Abstract
A concise, protecting group-free total synthesis of (−)-fusarisetin A (1) was efficiently achieved in 9 steps from commercially available (S)-(−)-citronellal. The synthetic approach was inspired by our proposed biosynthesis of 1. Key transformations of our strategy include a facile construction of the decalin moiety that sets the stage for a stereoselective IMDA reaction and a one-pot TEMPO induced radical cyclization/aminolysis that forms the C ring of 1. Our route is amenable to analogue synthesis for biological evaluation.
Isolated from the soil fungus Fusarium sp. FN080326, fusarisetin A (1) (Figure 1) has attracted considerable attention due to its unprecedented complex molecular architecture and remarkable bioactivity.1 The latter was shown to include potent inhibition of metastasis in MDA-MB-231 cells, a particularly invasive breast cancer cell line. Specifically, 1 was shown to inhibit acinar morphogenesis (77 nM), cell migration (7.7 nM) and cell invasion (26 nM) in these cell lines without any significant cytotoxicity.1 These biological observations are particularly important due to the fact that tumor metastasis is the primary cause of death of cancer patients.2 Thus, the chemical and biological investigation of fusarisetin could lead to the development of new and effective anticancer agents.3
Figure 1.
Fusarisetin A (1) and its proposed biosynthesis from equisetin (2)
From a structural point of view, fusarisetin is highlighted by the presence of an unprecedented pentacyclic motif containing 10 stereocenters. Close inspection of this framework reveals the fusion of a trans-decalin unit (AB ring system) with a tetramic acid moiety (E ring). These rings can also be found in the structure of equisetin (2),4,5 another secondary metabolite from fusarium sp., suggesting that both molecules arise from a common biosynthetic pathway. In fact, one possibility is that fusarisetin derives biogenetically from equisetin via a sequence that would involve formation of stabilized radical 3 (Figure 1). Radical cyclization at the pendant alkene followed by trapping by a reactive oxygen species (ROS)6 and hemiacetalization would then produce 1. Further evidence for this biosynthetic scenario was offered by a recent synthesis of 1 that revised its initially proposed structure as shown in Figure 1.7 Indeed, the revised structure of natural fusarisetin matches the absolute stereochemistry of equisetin.
Thorough evaluation of the pharmacological profile of fusarisetin A (1) would require a concise, high yielding and redox-economical synthetic process.8 With this in mind and inspired by its proposed biosynthesis, we devised a synthesis of 1, highlights of which are shown in Scheme 1.9 We envisioned that the pentacyclic motif of 1 could be constructed via a one-pot Dieckmann condensation and hemiacetalization of tricyclic precursor 4 (construction of DE rings). A subsequent one-pot radical cyclization and aminolysis would then produce compound 4 from bicyclic motif 5 (construction of C ring). Decalin 5 could arise from a Lewis acid-promoted intramolecular Diels-Alder (IMDA) reaction of polyene 6 (construction of AB rings). Lastly, compound 6 could be obtained from commercially available (S)-(−)-citronellal (7) via a Ru-carbene catalyzed olefin metathesis or a regioselective allylic oxidation followed by a regioselective Wittig olefination.
Scheme 1.
