate dehydrogenase (LDH) activity assay kit was from Clontech Laboratory (La Jolla, CA). ELISA kits for measuring rat interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα) were purchased from R&D Systems (Minneapolis, MN). Lidocaine was from Dai-Han Pharm. Co., LTD (Yeongdeungpo-gu, Seoul, South Korea). Normal goat serum was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and mouse monoclonal anti-ionized calcium binding adaptor molecule 1 (Iba1) antibody was purchased from Abcam Inc. (Cambridge, MA). Hoechst solution was from Thermo Scientific (Waltham, MA).
Cell culture
C8-B4 cells were cultured as we previously described.3,11 Briefly, they were cultured in Dulbecco’s Modified Eagle’s Medium containing 4 mM L-glutamine, 4500 mg/l glucose, 1 mM sodium pyruvate and 1500 mg/l sodium bicarbonate and supplemented with 10% heat inactivated fetal bovine serum, 100 U/ml penicillin and 100 pg/ml streptomycin in a humidified atmosphere of 95% air-5% CO2 at 37°C. The medium was changed every three days. The cells were plated at a density of 2.5 – 3 × 105 cells/ml on 6-well culture plates for various experiments.
Immunocytochemistry
To determine the purity of our cultures, we examined the expression of Iba1, a microglia-specific protein, in these cells. C8-B4 cells were fixed with ice-cold methanol for 5 min. After being rinsed with ice-cold phosphate buffered saline (PBS) twice, cells were incubated in PBS containing 0.25% Triton X-100 for 10 min. They were then washed with PBS three times for 5 min and incubated with 5% normal goat serum in PBS for 30 min. Cells were incubated with the anti-Iba1 antibody at a dilution of 1:50 for 1 h at room temperature. They were rinsed with PBS and were incubated in Alexa Fluor 488 goat anti-mouse IgG antibody at a dilution of 1:250 for 1 h at room temperature. All cells were counterstained using 1 µg/ml of Hoechst solution. They were rinsed with PBS, mounted with mounting medium and observed under a fluorescence microscope.
Trypan blue exclusion assay
To determine the viability of the cells under control conditions, the cells were detached from culture plates by incubation with 0.25% trypsin for 3 min at 2, 3 or 4 days after they were plated in the 6-well plates. The cells were then incubated in 0.04% trypan blue for 5 min at room temperature. Trypan blue stained or unstained cells were counted under a microscope with the use of a hemocytometer as we described previously.12
Cell stimulation and exposure to lidocaine and various chemicals
Cells were exposed to 1 µg/ml lipopolysaccharide and 10 U/ml IFNγ for 24 h at 24 h after the cells were plated. This incubation condition induced a significant LDH release in our previous study.3,11
In one set of experiments, lidocaine at 2, 10 or 20 µg/ml was applied for 1 h at 2 h after the onset of lipopolysaccharide and IFNγ stimulation. In another experiment, 20 µg/ml lidocaine was incubated with the cells for 1 h at 3 or 4 h after the onset of lipopolysaccharide and IFNγ stimulation. QX314, a permanently charged lidocaine analog, was added to the medium to make the final concentration at 25 µg/ml (73 µM that was the same molar concentration as 20 µg/ml lidocaine) for 1 h at 2 h after the addition of lipopolysaccharide and IFNγ. The mitochondrial KATP channel inhibitor 5-hydroxydecanoate (5-HD) was added 10 min before the application of lidocaine to make the final concentration 100 µM in the medium and was incubated with the cells for 70 min. After the completion of incubation with lidocaine, QX314 or 5-HD, the incubation solutions were replaced with fresh medium that did not contain these reagents but still included lipopolysaccharide and IFNγ. A similar procedure (incubation medium changing) was used in the other experimental groups in the same set of experiments.
Lactate dehydrogenase activity assay
As we described before,11 the culture medium was harvested at 24 h after the onset of lipopolysaccharide and IFNγ stimulation and was centrifuged at 1,000 g for 10 min. One hundred microliters cell-free supernatant was transferred to 96-well plates and incubated with the same amount of reaction mixture from the LDH detection kit. LDH activity was measured based on a colorimetric assay. The absorbance of samples was read at 492 nm with the reference wavelength of 655 nm in a spectrophotometry (Bio-Rad Laboratories). Background absorbance from the cell-free buffer solution was subtracted from all absorbance measurements. After removal of the culture medium from cells, cells were lysed with 1% triton X-100. The percentage of LDH released to culture medium in total LDH was calculated: 100 × LDH in the culture medium/(LDH in the culture medium + intracellular LDH released by triton X-100).
