Abstract
Objective
Insomnia and objectively measured sleep disturbances predict poor treatment outcomes in patients with major depressive disorder (MDD). However, prior research has utilized individual clinical trials with relatively small sample sizes and has focused on insomnia symptoms or objective measures, but not both. The present study is a secondary analysis that examines the degree to which insomnia, objective sleep disturbances, or their combination, predicts depression remission following pharmacotherapy and/or psychotherapy treatment.
Methods
Participants were 711 depressed patients drawn from six clinical trials. Remission status, defined as a score of ≤ 7 on the Hamilton Rating Scale for Depression (HDRS) over two consecutive months, served as the primary outcome. Insomnia was assessed via the 3 sleep items on the HDRS. Objectively measured short sleep duration (total sleep time < = 6 hours), and prolonged sleep latency (SL >30 minutes) or wakefulness after sleep onset (WASO>30) were derived from in-laboratory polysomnographic (PSG) sleep studies. Logistic regression predicted the odds of non-remission according to insomnia, each of the objective sleep disturbances, or their combination, after adjusting for age, sex, treatment modality, and baseline depressive symptoms.
Results
Prolonged sleep latency alone (OR = 1.82; CI: 1.23, 2.70) or in combination with insomnia (OR = 2.03; CI: 1.13, 3.95) predicted increased risk of non-remission. In addition, insomnia and sleep duration individually and in combination were each associated with a significantly increased risk of non-remission (p’s < .05).
Conclusion
Findings suggest that objectively measured prolonged sleep latency and short sleep duration independently, or in conjunction with insomnia are risk factors for poor depression treatment outcome.
Insomnia and objectively measured sleep disturbances are associated with slower treatment response and poorer treatment outcomes in patients with major depression.1–3 Although complete recovery is the goal of depression treatment, this is often an elusive goal, and sleep disturbances are among the most common residual symptoms.4–6 A recent study found a residual insomnia rate of 51% among patients who showed remission of other depressive symptoms following 20 weeks of either cognitive behavioral therapy or pharmacotherapy.7
Subjective complaints of insomnia and specific objective sleep disturbances measured with polysomnography (i.e., increased phasic rapid eye movement (REM) sleep, diminished slow wave sleep, and disturbed sleep continuity) predict symptom ratings, attrition and remission rates, stability of treatment response, and suicidal ideation in patients with depression.8–12 Furthermore, in previously remitted depressed persons, insomnia may be a prodromal symptom heralding the onset of a new depressive episode.13–15
Given that sleep disturbances are highly prevalent, portend poorer treatment outcomes, and when left untreated, increase risk for relapse in depressed populations, understanding the links between sleep disturbances and depression treatment outcome is critical. Previous studies of sleep and depression treatment outcome have been limited by the use of individual clinical trials with relatively small sample sizes and generally restricted to single treatment modalities and/or focused exclusively on insomnia complaints or EEG characteristics. However, the combination of insomnia with an objective marker of sleep disturbance may represent a biological marker of insomnia severity with added prognostic value. For instance, insomnia combined with polysomnographically-assessed short sleep duration has been linked with higher rates of hypertension,16 diabetes,17 and neurocognitive deficits.18 To our knowledge, no study to date has examined the impact of insomnia combined with an objective indicator of sleep disturbance on depression treatment outcome.
The present study represents a secondary analysis of data drawn from six clinical trials involving acute or maintenance treatment with psychotherapy, medication, or combination treatment, to examine the degree to which insomnia complaints and homologous PSG measures of disrupted sleep (defined as sleep duration <=6 hours, sleep latency > 30 minutes, or wakefulness after sleep onset > 30 minutes) predict depression remission status (defined as HDRS scores <=7 over two consecutive months) in a sample of 711 treated depressed patients. These specific PSG sleep disturbances, as opposed to other sleep architectural anomalies, were selected because they are most consistent with standard quantitative criteria typically used in clinical trials to define insomnia severity. Specifically, we examined the degree to which insomnia or PSG characteristics, alone or in combination, predict remission status in a large sample of clinically depressed adults. We predicted that insomnia symptoms and PSG sleep disturbances would individually be associated with increased risk of non-remission, and the combination of insomnia with an objective indicator would potentiate the risk. We also examined whether the combination of multiple sleep disturbances (defined as the total number of objective sleep disturbances and insomnia) increased risk of non-remission. Follow-up analyses explored whether the risk associated with sleep disturbances differed depending on treatment modality.
Methods
Overview
Data for the current study include 711 depressed patients drawn from 6 clinical trials conducted at the University of Pittsburgh between 1982 and 2001: Maintenance Therapies in Recurrent Depression,19 Maintenance Therapies in Late-Life Depression,20 Psychobiology of Recovery from Depression,21 Nocturnal Penile Tumescence in Depression Study,22 Social Zeitgebers in Depression,23 and Maintenance Psychotherapy in Recurrent Depression.24 Detailed methods and main hypotheses for each of these studies have been published previously.