Strategic bond disconnections of fusarisetin A
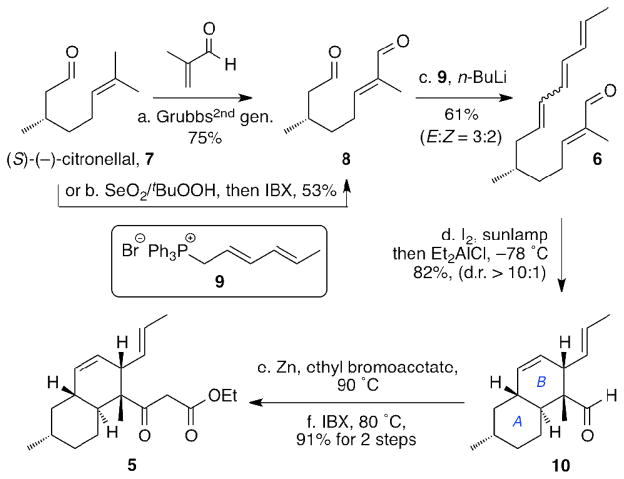
Despite the high efficiency and stereoselectivity of the IMDA reaction,10,11 the synthesis of decalin motifs via this process suffers from the preparation of the IMDA precursors that are usually synthesized via lengthy and tedious synthetic routes and/or proceed in low yields.5b–d,7,10,11 This observation prompted us to develop a more straightforward synthetic route toward 6 (Scheme 2). Starting with (S)-(−)-citronellal (7), we were able to synthesize dialdehyde 8 via a Ru-catalyzed olefin cross-metathesis (Grubbs catalyst, 2nd generation) with methacrolein12 in 75% yield (90% brsm). Alternatively, 8 was obtained via a regioselective allylic oxidation13 followed by sequential oxidation in 53% yield (64% brsm). Chemo-differentiation of the two aldehyde functionalities of 8 was expected to be a key factor of this approach. In fact, Horner–Wadsworth–Emmons reaction5b–c and Julia olefination14 of 8 produced only small amounts of the desired triene 6. We found, however, that Wittig olefination using phosphonium salt 915 led to isolation of triene 6 in 61% yield, albeit with moderate stereoselectivity (E:Z= ca. 3:2). This issue was addressed by an iodine-catalyzed photoisomerisation.16 Thus, treatment of 6 with iodine (5 mol%) in dichloromethane under a sunlamp (visible light, 5 min) led to almost exclusively the desired trans alkene. Without any further purification, this diastereomeric mixture was treated with diethyl aluminum chloride (1.0 equiv) at −78 °C to form decalin 10 in satisfactory yield (82%) and good diastereoselectivity (d.r.>10:1, the minor diastereomer unassigned). This approach allows facile construction of the fusarisetin decalin ring moiety and can also be applied to the synthesis of other natural products possessing similar structural features, such as maklamicin,17a apiosporamide, 17b simvastatin,17c lovastatin,17d oblongolides,17e–f and others.17g–o Treatment of the aldehyde functionality of 10 with ethyl bromoacetate under Reformatsky conditions5d followed by IBX oxidation of the resulting alcohol afforded β-ketoester 5 in 91% combined yield.
Scheme 2. Synthesis of decalin ester 5a.
aReagents and conditions: (a) methacrolein (2.0 equiv), Grubbs2nd gen. catalyst (5 mol%), CH2Cl2, 50 °C, 24 h, 75%, (90% brsm); (b) SeO2 (3 mol%), tBuOOH (4.0 equiv), salicylic acid (0.1 equiv), CH2Cl2, 36 h, then IBX (1.4 equiv), DMSO, 1.5 h, 53%, (64% brsm); (c) 9 (1.0 equiv), n-BuLi (1.0 equiv), THF, −60 °C, 1 h, then −78 °C, 8, 1 min (see SI), 61% (d) I2 (5 mol%), sunlamp (visible light), CH2Cl2, 5 min, then −78 °C, Et2AlCl (1.0 equiv), 18 h, 82%; (e) activated zinc dust (3.0 equiv), ethyl bromoacetate (1.2 equiv), PhH, 45 min, 90 °C; (f) IBX (2.0 equiv), DMSO, 80 °C, 10 min, 91% for 2 steps.
Next we sought to explore oxidative radical cyclization processes for the formation of the C ring of 1. Although radical reactions have often been used in natural products for the construction of C-C bonds, their application to the synthesis of C-O bonds remains very limited.18 In 2001, Jahn et al. reported the construction of 5-membered ring systems starting with a 1,3-dicarbonyl moiety and inactivated alkenes under TEMPO conditions.19–21 Inspired by the similarities between this method and the proposed biosynthesis of fusarisetin A, we decided to evaluate this unexplored method in our synthesis.22 Deprotonation of 5 with LiHMDS, followed by addition of TEMPO and ferrocenium hexafluorophosphate (11) as the oxidant, led to the isolation of 12 as an inseparable C-1 isomeric mixture in 99% yield. Importantly, the conversion of triene 6 to the key intermediate 12 needs only one column chromatography purification. Hence this method provides a convenient and scalable chemical process for the fusarisetin synthesis.