Quantification of IL-1β and TNFα
Culture medium was centrifuged at 1000 g for 10 min at 4°C. The supernatant was then assayed by ELISA kits for measuring rat IL-1β and TNFα according to the manufacturer’s instructions.
Data Analysis
Data are expressed as means ± S.D. (n ≥ 6 for each experimental condition). They were analyzed by one-way analysis of variance followed by the Tukey test for post hoc analysis after confirmation of normal distribution of the data or by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed. A P value < 0.05 was considered statistically significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
Results
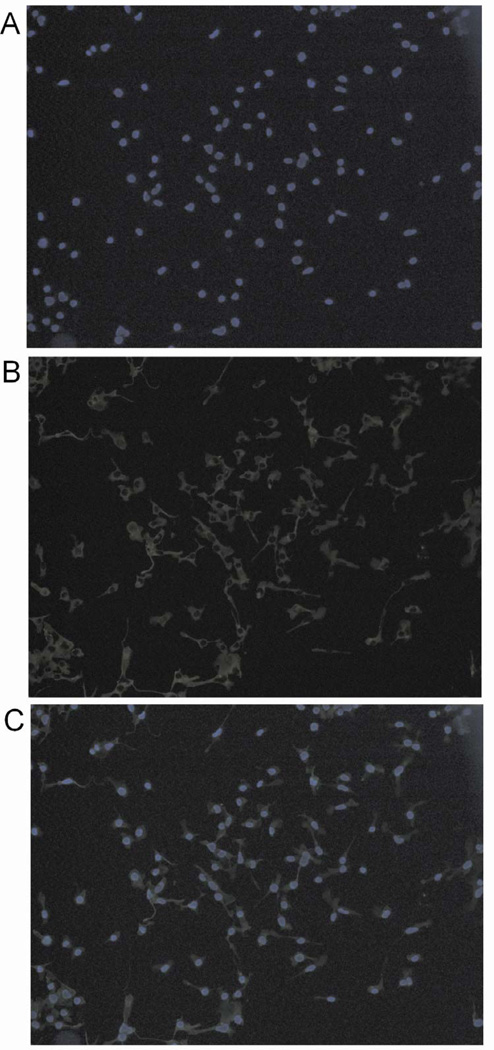
As shown in Figure 1, all cells that were stained by Hoechst were also positively stained by the anti-Iba1 antibody in our culture. Because Hoechst is a fluorescent DNA dye and should stain all cells, this result suggests that all cells in our culture have microglial phenotype.
Fig. 1.
Expression of the microglial marker ionized calcium binding adaptor molecule 1 (Iba1) in the mouse C8-B4 cells. (A) Hoechst staining; (B) immunocytochemical staining for Iba1 and (C) merged image.
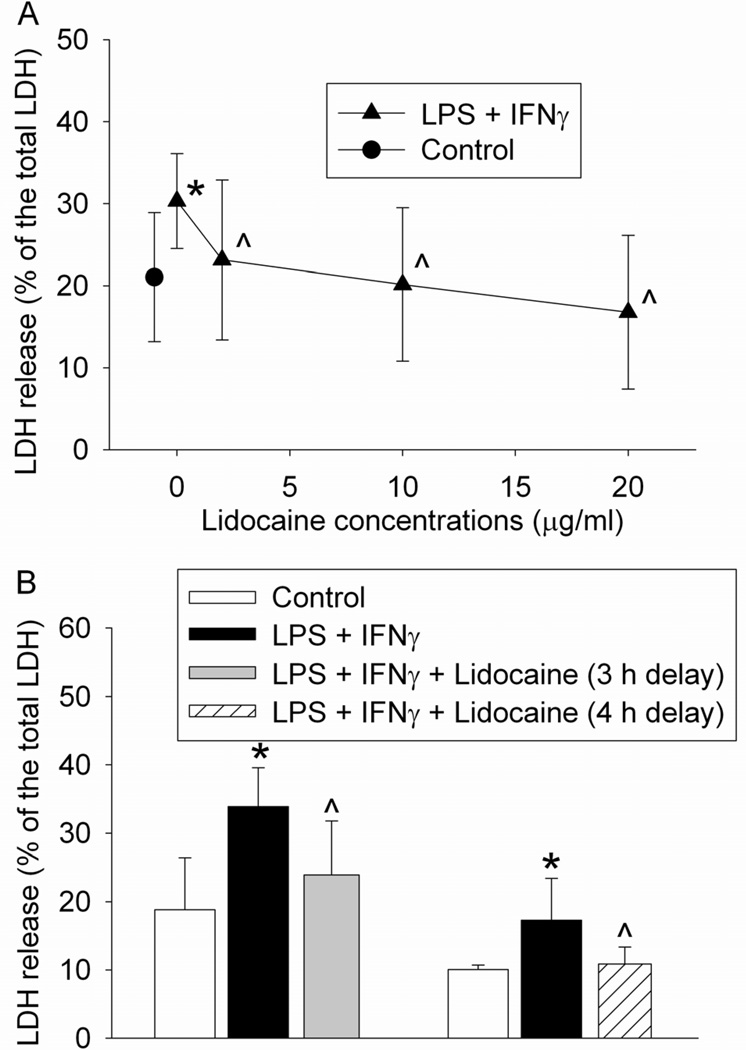