Herein, we briefly summarize methods germane to the present analyses and shared across the studies. Specifically, all 6 studies had the following in common: (1) an intake diagnosis of major depressive disorder (nonpsychotic, nonbipolar subtypes) determined according to DSM-III, DSM-III-R, or DSM-IV criteria on the basis of a semi-structured clinical interview conducted by a clinician and faculty psychiatrist; (2) medical stability determined by physical exam, laboratory studies, and electrocardiogram; (3) sleep measured via 1 or 2 nights of in-laboratory polysomnographic (PSG) sleep studies, following an adaptation sleep night; (4) baseline depressive symptoms as rated by the Hamilton Depression Rating Scale (HDRS25); (5) at least two consecutive monthly ratings of depressive symptoms using the HDRS following at least 8 weeks of treatment; and (6) written informed consent provided by all patients. As previously reported, psychotherapy modalities included interpersonal psychotherapy (IPT26) and cognitive-behavioral therapy (CBT27), which had similar rates of recovery and symptomatic symptoms.28 Pharmacologic treatments included tricyclic antidepressants (imipramine or nortriptyline), selective serotonin reuptake inhibitors (SSRIs, including fluoxetine and paroxetine) and bupropion. Based on the current report’s primary aim of examining the degree to which sleep disturbances predict treatment outcome, and that comparative efficacy data has been previously reported in a subset of this sample,28 the various treatment types were grouped and included as a binary covariate in all statistical models, coded as psychotherapy alone versus pharmacotherapy.
By design, the specific aims and eligibility criteria for each of the protocols resulted in large differences across demographic and clinical characteristics. Therefore, factors which were known to distinguish the individual protocols and to be risk factors for treatment prognosis (i.e., age, sex, depressive symptom severity, duration of follow-up, and treatment modality) were included as statistical covariates in all models.
Sleep Studies
Sleep studies were conducted after a minimum 14-day, psychotropic medication-free evaluation period. All protocols recorded sleep using in-laboratory PSG for 2 or 3 consecutive nights. The recording period for sleep studies varied somewhat depending on protocol. For sleep studies recorded prior to 1990 (n = 403), the recording period was set by the laboratory. For studies occurring after December 1990 (n = 289), sleep recordings were scheduled to be consistent with participants’ habitual “goodnight” and morning wake-up times (determined by self-report or sleep diaries). To minimize adaptation effects associated with sleep assessment,29 sleep data included only non-adaptation nights, i.e., Night 2 or the average of Nights 2 and 3. Sleep data were obtained using a standard PSG montage that includes one channel of electroence phalography (EEG; C3 or C4 referenced to A1–A2), bilateral electro-oculograms, and bipolar submental electromyograms (EMGs) with sleep records scored in one-minute epochs according to standard criteria,30 and consistent with scoring conventions at the time the studies were conducted.31 The derived sleep variables of consideration in the present study included total sleep time (TST; time spent asleep), sleep latency (SL; time from beginning of the recording period to the first of 10 consecutive minutes of Stage 2, slow wave, or REM sleep uninterrupted by no more than 1 minute of wakefulness, or 2 minutes of stage 1 sleep), and wakefulness after sleep onset (WASO; minutes of intermittent wakefulness). In accordance with quantitative severity criteria typically used in insomnia research,32;33 each of these sleep measures were categorized into binary variables to reflect the presence of a clinically significant sleep disturbance as follows: short sleep duration (TST < 6 hours), prolonged sleep latency (SL >30 minutes), and prolonged WASO (WASO >30 minutes). We also analyzed each of the objective sleep disturbances as continuous predictors and results were similar (analyses available upon request). Therefore, analyses presented herein are based on dichotomous sleep disturbances derived from clinically-based quantitative thresholds.
