Abstract
Objectives
Scutellaria baicalensis has been a subject of research interests due to its potential multiple therapeutic benefits. This study was to examine the distribution of baicalein, wogonin, oroxylin A and their glucuronide/sulfate conjugated metabolites in plasma, colon, small intestine, lung, liver, pancreas, kidney, and prostate tissues and in pancreatic tumor in a xenograft animal model. In addition, we examined metabolic stability of baicalin in these tissues.
Methods
A mouse xenograft model was prepared by injection of 3×106 human pancreatic cancer MiaPaCa-2 cells subcutaneously into nude mice. Mice were randomly allocated to control diet (AIN76A) and 1% SB diet (n=8 per group) for 13 weeks. Levels of baicalein, wogonin, oroxylin A, and their conjugates in mouce tissues were measured by high-pressure liquid chromatography following enzymatic hydrolysis and then extraction.
Results
A substantial amount of baicalin (34–63%) was methylated to oroxylin A and its conjugates in various organs during absorption. While plasma contained predominantly conjugates of baicalein, wogonin, and oroxylin A, both aglycones and conjugates were found in all other tissues investigated and in tumor.
Conclusions
Substantial accumulation of bioactive metabolites are found in target tissues, suggesting strong potential for SB use as a preventive or adjuvant supplement for pancreatic cancer.
Keywords: Scutelleria baicalensis, baicalin, baicalein, oroxylin A, pancreatic cancer, in vivo
Scutellaria baicalensis Georgi (Chinese Skullcap) is a member of the Lamiaceae family and has been used in China and Japan for centuries to treat inflammation, respiratory, and gastrointestinal bacterial and viral infections. It is known to the Chinese as Huang-Qin and to the Japanese as Ogon and is a major component of several herbal medicine products.1 Recent studies on animal models have demonstrated that Scutellaria baicalensis (SB) or its major active constituent baicalin exhibits multiple activities against severe acute pancreatitis,2 obesity,3 metabolic disorders,4 and cancer.5, 6
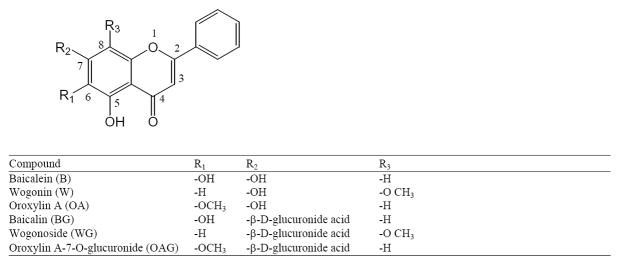
The most abundant bioactive component in Scutellaria baicalensis root extract is baicalin (5, 6, 7-trihydetroxyflavone-7-β-D-glucuronide or baicalein-7-O-glucuronide; BG). Other flavones identified are wogonoside (wogonin-7-O-glucuronide; WG), oroxylin A-7-O-glucuronide (OAG) and their aglycones, baicalein (B), wogonin (W), and oroxylin A (OA)7 (Figure 1). There have been a number of pharmacokinetics studies to investigate absorption of BG and B.8–11 Akao et al compared in vivo absorption of BG and B in regular rats to that in germ-free rats and suggested that BG underwent intestinal conversion to B, which is absorbed rapidly and converted back to BG in the systemic circulation.11 An in situ absorption experiment revealed the existence of double-site absorption of BG. The first absorption site was in upper intestine and might be due to the directly absorption of BG and the second site was in colon in the form of aglygone.10 In rat plasma glucuronic acid conjugates were the predominant forms, the result of first-pass metabolism.11–14 A few recent pharmacokinetics studies identified and quantified rat plasma OA, a 6-O-methylated B.1, 15 Hou et al reported the distribution of B and W and their conjugates in the lung, liver, kidney, and brain of rats to which a decoction of SB roots at 2.0 g/4 mL/kg three times daily for 7 doses was administered via gastric gavage. We are unaware of any report of tissue absorption and distribution after long-term oral administration of SB in animal models.
Figure 1.
Chemical structures of major flavonoids found in Scutellaria baicalensis.
Our research team has demonstrated that B induces apoptosis in human pancreatic cancer BxPC-3, HPAF-II, Panc-1 and MicPaCa-2 cell lines which is mediated through the inhibition of Mcl-1 activity.16 Furthermore, we have shown that a diet containing an extract from the root of SB inhibited the growth of pancreatic tumor xenograft in a mouse in vivo model after a long-term oral administration (unpublished data). To exert its biologic activity, BG must be sufficiently absorbed in the gastrointestinal tract and reach pharmacological concentrations in the target tissue. In order to understand how BG is absorbed and metabolized in mice, we investigated uptake of BG and presence of metabolites in pancreas and tumor tissue, and in the respiratory and gastrointestinal organs including lung, colon, small intestine, as well as in liver, kidney, and prostate in xenograft-bearing mice. In addition, we examined metabolic stability of BG in the tissues sampled.