As we reported previously,11 the combination of lipopolysaccharide and IFNγ increased LDH release from the microglial cells. Because LDH release from cells may require cell plasma membrane damage, this result suggests that lipopolysaccharide and IFNγ cause microglial injury. This injury was dose-dependently attenuated by lidocaine applied at 2 h after the onset of lipopolysaccharide and IFNγ stimulation. This protection was already apparent with lidocaine at 2 µg/ml (30.3 ± 5.8 and 23.1 ± 9.7%, respectively, for stimulation alone and the stimulation in the presence of lidocaine, n = 18, P = 0.025) (Fig. 2A). Application of 20 µg/ml lidocaine at 3 or 4 h after the onset of lidocaine also reduced lipopolysaccharide and IFNγ-induced microglial cell injury (Fig. 2B).
Fig. 2.
Protective effects of lidocaine on lipopolysaccharide (LPS) and interferon γ (IFNγ)-induced cell injury. (A) Dose-response of the lidocaine effects. The mouse C8-B4 microglial cells were incubated with 1 µg/ml LPS and 10 U/ml IFNγ for 24 h. Cells were exposed to 2, 10 or 20 µg/ml lidocaine for 1 h at 2 h after the initiation of the LPS and IFNγ stimulation. Cell injury was quantified by lactate dehydrogenase (LDH) release assay. Results are mean ± S.D. (n = 15 – 18). (B) Time-window of delayed isoflurane treatment. The mouse C8-B4 microglial cells were incubated with 1 µg/ml LPS and 10 U/ml IFNγ for 24 h. Cells were exposed to 20 µg/ml lidocaine for 1 h at 3 or 4 h after the initiation of the LPS and IFNγ stimulation. Results are mean ± S.D. (n = 6 – 17). * P < 0.05 compared to control. ^ P < 0.05 compared to LPS plus IFNγ only.