The radical cyclization of 12 proceeded cleanly upon heating in toluene at 90 °C over a period of 36 h. Tricyclic product 15 was isolated as a mixture of diastereomers at the C-5 center in good overall yield (80 %, d.r.= ca. 1:1) (Scheme 3). A reasonable mechanism of this cyclization would involve reversible generation of the stable radical 14 under heating followed by cyclization at the C-6 center to create the C-5 radical (5-exo-trig cyclization). Remarkably, the stereoselectivity of this cyclization is substratecontrolled and forms the desired isomer at the C-1 and C-6 centers. Subsequent irreversible trapping of the C-5 radical with TEMPO can give rise to compound 15. To enhance the overall synthetic efficiency, we further performed this radical reaction in the presence of the amino acid 13.23 We were pleased to find that 13 did not interfere with the cyclization and readily aminolysed the C-2 ester, to afford 4 and C5-epi-4 (d.r.= ca. 1:1) in one-pot and 70% overall yield. Despite the moderate diastereoselectivity, this onepot radical cyclization/aminolysis reaction cascade24 offers a concise way to build up the fusarisetin core structure. Subsequently, the C-5 hydroxy group of compound 4 was liberated under Zn/AcOH conditions25 Treatment of the resulting C-5 alcohol under basic conditions (NaOMe) induced a one-pot Dieckmann condensation/ hemiacetaliza- tion5,7 (construction of DE rings) ultimately producing fusarisetin A (1) in 42% overall yield. Importantly, the C5- epi-4 could be also converted to fusarisetin A via a 3-step sequence that included: a) oxidative cleavage of the N-O bond with mCPBA26 to form ketone 16; b) regioselective and stereoselective reduction of the C-5 ketone with NaBH4 (d.r.= ca. 3:1) and c) one-pot Dieckmann condensation/ hemiacetalization (38% yield over 3 steps).
Scheme 3. Completion of the synthesisa.
aReagents and conditions: (a) LiHMDS (1.5 equiv), 1,2- dimethoxyethane, −78 °C, 30 min, then 0 °C, TEMPO (1.05 equiv), Cp2FePF6 (2.0 equiv), 5 min, 99%; (b) 14, 4-DMAP (2 equiv), PhMe, 4Å MS, 90 °C, 36 h, 70% (d.r. = ca. 1:1); (c) activated zinc dust (100 equiv), AcOH/THF/H2O (3:1:1), 70 °C, 12 h; (d) NaOMe (5.0 equiv), MeOH, 10 min, 42% for 2 steps; (e) mCPBA (1.2 equiv), CH2Cl2, 0 °C, 15 min, 95%; (f) NaBH4 (0.6 equiv), MeOH, −78 °C (d.r. = ca. 3:1); (g) NaOMe (5.0 equiv), MeOH, 10 min, 39% for 2 steps.
The isolated sample of synthetic 1 was found to be identical in all aspects with naturally occurring fusarisetin A (1H-NMR, 13C-NMR and HR-MS), except for the optical rotation [synthetic: [α]D23 = −86.2 (c = 0.065 in MeOH); natural: [α]D25 = +84.6 (c = 0.2 in MeOH)1, reported synthetic (−)-1: [α]D27 = −88.0 (c = 0.15 in MeOH)7]. The chemical synthesis of 1 confirmed that the absolute stereochemistry of natural fusarisetin A is opposite to our synthetic 1 and provides support for its proposed biosynthesis.
In conclusion, we have accomplished a concise, natureinspired and protecting-group-free27 total synthesis of (−)-fusarisetin A (1). The overall synthesis proceeds in 9 steps and 10% overall yield. Our strategy is highlighted by: a) a rapid stereoselective construction of decalin 5 (AB ring system) using an optimized sequence; b) an efficient onepot radical cyclization cascade/aminolysis that for the C ring of 1 and installs the C-5 hydroxy group; and c) a rapid Dieckman condensation/hemiacetalization reaction cascade that produces the DE rings of 1. Our strategy also demonstrates for the first time the applicability of the TEMPO induced oxidative radical cyclization reaction in the context of complex molecules synthesis. We expect that this approach would be able to provide sufficient amounts of the natural (+)-fusarisetin A and related analogs for further biological investigations en route to the development of novel small molecule inhibitors of tumor metastasis.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institutes of Health (NIH) for financial support of this work through Grant Number R01 GM081484-01. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Anthony Mrse (UCSD NMR Facility), and Dr. Yongxuan Su (UCSD MS Facility). We also thank C.-I. Hung for technical assistance.