Baseline clinical severity, insomnia, and treatment outcome
For each of the study protocols, at study entry and regularly throughout the treatment protocol, patients were administered the HDRS25 by a trained, independent clinician. The HDRS is a clinician-administered interview scale that assesses the presence and severity of 17 symptoms of depression experienced in the past week using a varied response format ranging from 0–2 to 0–4 (with higher scores indicating greater depression severity), and exhibits well-documented reliability and validity.34 The HDRS includes 3 sleep disturbance items which pertain to early, middle, and late insomnia (i.e., difficulty falling asleep, difficulty staying asleep, and early morning awakenings). Patients who endorsed a clinically significant sleep complaint on any of these sleep items (as indicated by a score of >=2) were categorized as having insomnia. Baseline depressive symptoms were derived from HDRS scores with sleep items removed and included as a statistical covariate in all models. In addition, anxiety symptoms as defined by two items on the HDRS assessing psychic and somatic anxiety symptoms, were also included as a covariate in follow-up analyses. The primary outcome in the current analyses was remission status, defined by an HDRS score of ≤7 for two consecutive monthly ratings, as this threshold is one of the most commonly used and recommended criteria for defining remission status in depression treatment studies.35
Analyses
Study hypotheses were examined using a series of logistic regression models, adjusted for age, sex, treatment modality, number of weeks of follow-up, and baseline depression severity (HDRS with sleep items removed). Specifically, the first set of logistic regression models regressed remission status (with non-remission coded as 1 and remission coded as 0) on the presence of subjective insomnia symptoms or an objective marker or sleep disturbance (SL>30, WASO >30, or TST <=6 hours, each entered individually). Next, to examine whether the combination of insomnia symptoms with an objective sleep disturbance potentiates the risk for non-remission, we entered the insomnia complaint, each of the objective sleep disturbances (in separate models) and the interaction between insomnia and the objective marker into the logistic regression models. For significant interaction effects, we used dummy-coded contrasts to examine the nature of the interaction. That is, we compared the referent group (those without insomnia and without the particular objective sleep disturbance) to the following contrasts: those with insomnia only, those with the objective marker only (no insomnia), or those with insomnia and the objective marker. For significant effects, we examined the 2-or 3-way interaction terms, respectively, between individual sleep disturbances, or the combination of objective and subjective sleep disturbances, and treatment modality (pharmacotherapy or combined treatment versus psychotherapy alone). Finally, we examined whether increasing numbers of sleep disturbances (defined as the sum of each objective sleep disturbance and the insomnia complaint) were associated with increased risk of non-remission.
Given that anxiety symptoms have been shown to predict poor treatment response in depression,36;37 for all results reported, we conducted analyses with baseline anxiety symptoms (from HDRS) covaried in place of baseline depressive symptoms. In all cases, results were unaffected by covarying anxiety symptoms. Therefore, we report results covarying only for depressive symptoms.
Results
Demographic, clinical, and sleep characteristics for the total sample and according to the presence of each of the objective sleep disturbances and insomnia complaint are reported in Table 1. Overall, the sample was predominantly female (74%) and Caucasian (93%) with a mean age of 43.5 years (SD = 15.2). Overall, baseline depressive symptoms were in the moderate range of clinical severity for the total sample. Roughly three-quarters of patients (73%) evidenced a subjective complaints of insomnia. The majority of those with a significant insomnia complaint had more than one insomnia symptom and the frequency of each insomnia symptom was roughly equivalent (37% with initial insomnia, 40% for middle insomnia, and 39% for “late” insomnia; i.e., early morning awakenings). The prevalence of objective markers of sleep disturbances was considerably lower (ranging from 20% for prolonged SL to 37% for WASO >30). Individuals with insomnia or any of the objective sleep disturbances were older, had higher depressive symptoms at baseline (with or without sleep items included), and were more likely to have received pharmacologic treatment than those without the particular sleep disturbance. Thirty-seven percent of the population did not meet remission criteria, and those with objectively prolonged sleep latency were more likely to be non-remitters relative to those who had sleep latencies <=30 minutes.
Table 1.
Sample Characteristics (N=711)
| Mean | SD | |
|---|---|---|
| Sociodemographics | ||
| Age | 43.5 | 15.0 |
| N | % | |
| Female | 526 | 74 |
| Caucasian | 660 | 93 |
| Clinical Characteristics | Mean | SD |
| Current episode duration (weeks) | 30.8 | 36.9 |
| Duration of follow-up (weeks) | 15.9 | 11.6 |
| Total HDRSa score | 20.7 | 4.3 |
| HDRS score (Sleep Items Removed) | 17.6 | 3.6 |
| Treatment Modality/Outcome | N | % |
| Psychotherapy only | 325 | 46 |
| Non-Remission | 260 | 37 |
| Subjective Sleep Disturbance | N | % |
| Insomnia Complaint (endorses) | 517 | 73 |
| EEG Sleep Disturbance | ||
| Sleep Latency >30 minutes | 144 | 20 |
| Wake after Sleep Onset >30 minutes | 264 | 37 |
| Total Sleep Time <= 6 hours | 206 | 29 |
Note.
Hamilton Depression Rating Scale.