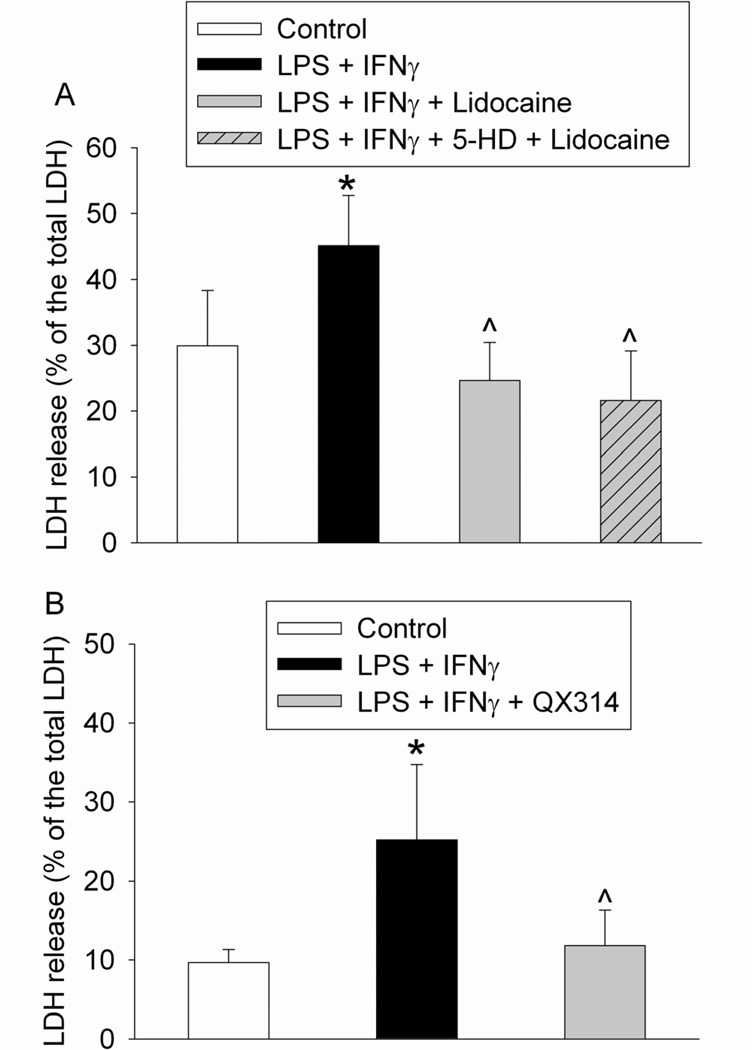
The protective effect of lidocaine was not affected by 5-HD (Fig. 3A). Similar to lidocaine, 25 µg/ml QX314 also attenuated lipopolysaccharide and IFNγ-induced cell injury (Fig. 3B).
Fig. 3.
Protective effects of lidocaine or QX314 on lipopolysaccharide (LPS) and interferon γ (IFNγ)-induced cell injury. The mouse C8-B4 microglial cells were incubated with 1 µg/ml LPS and 10 U/ml IFNγ for 24 h. Cells were exposed to 20 µg/ml lidocaine or 25 µg/ml QX314 for 1 h at 2 h after the initiation of the LPS and IFNγ stimulation. Cell injury was quantified by lactate dehydrogenase (LDH) release assay. Results are mean ± S.D. (n = 6 for panel A and 36 for panel B). * P < 0.05 compared to control. ^ P < 0.05 compared to LPS plus IFNγ only. 5-HD: 5-hydroxydecanoate.
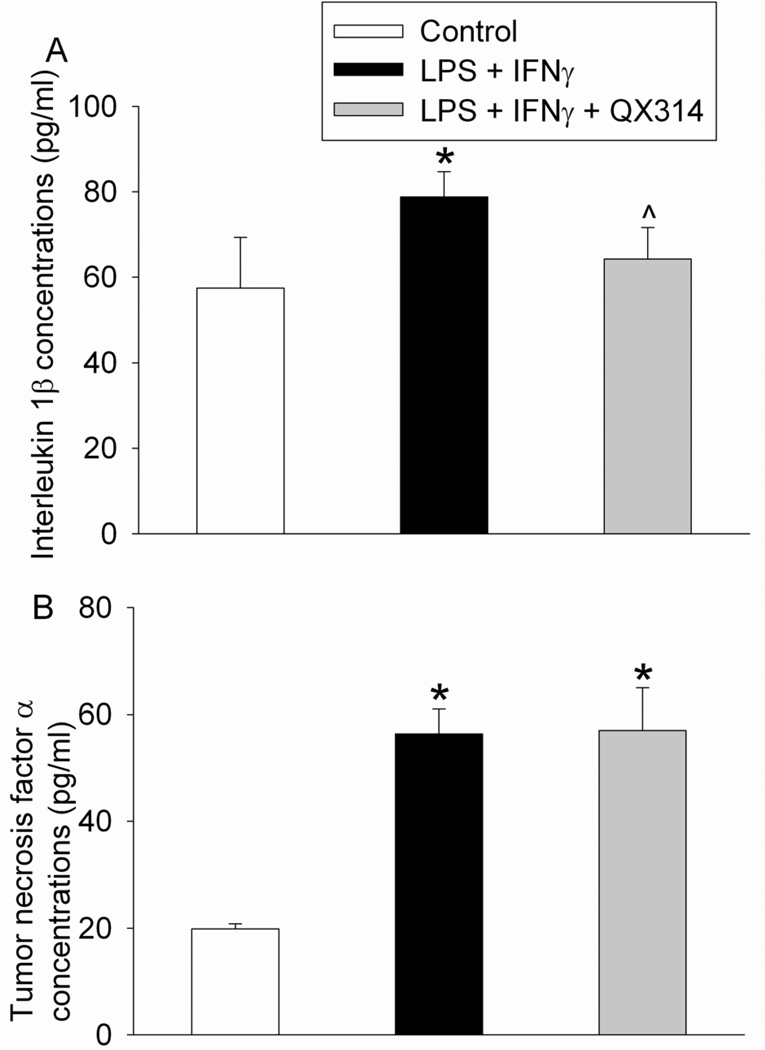
Lipopolysaccharide and IFNγ increased the production of IL-1β and TNFα from the microglial cells. Application of QX314 attenuated the lipopolysaccharide and IFNγ-induced increase of IL-1β but did not affect the increase of TNFα (Fig. 4).