Footnotes
Supporting Information. Experimental details, spectral data for key compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jang JH, Asami Y, Jang JP, Kim SO, Moon DO, Shin KS, Hashizume D, Muroi M, Saito T, Oh H, Kim BY, Osada H, Ahn JS. J Am Chem Soc. 2011;133:6865–6867. doi: 10.1021/ja1110688. [DOI] [PubMed] [Google Scholar]
- 2.(a) Chaffer CL, Weinberg RA. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]; (b) Chiang AC, Massagué J. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bacac M, Stamenkovic I. Annu Rev Pathol Mech Dis. 2008;3:221–247. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- 3.For impressive studies on (+)-migrastatin, a potent matastasis inhibitor, see: Gaul C, Njardarson JT, Danishefsky S. J J Am Chem Soc. 2003;125:6042–6043. doi: 10.1021/ja0349103.Shan DD, Chen L, Njardarson JT, Gaul C, Ma XM, Danishefsky SJ, Huang XY. Proc Nat Acad Sci USA. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102.Geho DH, Bandle RW, Clair T, Liotta LA. Physiology. 2005;20:194–200. doi: 10.1152/physiol.00009.2005.
- 4.For equisetin isolation, characterization and biosynthesis see: Burmeister HR, Bennett GA, Vesonder RF, Hesseltine CW. Antimicrob Agents Chemother. 1974;5:634–639. doi: 10.1128/aac.5.6.634.Phillips NJ, Goodwin JT, Fraiman A, Cole RJ, Lynn DG. J Am Chem Soc. 1989;111:8223–8231.Sims JW, Fillmore JP, Warner DD, Schmidt EW. Chem Commun. 2005:186–188. doi: 10.1039/b413523g.
- 5.For equisetin synthesis see: Turos E, Audia JE, Danishefsky SJ. J Am Chem Soc. 1989;111:8231–8236.Burke LT, Dixon DJ, Ley SV, Rodríguez F. Org Lett. 2000;2:3611–3613. doi: 10.1021/ol006493u.Burke LT, Dixon DJ, Ley SV, Rodríguez F. Org Biomol Chem. 2005;3:274–280. doi: 10.1039/b411350k.Kumiko Yuki K, Shindo M, Shishido K. Tetrahedron Lett. 2001;42:2517–2519.
- 6.(a) Dickinson BC, Chang CJ. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Apel K, Hirt H. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]; (c) Müller K, Gawlik I. Free Radical Bio Med. 1997;23:321–330. doi: 10.1016/s0891-5849(97)00092-0. [DOI] [PubMed] [Google Scholar]; (d) Corey EJ, Wang Z. Tetrahedron Lett. 1994;35:539–542. [Google Scholar]
- 7.Deng J, Zhu B, Lu ZY, Yu HX, Li A. J Am Chem Soc. 2012;134:920–923. doi: 10.1021/ja211444m. [DOI] [PubMed] [Google Scholar]
- 8.For reviews of atom economic and redox economic synthesis see: Burns NZ, Baran PS, Hoffmann RW. Angew Chem Int Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086.Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010–3021. doi: 10.1039/b821200g.Gaich T, Baran PS. J Org Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812.
- 9.For selected total synthesis efforts from the Theodorakis group see: Ling TT, Xiang AX, Theodorakis EA. Angew Chem Int Ed. 1999;38:3089–3091. doi: 10.1002/(sici)1521-3773(19991018)38:20<3089::aid-anie3089>3.0.co;2-w.Ling TT, Poupon E, Rueden EJ, Kim SH, Theodorakis EA. J Am Chem Soc. 2002;124:12261–12267. doi: 10.1021/ja027517q.Brady TP, Kim SH, Wen K, Theodorakis EA. Angew Chem Int Ed. 2004;43:739–742. doi: 10.1002/anie.200352868.Tisdale EJ, Slobodov I, Theodorakis EA. Proc Nat Acad Sci USA. 2004;101:12030–12035. doi: 10.1073/pnas.0401932101.Chantarasriwong O, Batova A, Chavasiri W, Theodorakis EA. Chem–Eur J. 2010;16:9944–9962. doi: 10.1002/chem.201000741.Xu J, Trzoss L, Chang WK, Theodorakis EA. Angew Chem Int Ed. 2011;50:3672–3676. doi: 10.1002/anie.201100313.
- 10.For reviews of IMDA reaction in total synthesis see: Juhl M, Tanner D. Chem Soc Rev. 2009;38:2983–2992. doi: 10.1039/b816703f.Takao K, Munakata R, Tadano K. Chem Rev. 2005;105:4779–4807. doi: 10.1021/cr040632u.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem Int Ed Engl. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z.