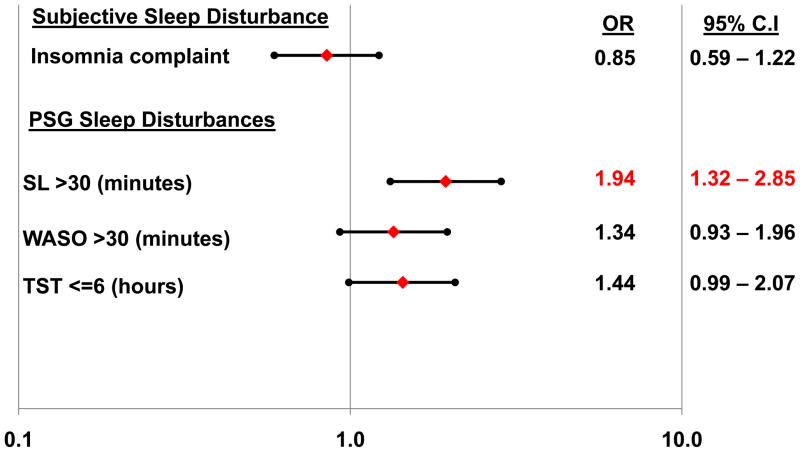
Logistic regression models which regressed each of the individual sleep disturbances on remission status, after adjusting for covariates, revealed that prolonged SL was an independent predictor of non-remission (Figure 1). Neither the subjective complaint of insomnia, nor TST or WASO predicted remission status independently, although the odds ratios for each of the objective markers were in the direction of increased risk. There were no significant interactions between individual sleep disturbances and treatment modality (analyses not shown).
Figure 1. Logistic regression model predicting the odds of non-remission according to insomnia or individual objective sleep disturbances.
Filled diamonds depict adjusted odds ratios predicting non-remission; filled circles depict lower and upper 95% confidence limits. Odds ratios are adjusted for age, sex, antidepressant medication usage (yes/no), duration of follow-up, and baseline depressive symptom severity (HRSD with sleep items removed). SL = sleep latency; WASO = wakefulness after sleep onset; TST = total sleep time.
Next, we examined the interaction between insomnia and each of the objective markers of sleep disturbance. As shown in Table 2, Model 1, when the main effects of insomnia, objectively measured SL, and their interaction were included, there was a significant main effect of prolonged SL and a statistically significant interaction between insomnia and prolonged SL. For the model including TST (Model 3), there were significant main effects of both insomnia and TST, and their interaction was also statistically significant. There was not a significant main effect of WASO nor a WASO*insomnia interaction (Model 2). There were no significant three-way interactions between objective EEG sleep disturbances, insomnia, and treatment modality (analyses not shown).
Table 2.
Logistic Regression Model Predicting Odds of Non-Remission Status according to Insomnia, EEG Sleep Disturbances, and their interaction, adjusted for clinical and demographic risk factors (N = 711).
| OR | 95% CI | |
|---|---|---|
| Model 1 | ||
| Insomnia | 1.36 | 0.91, 2.05 |
| SL >30 | 4.71** | 1.92, 11.57 |
| Insomnia × SL>30 | 0.33* | 0.12, 0.88 |
| Model 2 | ||
| Insomnia | 1.33 | 0.85, 2.08 |
| WASO>30 | 1.84 | 0.92, 3.67 |
| Insomnia × WASO>30 | 0.65 | 0.30, 1.41 |
| Model 3 | ||
| Insomnia | 1.55* | 1.01, 1.05 |
| TST <=6 hours | 3.43** | 1.64, 7.17 |
| Insomnia × TST<=6 | 0.32** | 0.14, 0.74 |
Note.
p< .05,
p< .01. Insomnia= Endorses one or more insomnia complaint on HDRS sleep items. SL >30 = sleep latency > 30 minutes versus referent (SL <=30); WASO>30= wakefulness after sleep onset >30 minutes versus referent (WASO <=30); TST<=6 = total sleeptime<= 6 hours versus referent (TST>6). Covariates include: age, sex, antidepressant medication usage (yes/no), duration of follow-up, and baseline depressive symptom severity (HDRSwith sleep items removed).