Fig. 4.
Effects of QX314 on lipopolysaccharide (LPS) and interferon γ (IFNγ)-induced cytokine production. The mouse C8-B4 microglial cells were incubated with 1 µg/ml LPS and 10 U/ml IFNγ for 24 h. Cells were exposed to 25 µg/ml QX314 for 1 h at 2 h after the initiation of the LPS and IFNγ stimulation. Cytokine concentrations in the culture medium during the 24-h stimulation were assayed. Results are mean ± S.D. (n = 6). * P < 0.05 compared to control. ^ P < 0.05 compared to LPS plus IFNγ only.
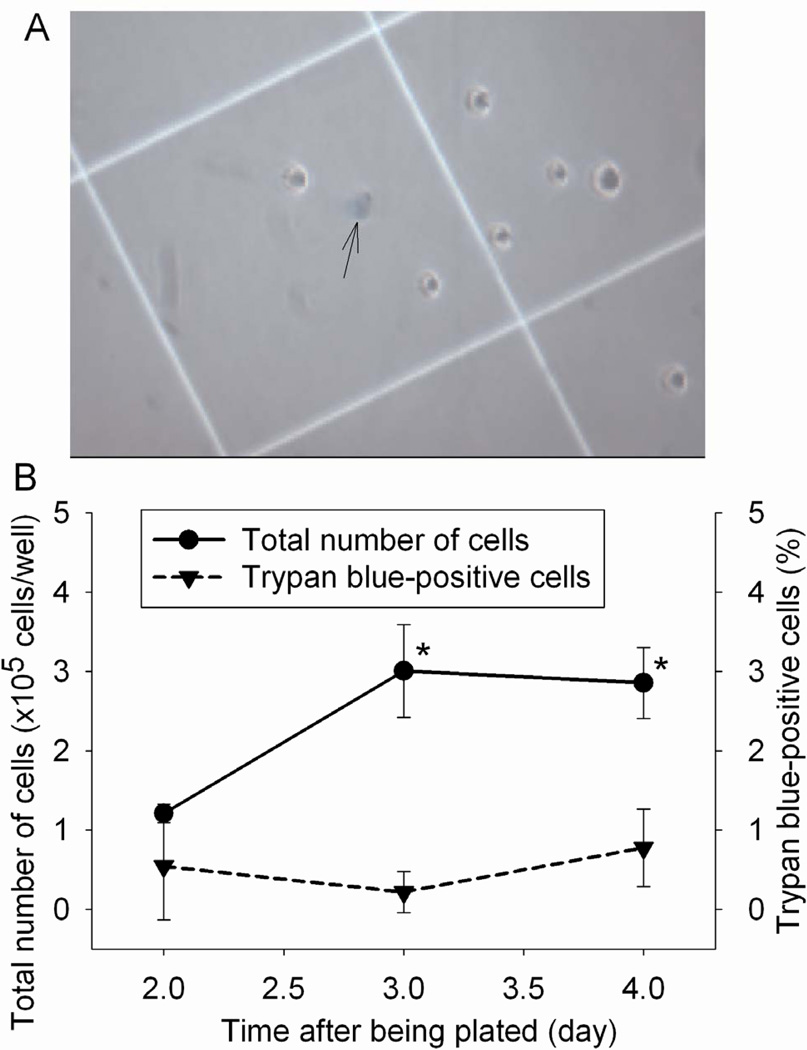
Because there was a high percentage of extracellular LDH in our microglial cell cultures under control conditions, we used trypan blue exclusion assay to determine whether these cells were injured. As shown in Figure 5, cell numbers were increased with the time in culture. This increase was stopped on the third day in culture because they were confluent. However, trypan blue-positive cells were not increased with the time in culture. Of note, LDH release assay performed in other experiments occurred during the second day after the cells were plated in the 6-well plates.