- 11.(a) Williams DR, Kammler DC, Donnell AF, Goundry WRF. Angew Chem Int Ed. 2005;44:6715–6718. doi: 10.1002/anie.200502015. [DOI] [PubMed] [Google Scholar]; (b) Inoue A, Kanematsu M, Yoshida M, Shishido K. Tetrahedron Lett. 2010;51:3966–3968. [Google Scholar]; (c) Wilson RM, Jen WS, MacMillan DWC. J Am Chem Soc. 2005;127:11616–11617. doi: 10.1021/ja054008q. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 13.Beckett JS, Beckett JD, Hofferberth JE. Org Lett. 2010;12:1408–1411. doi: 10.1021/ol100077z. [DOI] [PubMed] [Google Scholar]
- 14.(a) Julia M, Paris JM. Tetrahedron Lett. 1973;14:4833–4836. [Google Scholar]; (b) Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26–28. [Google Scholar]
- 15.(a) Chen X, Millar JG. Synthesis. 2000:113–118. [Google Scholar]; (b) Jacobs WC, Christmann M. Synlett. 2008:247–251. [Google Scholar]; (c) Kim T, Mirafzal GA, Liu JP, Bauld NL. J Am Chem Soc. 1993;115:7653–7664. [Google Scholar]; (d) Tilley SD, Reber KP, Sorensen EJ. Org Lett. 2009;11:701–703. doi: 10.1021/ol802768p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner CI, Williamson RM, Turner P, Sherburn MS. Chem Commun. 2003:1610–1611. [Google Scholar]
- 17.For references on selected natural products possess similar decalin functionalities see: Igarashi Y, Ogura H, Furihata K, Oku N, Indananda C, Thamchaipenet A. J Nat Prod. 2011;74:670–674. doi: 10.1021/np100727h.Alfatafta AA, Gloer JB, Scott JA, Malloch D. J Nat Prod. 1994;57:1696–1702. doi: 10.1021/np50114a012.Askin D, Verhoeven TR, Liu TM-H, Shinkai I. J Org Chem. 1991;56:4929–4932.Endo A, Hasumi K. J Antibiot. 1979;32:852–854. doi: 10.7164/antibiotics.32.852.Bunyapaiboonsri T, Yoiprommarat S, Srikitikulchai P, Srichomthong K, Lumyong S. J Nat Prod. 2010;73:55–59. doi: 10.1021/np900650c.Lin T, Lin X, Lu C-H, Hu Z-Y, Huang W-Y, Huang Y-J, Shen Y-M. Eur J Org Chem. 2009:2975–2982.Hellwig V, Grothe T, Mayer-Bartschmid A, Endermann R, Geschke FU, Henkel T, Stadler M. J Antibiot. 2002;55:881–892. doi: 10.7164/antibiotics.55.881.Tsukamoto S, Miura S, Yamashita Y, Ohta T. Bioorg Med Chem Lett. 2004;14:417–420. doi: 10.1016/j.bmcl.2003.10.053.Sugie Y, Inagaki S, Kato Y, Nishida H, Pang CH, Saito T, Sakemi S, Dib-Hajj F, Mueller JP, Sutcliffe J, Kojima Y. J Antibiot. 2002;55:25–29. doi: 10.7164/antibiotics.55.25.Li JY, Strobel G, Harper J, Lobkovsky E, Clardy J. Org Lett. 2000;2:767–770. doi: 10.1021/ol000008d.Endo A, Hasumi K, Nakamura T, Kunishima M, Masuda M. J Antibiot. 1985;38:321–327. doi: 10.7164/antibiotics.38.321.Singha SB, Zinka DL, Goetza MA, Dombrowskia AW, Polishooka JD, Hazuda DJ. Tetrahedron Lett. 1998;39:2243–2246.Lang G, Blunt JW, Cummings NJ, Cole ALJ, Munro MHG. J Nat Prod. 2005;68:810–811. doi: 10.1021/np0500979.Shibazaki M, Taniguchi M, Yokoi T, Nagai K, Watanabe M, Suzuki K, Yamamoto T. J Antibiot. 2004;57:379–382. doi: 10.7164/antibiotics.57.379.Marfori EC, Kajiyama S, Fukusaki E, Kobayashi AZ. Naturforsch C. 2002;57:465–470. doi: 10.1515/znc-2002-5-611.