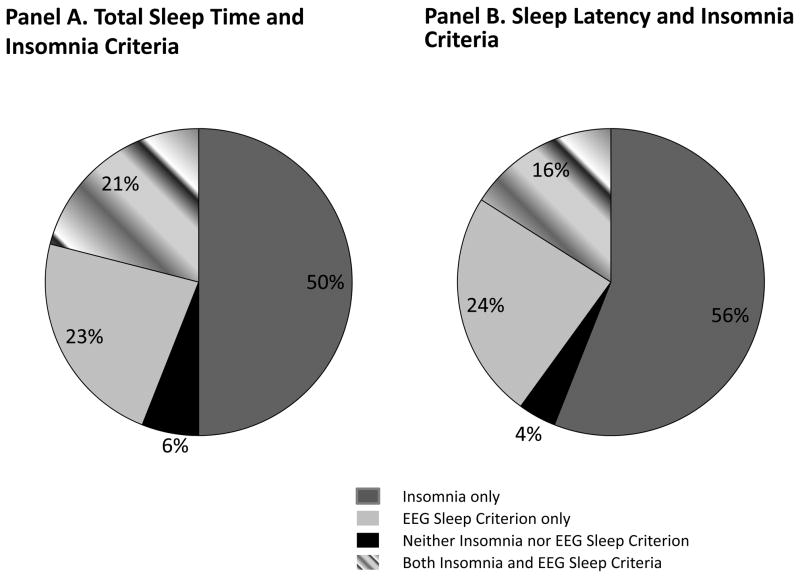
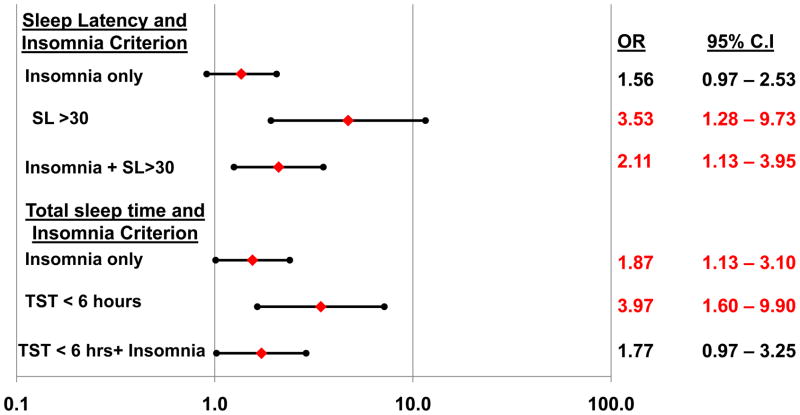
To further explore the significant insomnia*SL and insomnia*TST interactions, we conducted follow-up logistic regression models which included dummy-coded contrasts for insomnia without EEG sleep disturbance (i.e., either SL>30 or TST <6), EEG sleep disturbance without insomnia, or the combination of insomnia with EEG sleep disturbance, in comparison to the referent group (neither insomnia nor EEG sleep disturbance). The percentage of patients in each of these subgroups is depicted in Figure 2. As shown in Figure 3, prolonged sleep latency without insomnia, or in conjunction with insomnia was associated with significantly increased risk of non-remission. For the TST criterion, insomnia without PSG-measured short sleep, short sleep without insomnia, and their combination each were associated with significantly increased risk of non-remission.
Figure 2.
Distribution of sleep subgroups according to insomnia and objective Sleep Criterion (TST < 6 hours or SL > 30 minutes, respectively).
Figure 3. Logistic regression model predicting the odds of non-remission according to the presence or absence of insomnia and objective sleep disturbances.
Filled diamonds depict adjusted odds ratios predicting non-remission; filled circles depict lower and upper 95% confidence limits. Odds ratios are adjusted for age, sex, antidepressant medication usage (yes/no), duration of follow-up, and baseline depressive symptom severity (HRSD with sleep items removed). SL = sleep latency; WASO = wakefulness after sleep onset; TST = total sleep time.
Given these differences, we explored potential demographic (age at baseline, sex) and clinical characteristics (age of depression onset, duration of depressive episode, baseline depressive symptoms, and baseline anxiety symptoms) which might distinguish these sleep subgroups. Neither age, sex, age of onset, or duration of depressive episode were associated with sleep subgroups, independent of study entry criteria. However, baseline depressive symptoms (with sleep items removed) and anxiety symptoms did differ among the groups. Specifically, the combination of insomnia with either objective marker (prolonged sleep latency or short sleep time) was associated with significantly higher baseline depressive and anxiety symptoms (p < .05).
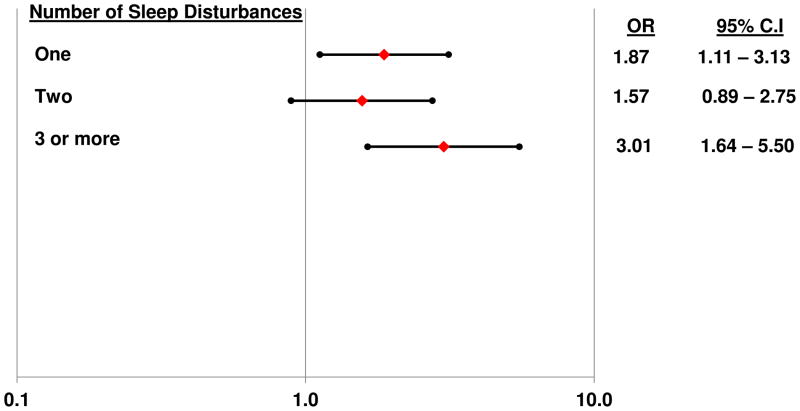
Finally, we examined whether the total number of sleep disturbances (ranging from 0 to 4) was associated with increased risk of non-remission. Due to the distribution of this summary score, individuals with 3 or 4 sleep disturbances were combined into a single group. As shown in Figure 4, compared to the referent group with no sleep disturbances, those with 3 or more sleep disturbances were 3 times as likely to be non-remitters.