Fig. 5.
Cell viability under control condition. The viability of mouse C8-B4 microglial cells was evaluated by the trypan blue exclusion test at various times after they were plated. A representative microscopic view is presented in panel A and the pooled results are in panel B. The arrow in panel A indicates a trypan blue-positive cell. Results are means ± S.D. (n = 6). * P < 0.05 compared with the corresponding value at 1 day after the cells were plated.
Discussion
Our results clearly show that delayed treatment with lidocaine attenuates lipopolysaccharide and IFNγ-induced microglial injury. Lidocaine has been shown to provide neuroprotection in animal studies.7,8 Our finding adds a novel mechanism for this neuroprotective effect because microglial cells are brain cells and protection of microglial cells may reduce secondary injury to other brain cells, such as neurons, due to the release of intracellular toxic chemicals from microglial cells. Our results indicate that lidocaine at 2 µg/ml is protective. It has been proposed that the plasma concentrations of lidocaine at 2 to 5 µg/ml are an accepted therapeutic range for its effects, such as suppression of seizures.13 Although the brain lidocaine concentrations corresponding to this plasma therapeutic range are not known, our findings suggest that protection of microglial cells by lidocaine may occur at clinically relevant concentrations. In our study, the latest application of lidocaine was at 4 h after the onset of lipopolysaccharide and IFNγ stimulation. This delayed treatment also attenuated lipopolysaccharide and IFNγ-induced microglial injury. Further delay of the treatment was not performed due to the concern that culture medium change that is needed for removal of lidocaine at a very later time point may affect the cell response to lipopolysaccharide and IFNγ and will decrease the amount of LDH in the culture medium used for LDH release assay.
The protective effects of lidocaine on microglial cells may be mediated by cell surface target(s) because extracellular application of QX314 also induces a similar protection. QX314 is a permanently charged lidocaine analog whose charge does not appear to be affected by pH.14,15 Thus, QX314 should not be able to permeate through the plasma membrane. Consistent with this possibility, we and other people have shown that intracellular, but not external, application of QX314 can affect the glutamate transporter type 3 activity and N-methy-D-aspartate receptor signaling.16,17 The effects of lidocaine on glutamate transporter type 3 and N-methy-D-aspartate receptor signaling are shown to be mediated by intracellular protein kinase C.16,17 Also, our results show that lidocaine's effects were not inhibited by 5-HD, suggesting that mitochondrial KATP channels may not be involved in lidocaine's protection. Mitochondrial KATP channels may be involved in lidocaine's protection of cytokines-induced endothelial cell injury.18 The failed inhibition of lidocaine's protection by 5-HD as observed in this study is consistent with the possibility that lidocaine's effects may be through cell surface target(s).
Lidocaine has been shown to reduce lipopolysaccharide-induced cytokine release in rat lungs and adenosine triphosphate-induced cytokine release from rat microglial cultures.5,19 Consistent with these findings, we showed here that QX314 attenuated lipopolysaccharide and IFNγ-induced IL-1β release from mouse microglial cells. Because production of cytokines is an indicator of microglial cell activation, this result suggests that QX314/lidocaine can reduce the microglial activation caused by lipopolysaccharide and IFNγ. Previous studies show that IL-1β and TNFα release from rat lungs stimulated by lipopolysaccharide or from microglial cultures stimulated by ATP is inhibited by lidocaine.5,19 However, TNFα release in our study is not affected by QX314. The reasons for the selective effect of QX314 on IL-1β are not known from our study. Further studies are needed to reveal the mechanisms for this interesting finding.