- 18.For reviews, see: Jasperse CP, Curran DP, Fevig TL. Chem Rev. 1991;91:1237–1286.Justicia J, Cienfuegos LÁ, Campaña AG, Miguel D, Jakoby V, Gansäuer A, Cuerva JM. Chem Soc Rev. 2011;40:3525–3537. doi: 10.1039/c0cs00220h.Snider BB. Chem Rev. 1996;96:339–364. doi: 10.1021/cr950026m.Hawker CJ, Bosman AW, Harth E. Chem Rev. 2001;101:3661–3688. doi: 10.1021/cr990119u.
- 19.For initial studies, see: Jahn U. Chem Commun. 2001:1600–1601. doi: 10.1039/b104415j.Jahn U, Hartmann P, Dix I, Jones PG. Eur J Org Chem. 2001:3333–3355.
- 20.For related studies see: Jahn U, Müller M, Aussieker S. J Am Chem Soc. 2000;122:5212–5213.Wetter C, Jantos K, Woithe K, Studer A. Org Lett. 2003;5:2899–2902. doi: 10.1021/ol034994k.Vogler T, Studer A. Synthesis. 2006:4257–4265.Molawi K, Schulte T, Siegenthaler KO, Wetter C, Studer A. Chem–Eur J. 2005;11:2335–2350. doi: 10.1002/chem.200400936.Wetter C, Studer A. Chem Commun. 2004:174–175. doi: 10.1039/b313139d.Schulte B, Studer A. Synthesis. 2006:2129–2138.
- 21.For applications see: Jahn U, Hartmann P, Dix I, Jones PG. Eur J Org Chem. 2002:718–735.Siegenthaler KO, Schäfer A, Studer A. J Am Chem Soc. 2007;129:5826–5827. doi: 10.1021/ja0686716.Wienhöfer IC, Studer A, Rahman MT, Fukuyama T, Ryu I. Org Lett. 2009;11:2457–2460. doi: 10.1021/ol900713d.
- 22.For selected recent natural products synthesis using oxidative radical cyclizations, see: Chen P-H, Cao L-D, Tian W-H, Wang X-F, Li C-Z. Chem Commun. 2010;46:8436–8438. doi: 10.1039/c0cc03428b.Stoye A, Opatz T. Org Lett. 2010;12:2140–2141. doi: 10.1021/ol100652b.Davies JJ, Krulle TM, Burton JW. Org Lett. 2010;12:2738–2741. doi: 10.1021/ol100794k.for recent natural product total synthesis using reductive radical cyclizations see: Beemelmanns C, Reissig HU. Angew Chemie Int Ed. 2010;49:8021–8025. doi: 10.1002/anie.201003320.Li Z, Nakashige M, Chain WJ. J Am Chem Soc. 2011;133:6553–6556. doi: 10.1021/ja201921j.
- 23.White KN, Konopelski JP. Org Lett. 2005;7:4111–4112. doi: 10.1021/ol051441w. [DOI] [PubMed] [Google Scholar]
- 24.For recent reviews of cascade reactions see: Nicolaou KC, Chen JS. Chem Soc Rev. 2009;38:2993–3009. doi: 10.1039/b903290h.Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872.Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n.
- 25.(a) Howell AR, Pattenden G. J Chem Soc, Perkin Trans. 1990;1:2715–2720. [Google Scholar]; (b) Gong JX, Lin G, Sun WB, Li CC, Yang Z. J Am Chem Soc. 2010;132:16745–16746. doi: 10.1021/ja108907x. [DOI] [PubMed] [Google Scholar]
- 26.Wang YF, Toh KK, Lee JY, Chiba S. Angew Chem Int Ed. 2011;50:5927–5931. doi: 10.1002/anie.201101009. [DOI] [PubMed] [Google Scholar]
- 27.For reviews and recent examples of protecting-group-free total synthesis see: Young IS, Baran PS. Nature Chem. 2009;1:193–205. doi: 10.1038/nchem.216.Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r.McFadden RM, Stoltz BM. J Am Chem Soc. 2006;128:7738–7739. doi: 10.1021/ja061853f.Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569.Gademann K, Bonazzi S. Angew Chem Int Ed. 2007;46:5656–5658. doi: 10.1002/anie.200701881.Zhou Q, Chen X, Ma DW. Angew Chem Int Ed. 2010;49:3513–3516. doi: 10.1002/anie.201000888.Hickmann V, Alcarazo M, Fürstner A. J Am Chem Soc. 2010;132:11042–11044. doi: 10.1021/ja104796a.Gerfaud T, Xie CS, Neuville L, Zhu JP. Angew Chem Int Ed. 2011;50:3954–3957. doi: 10.1002/anie.201100257.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.