Figure 4. Logistic regression model predicting the odds of non-remission according to increasing numbers of sleep disturbances.
Filled diamonds depict adjusted odds ratios predicting non-remission; filled circles depict lower and upper 95% confidence limits. Odds ratios are adjusted for age, sex, antidepressant medication usage (yes/no), duration of follow-up, and baseline depressive symptom severity (HRSD with sleep items removed). Referent group are those with no sleep disturbances.
Discussion
In this secondary analysis of a large, well-characterized sample of clinically depressed patients, we identify specific objective sleep disturbances that are associated with poor treatment outcome in depression. In particular, we found that objectively measured prolonged sleep latency (>30 minutes) is associated with significantly increased risk of non-remission following pharmacologic and/or psychotherapeutic treatment for depression. Results were independent of baseline clinical characteristics (depression or anxiety symptoms), length of follow-up, treatment modality (psychotherapy alone versus pharmacotherapy with or without psychotherapy) and demographic characteristics (age, sex) which are known to influence treatment outcomes. We also found that increasing numbers of sleep disturbances, particularly those with 3 or more disturbances were 3 times more likely to be non-remitters than those without any sleep disturbances. These findings are consistent with previous evidence linking prolonged sleep latency with adverse physical health outcomes, including risk of developing the metabolic syndrome38 and mortality.39 In contrast, subjective insomnia complaints alone were not associated with increased risk of poor treatment outcome. In populations in which insomnia is commonly comorbid, perhaps only the more severe insomnia phenotypes, characterized by objective sleep disturbance, are associated with increased risk for poor outcomes.
Indeed, we found that insomnia in combination with objectively measured prolonged sleep latency predicted increased risk of non-remission. In addition, insomnia and short sleep duration individually and in combination were associated with a significantly increased risk of non-remission. Notably, with regards to both combinations of objective criterion with or without insomnia, the population with the objective marker but lacking the insomnia complaint were the smallest subgroups (n=27 for prolonged sleep latency without insomnia and n=44 for short sleep duration without insomnia). Thus, caution is warranted in interpreting the odds ratios associated with these specific subgroups due to the relatively small sample sizes.
Nevertheless, these findings suggest important avenues for future research as they highlight the heterogeneity in traditional characterizations of sleep disturbances. This is perhaps most striking when considering the consistently reported health risks associated with short sleep duration. Epidemiologic investigations typically define short sleepers on the basis of subjective responses to a single-item question assessing typical sleep duration, without assessing distress associated with short sleep (necessary for identifying insomnia), and without assessing sleep duration objectively. Therefore, the question remains whether the risk associated with short sleep duration is due to the pathophysiological effects of short sleep in the absence of distress (i.e., non-complaining short sleepers), or the combination of short sleep with concomitant distress that may potentiate risk.
Investigation of these sleep subgroups has clear clinical implications as they may be characterized by differential risk trajectories and may require different treatment approaches. For instance, a limited number of studies have sought to characterize “non-complaining short sleepers,” with some evidence suggesting higher rates of subclinical hypomanic symptoms in this subgroup40. Other studies have shown that non-complaining short sleepers primarily differ from their complaining, short sleeping counterparts, in their lack of psychological distress.41–43
Our exploratory analysis did not identify specific clinical or demographic characteristics that distinguished the subgroup with short sleep duration or prolonged sleep latency, without insomnia (the smallest subgroups). Rather, consistent with our hypothesis that the combination of an objective marker of sleep disturbance with insomnia may represent a more biologically severe phenotype of insomnia, we found that the subjective + objective disturbance groups had significantly higher baseline measures of depressive and anxiety symptoms. These findings are also consistent with Vgontzas and colleagues’ work showing that the combination of insomnia with objectively measured short sleep duration potentiates the risk for adverse outcomes, ranging from neurocognitive functioning to hypertension, diabetes, and mortality.16–18;44 Given robust links between depression and cardiometabolic consequences,45–48 the combination of insomnia with objectively measured sleep disturbances may confer increased risk for poor depression outcomes, as well as accelerated risk trajectories for cardiometabolic morbidity and mortality. Previous evidence also suggests that the combination of insomnia with high stress responsivity may represent a distinct endophenotype of depression49–52.