Cytokines are important mediators for inflammation that can lead to cell injury. The reduction of lipopolysaccharide and IFNγ-induced IL-1β release by QX314 may be a mechanism for its protective effects on microglial cells. Consistent with this proposal, direct application of cytokines has been shown to induce cell injury.18
We plated the same number of cells in each culture well and harvested the culture medium for cytokine measurement after the cells were stimulated by lipopolysaccharide plus IFNγ for 24 h. We did not normalize the cytokine results by the final number of cells or protein amount at the end of the study because cytokines should be produced at all times during the 24-h incubation and it is difficult to determine at which time points cell number or protein amount should be used to normalize these cytokine data. In addition, assuming that the change in direction of intact cell numbers at the end of the 24-h incubation is opposite to that of LDH release data among different experimental conditions (i.e., the cultures exposed to lipopolysaccharide and IFNγ alone have the highest LDH release and lowest intact cell number), normalization of the cytokine data by the final number of intact cells at the end of the incubation should increase the difference among the groups but should not alter the change in direction shown in Figure 4.
Our findings may have significant implications. Sepsis or infections are common clinical entities. Neuroinflammation and activation of brain cells that are involved in neuroinflammation are proposed to participate in the pathophysiology of a broad spectrum of diseases including stroke and neurodegenerative diseases. Preservation of brain cells and modulation of neuroinflammation participating cells under these clinical situations will obviously be beneficial. Delayed treatment with lidocaine as shown in this study may be a useful approach for this purpose.
Interestingly, in contrast to the neuroprotective effects reported in humans and animals,7,8,20,21 lidocaine has also been reported to induce neurotoxicity. Cauda equine syndrome and transient neurological symptoms occur after spinal anesthesia with lidocaine.22 Lidocaine at very high systemic concentrations can cause neurotoxicity, such as seizure, in humans. A systemic subconvulsive dose of lidocaine induces neuronal death in rat brain.23 These neurotoxic effects are caused very likely due to high local concentrations in neural tissues. It is not known whether the protective effects of lidocaine at concentrations within its therapeutic window on microglia as shown in this study have an implication in lidocaine-induced neurotoxicity.
Our study has limitations. We used microglial cell cultures to obtain cell-specific effects. It is not appropriate to directly extrapolate our findings to more complex biological systems, such as in vivo conditions. Also, we have not identified which cell surface target(s) may be involved in the lidocaine effect. Further studies are needed to reveal the molecular mechanisms for this lidocaine effect. Finally, a high percentage (~20%) of total LDH was in the culture medium of the control cells. This percentage is very similar to that reported in our previous study also using C8-B4 cells.24 Other than our data, studies reporting LDH release in these cells under control conditions have not been identified in the literature. The mechanisms for this high percentage of extracellular LDH in control cells are not known yet. Since trypan blue-positive cells were not increased over the time in culture under the control conditions, cell injury or sick cells may not be the reason for the high extracellular LDH fraction in control cells.
In conclusion, our study has shown that delayed treatment with lidocaine attenuates lipopolysaccharide and IFNγ-induced microglial cell activation and injury. These effects may be mediated by cell surface target(s).
Acknowledgments
Funding: This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grantin-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland and the Robert M. Epstein Professorship endowment, University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
DISCLOSURES:
Name: Hae-Jeong Jeong, M.D., Ph.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Hae-Jeong Jeong has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Daowei Lin, M.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Daowei Lin has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Liaoliao Li, Ph.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Liaoliao Li has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Zhiyi Zuo, M.D., Ph.D.
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Attestation: Zhiyi Zuo has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Gregory J. Crosby, M.D.