Several inherent limitations associated with this secondary data analysis warrant caution in interpreting the findings. Based on stringent eligibility criteria for each of the included protocols, patients with severe, comorbid psychiatric diagnoses were excluded, which may limit generalizability to the broader population of depressed individuals. Other limits to generalizability concern the fact that the sample was predominantly female and Caucasian, and included a disproportionate number of patients with recurrent depression. In addition, the outcome in the current study, remission status, was based on HDRS scores of ≤7 for two consecutive monthly ratings, as this threshold is one of the most commonly used and recommended criteria for defining remission status in depression treatment studies.35 However, given that the total score includes sleep items, it is possible that non-remission reflects stability of sleep complaints rather than non-remission of depression per se. However, in follow-up analyses that restricted the sample to those with scores on the HDRS sleep items at baseline <4 (29% of the sample), results were unchanged, suggesting that our definition of non-remission was reflecting symptoms other than sleep complaints present at baseline. More generally, pooling data from multiple protocols inherently introduces heterogeneity which may pose a threat to the validity of the findings. However, these threats were mitigated by statistical covariation for patient characteristics that differentiated the individual protocols, by the absence of significant interactions based on treatment modality, and the fact that all patients were selected from the same stable community population, were diagnosed and assessed using standard, reliable measures, and were evaluated and treated at the same institution by affiliated investigators. Regarding the in-laboratory sleep studies, heterogeneity may also have been introduced due to differences in the protocols for the timing of sleep recordings (based on fixed lab time or according to patients’ habitual sleep-wake patterns). However, we conducted follow-up analyses controlling for sleep recording methodology (habitual sleep/wake times versus fixed laboratory time) and results were unchanged. Finally, given that the majority of patients included in these protocols conducted between 1982 and 2001 were treated with tricyclic antidepressants, rather than SSRIs and other current medications, it is possible that findings may differ, in a more contemporaneous pharmacologically treated population. These caveats notwithstanding, this approach of pooling data across individual clinical trials which utilize common assessment tools and standardized treatment protocols offers the powerful opportunity to address research questions that would otherwise be unanswerable by individual clinical trials.
Clinical implications of this research suggest that more aggressive depression treatment, including treatment of sleep disturbances, is warranted in depressed individuals who evidence subjective sleep complaints as well as objective sleep disturbances. Importantly, while the presence of insomnia was nearly ubiquitous in the total sample of depressed patients (73%), only 16–20% of the population had both insomnia and short sleep duration or prolonged sleep latency, respectively. The use of non-invasive, and relatively inexpensive sleep methodologies, such as actigraphy may facilitate the identification of these specific subgroups who may be at increased risk for poor treatment outcome as well as downstream health consequences.
Clinical Points.
Sleep problems are highly prevalent, often intractable symptoms which increase the risk of poor depression treatment response and recurrence.
Insomnia combined with objectively measured sleep disturbance may represent a biologically more severe phenotype of insomnia.
Treating sleep problems using empirically-supported behavioral or pharmacologic interventions may play an important role in optimizing depression treatment and prevention efforts.
Acknowledgments
This research was supported in part by grants from the National Institutes of Mental Health (MH-30915, MH-29618, MH-43832, MH-41884, MH-40023, MH-37869, MH71944, MH-49115, MH-24652), National Center on Research Resources (RR-00056, RR-024153) and the National Heart Lung Blood Institute (HL-093220).The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the National Institutes of Health.
Footnotes
The authors have no financial conflicts of interest to disclose with regard to the data presented herein.
Reference List
- 1.Kupfer DJ, Frank E, McEachran AB, et al. Delta sleep ratio: A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–1105. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 2.Pigeon WR, Hegel M, Unutzer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31:481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reynolds CF, Hoch CC, et al. Longitudinal effects of nortriptyline on EEG sleep and the likelihood of recurrence in elderly depressed patients. Neuropsychopharmacology. 1996;14:243–252. doi: 10.1016/0893-133X(95)00114-S. [DOI] [PubMed] [Google Scholar]
- 4.Kanai T, Takeuchi H, Furukawa TA, et al. Time to recurrence after recovery from major depressive episodes and its predictors. Psychol Med. 2003;33:839–845. doi: 10.1017/s0033291703007827. [DOI] [PubMed] [Google Scholar]
- 5.Karp JF, Buysse DJ, Houck PR, et al. Relationship of variability in residual symptoms with recurrence of major depressive disorder during mainentance treatment. Am J Psychiatry. 2004;161:1877–1884. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon WR, May PE, Perlis ML, et al. The effect of interpersonal psychotherapy for depression on insomnia symptoms in a cohort of women with sexual abuse histories. J Trauma Stress. 2009;22:634–638. doi: 10.1002/jts.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carney CE, Segal ZV, Edinger JD, et al. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–260. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF, Houck PR, et al. Does lorazepam impair the antidepressant response to nortriptyline and psychotherapy? J Clin Psychiatry. 1997;58:426–432. doi: 10.4088/jcp.v58n1003. [DOI] [PubMed] [Google Scholar]