This report was previously presented, in part, at the ASA Annual Meeting 2011
Contributor Information
Hae-Jeong Jeong, Department of Anesthesiology, University of Virginia, Charlottesville, Virginia, (Current Affiliation: Department of Anesthesiology and Pain Medicine, National Cancer Center, Goyang-si, South Korea)
Daowei Lin, Department of Anesthesiology, University of Virginia, Charlottesville, Virginia, (Current Affiliation: Department of Anesthesiology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangdong, China)
Liaoliao Li, Department of Anesthesiology, University of Virginia, Charlottesville, Virginia
Zhiyi Zuo, Department of Anesthesiology, University of Virginia, Charlottesville, Virginia
References
- 1.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 2.Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neuroscience. 2008;154:1002–1008. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flondor M, Listle H, Kemming GI, Zwissler B, Hofstetter C. Effect of inhaled and intravenous lidocaine on inflammatory reaction in endotoxaemic rats. Eur J Anaesthesiol. 2010;27:53–60. doi: 10.1097/EJA.0b013e32832b8a70. [DOI] [PubMed] [Google Scholar]
- 6.Caracas HC, Maciel JV, Martins PM, de Souza MM, Maia LC. The use of lidocaine as an anti-inflammatory substance: a systematic review. J Dent. 2009;37:93–97. doi: 10.1016/j.jdent.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Lei B, Cottrell JE, Kass IS. Neuroprotective effect of low-dose lidocaine in a rat model of transient focal cerebral ischemia. Anesthesiology. 2001;95:445–451. doi: 10.1097/00000542-200108000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Lei B, Popp S, Capuano-Waters C, Cottrell JE, Kass IS. Effects of delayed administration of low-dose lidocaine on transient focal cerebral ischemia in rats. Anesthesiology. 2002;97:1534–1540. doi: 10.1097/00000542-200212000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Cao H, Kass IS, Cottrell JE, Bergold PJ. Pre- or postinsult administration of lidocaine or thiopental attenuates cell death in rat hippocampal slice cultures caused by oxygen-glucose deprivation. Anesth Analg. 2005;101:1163–1169. doi: 10.1213/01.ane.0000167268.61051.41. [DOI] [PubMed] [Google Scholar]
- 10.Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol. 2006;13:281–288. doi: 10.1128/CVI.13.2.281-288.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Li L, Zuo Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon γ-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111:566–573. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–650. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 13.Lemmen LJ, Klassen M, Duiser B. Intravenous lidocaine in the treatment of convulsions. JAMA. 1978;239:2025. doi: 10.1001/jama.239.19.2025. [DOI] [PubMed] [Google Scholar]
- 14.Modest VE, Butterworth JF., 4th Effect of pH and lidocaine on beta-adrenergic receptor binding. Interaction during resuscitation? Chest. 1995;108:1373–1379. doi: 10.1378/chest.108.5.1373. [DOI] [PubMed] [Google Scholar]
- 15.Nettleton J, Wang GK. pH-dependent binding of local anesthetics in single batrachotoxin-activated Na+ channels. Cocaine vs. quaternary compounds. Biophys J. 1990;58:95–106. doi: 10.1016/S0006-3495(90)82356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do S-H, Fang HY, Ham BM, Zuo Z. The effects of lidocaine on the activity of glutamate transporter EAAT3: the role of protein kinase C and phosphatidylinositol 3-kinase. Anesth Analg. 2002;95:1263–1268. doi: 10.1097/00000539-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Hahnenkamp K, Durieux ME, Hahnenkamp A, Schauerte SK, Hoenemann CW, Vegh V, Theilmeier G, Hollmann MW. Local anaesthetics inhibit signalling of human NMDA receptors recombinantly expressed in Xenopus laevis oocytes: role of protein kinase C. Br J Anaesth. 2006;96:77–87. doi: 10.1093/bja/aei271. [DOI] [PubMed] [Google Scholar]
- 18.de Klaver MJ, Buckingham MG, Rich GF. Lidocaine attenuates cytokine-induced cell injury in endothelial and vascular smooth muscle cells. Anesth Analg. 2003;97:465–470. doi: 10.1213/01.ANE.0000073162.27208.E9. [DOI] [PubMed] [Google Scholar]
- 19.Su D, Gu Y, Wang Z, Wang X. Lidocaine attenuates proinflammatory cytokine production induced by extracellular adenosine triphosphate in cultured rat microglia. Anesth Analg. 2010;111:768–774. doi: 10.1213/ANE.0b013e3181e9e897. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg. 1999;67:1117–1124. doi: 10.1016/s0003-4975(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–1141. doi: 10.1097/00000539-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Johnson ME. Neurotoxicity of lidocaine: implications for spinal anesthesia and neuroprotection. J Neurosurg Anesthesiol. 2004;16:80–83. doi: 10.1097/00008506-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Blas-Valdivia V, Cano-Europa E, Hernandez-Garcia A, Ortiz-Butron R. Hippocampus and amygdala neurotoxicity produced by systemic lidocaine in adult rats. Life Sci. 2007;81:691–694. doi: 10.1016/j.lfs.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Gwak MS, Li L, Zuo Z. Morphine preconditioning reduces lipopolysaccharide and interferon-gamma-induced mouse microglial cell injury via delta 1 opioid receptor activation. Neuroscience. 2010;167:256–260. doi: 10.1016/j.neuroscience.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]