- 9.Dew MA, Reynolds CF, Houck PR, et al. Temporal profiles of the course of depression during treatment: Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- 10.Thase ME, Buysse DJ, Frank E, et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am J Psychiatry. 1997;154:502–509. doi: 10.1176/ajp.154.4.502. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME. Depression, sleep, and antidepressants. J Clin Psychiatry. 1998;59:55–65. [PubMed] [Google Scholar]
- 12.Dombrovski AY, Cyranowski JM, Mulsant BH, et al. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress Anxiety. 2008;25:1060–1066. doi: 10.1002/da.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 14.Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Discord. 1997;42:209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 15.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Liao D, Bixler EO, et al. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank E, Kupfer DJ, Perel JM, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47:1093–1099. doi: 10.1001/archpsyc.1990.01810240013002. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds CF, Frank E, Perel JM, et al. Combined pharmacotherapy and psychotherapy in the acute and continuation treatment of elderly patients with recurrent major depression: A preliminary report. Am J Psychiatry. 1992;149:1687–1692. doi: 10.1176/ajp.149.12.1687. [DOI] [PubMed] [Google Scholar]
- 21.Simons AD, Thase ME. Biological markers, treatment outcome, and one-uear follow-up in endogenous depression: EEG sleep studies and response to cognitive therapy. J Consult Clin Psychol. 1992;60:392–401. doi: 10.1037//0022-006x.60.3.392. [DOI] [PubMed] [Google Scholar]
- 22.Nofzinger EA, Thase ME, Reynolds CF, et al. Sexual function in depressed men Assessment by self-report, behavioral, and nocturnal penile tumescence measures before and after treatment with cognitive behavior therapy. Arch Gen Psychiatry. 1993;50:24–30. doi: 10.1001/archpsyc.1993.01820130026005. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Kupfer DJ, Frank E, et al. Electroencephalographic sleep studies in depressed outpatients treated with interpersonal psychotherapy. II. Longitudinal studies at baseline and recovery. Psychiatry Res. 1992;40:27–40. doi: 10.1016/0165-1781(92)90036-3. [DOI] [PubMed] [Google Scholar]
- 24.Frank E, Kupfer DJ, Buysse DJ, et al. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164:761–767. doi: 10.1176/appi.ajp.164.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klerman GL, Weissman MM, Rounsaville BJ, et al. Interpersonal Psychotherapy of Depression. New York: Academic Press, Basic Books Inc; 1984. [Google Scholar]
- 27.Beck AT, Rush AJ, Shaw BF, et al. Cognitive therapy of depression: A treatment manual. New York: Guilford Press; 1979. [Google Scholar]
- 28.Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54:1009–1015. doi: 10.1001/archpsyc.1997.01830230043006. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt HS, Kaelbling R. The differential laboratory adaptation of sleep parameters. Biol Psychiatry. 1971;3:33–45. [PubMed] [Google Scholar]
- 30.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects NIH Publication 204. Washington, D.C: U.S. Government Printing Office, Department of Health Education and Welfare; 1968. [Google Scholar]
- 31.Kupfer DJ, Ehlers CL, Frank E, et al. EEG sleep profiles and recurrent depression. Biol Psychiatry. 1991;30:641–655. doi: 10.1016/0006-3223(91)90010-j. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 33.Lichstein KL, Durrence HH, Taylor DJ, et al. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 35.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 36.Andreescu C, Lenze EJ, Dew MA, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: Controlled study. The British Journal of Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- 37.Fava M, Uebelacker LA, Alpert JE, et al. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- 38.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–1640. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 40.Monk TH, Reynolds CF, Machen MA, et al. Daily social rhythms in the elderly and their relation to objectively recorded sleep. Sleep. 1992;15:322–329. doi: 10.1093/sleep/15.4.322. [DOI] [PubMed] [Google Scholar]
- 41.Edinger JD, Fins AI, Glenn DM, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68:586–593. [PubMed] [Google Scholar]
- 42.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fichten CS, Creti L, Amsel R, et al. Poor sleepers who do not complain of insomnia: myths and realities about psychological and lifestyle characteristics of older good and poor sleepers. J Behav Med. 1995;18:189–223. doi: 10.1007/BF01857869. [DOI] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nabi H, Kivimaki M, Suominen S, et al. Does depression predict coronary heart disease and cerebrovascular disease equally well? The Health and Social Support Prospective Cohort Study. Int J Epidemiol. 2010;39:1016–1024. doi: 10.1093/ije/dyq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart RA, North FM, West TM, et al. Depression and cardiovascular morbidity and mortality: cause or consequence? Eur Heart J. 2003;24:2027–2037. doi: 10.1016/j.ehj.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 48.Dobbels F, De Geest S, Vanhees L, et al. Depression and the heart: a systematic overview of definition, measurement, consequences and treatment of depression in cardiovascular disease. European Journal of CArdiovascular Nursing. 2002;1:45–55. doi: 10.1016/S1474-5151(01)00012-3. [DOI] [PubMed] [Google Scholar]
- 49.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 50.Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- 51.Shaffery J, Hoffmann R, Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9:82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- 52.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]