MATERIALS AND METHODS
Materials
All solvents used were HPLC grade (Fisher Scientific, Fairlawn, NJ). BG and β-glucuronidase/sulfatase (type H-5 from Helix Pomatia) were purchased from Sigma-Aldrich (St. Louis, MO). B and W were from Chromadex (Irvine, CA) and OA from Nanjing Zelang Medical Technology Co. Ltd. China. Internal standard 3, 3′, 4′-trihydroxyflavone was purchased from Indofine (Hillsborough, NJ).
Extract of Scutellaria baicalensis
Extract of SB was purchased from Cortex Scientific Botanicals (Ojai, CA) in 2008. Scutellaria baicalensis Georgi was harvested from Hebei Province, China in 2006 and root samples were extracted with 50% ethanol and dried completely. Reference specimens of the extract and the crude raw material were kept at Cortex Scientific Botanicals in Beijing, China. The extract was stored in −20°C protected from light. Flavonoid content was measured by high-performance liquid chromatography (HPLC) after the extract was completely dissolved in a 1:1 ratio of water and methanol.
Animals and diets
Animal studies were approved by the Chancellor’s Animal Research Committee of the University of California, Los Angeles, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Nude mice (Charles River Laboratories, San Diego, CA) were housed 4/cage in a room with controlled temperature (20–22°C). The base diet was AIN-76A (a low soy content diet) purified rodent diet (Dyets, Bethlehem, PA) supplement with or without SB extract (1% w/w). Diet was replaced every 2 or 3 days and the stability of BG, B, and W were analyzed in day 0 (as control) and day 5 by HPLC following vigorous extraction with methanol containing BHT as an antioxidant. The xenograft model was performed as described earlier.17 Briefly, 3×106 MiaPaCa-2 cells were injected subcutaneously into the flank of nude mice. Animals were divided into 2 groups of 8 animals each and fed either control diet or diet with SB extract, which was administered orally mixed with the powder diet 8 days prior to the cell inoculation and continued for thirteen weeks. Animals were then anesthetized and blood was taken by cardiac puncture until exsanguination. The tissues of interest were harvested, immediately frozen in liquid nitrogen, and stored at −80° C.
Mouse plasma and tissue extraction
Plasma samples were acidified with 0.1 volume of 0.58 M acetic acid to stabilize flavones.18 To the acidified mice plasma samples (100 μL) in a 2 mL microcentrifuge tube was added 58 μL of β-glucuronidase in 0.2 M sodium acetate buffer (pH 5.0) containing 1000 U β-glucuronidase and 25 U sulfatase. An aliquot of 42 μL of 0.2 M sodium acetate buffer (pH 5.0) containing 0.5% ascorbic acid was added. The mixture was vortexed and then incubated at 37°C for 1.5 hours. After the incubation period, 800 μL of ethyl acetate was added followed by addition of 40 μL of internal standard 3, 3′, 4′-trihydroxyflavone in ethanol (25 μM). B and other metabolites were extracted with 800 μL ethyl acetate twice. Each extraction mixture was vortexed for 1 min and then centrifuged at 600 rpm for 5 min. Supernatant was transferred to a glass test tube and combined. Solvent was removed in a SpeedVac at RT until completely dry. The residue was reconstituted in 100 μL of MeOH:H2O 1:1, vortexed, and then sonicated for 1 min. and a 25 μL aliquot of the mixture was injected into the HPLC. For analysis of mouse tissue, frozen tissue (0.1–0.2 g) was weighed and homogenized in buffer containing 1% ascorbic acid using a tissue grinder, and internal standard was added. The mixture was then hydrolyzed enzymatically (or without hydrolysis) and extracted similarly as plasma samples.
Stability of BG in mouse tissue homogenates
An ex vivo stability study was conducted to determine the extent that BG is converted to B in tissue homogenates. An aliquot of BG in ethanol (equivalent to 2.24 and 22.40 nmol/g tissue) was spiked in various control mouse tissue homogenates in pH 7 buffer containing 1% ascorbic acid followed by the addition of internal standard. Samples were incubated at 37°C for 2 hr under anaerobic and dark conditions. Liver homogenates containing 1% ascorbic acid (pH 5 and 7) and acidified plasma (pH 5) samples were incubated similarly with BG (equivalent to 2.24 nmol/g tissue or 2.24 nmol/ml plasma) and internal standard. Samples were taken at 0, 30, 60, 90, and 120 min and ethyl acetate added immediately, followed by extraction and HPLC analysis as described.
High-Pressure Liquid Chromatography (HPLC)
HPLC analysis was performed with a RP-18 Luna column (150 × 4.6 mm, 3 μm, Phenomenex, Torrance, CA) on an Agilent 1100 HPLC system (Santa Clara, CA) comprised of an autosampler and quaternary pump coupled to a photodiode array detector. The mobile phase consisted of a binary gradient of 0.1% (v/v) ortho-phosphoric acid in water (eluent A) and acetonitrile (eluent B), used with a flow rate of 0.6 mL/min in the following conditions: 25% B (0–2 min); 25–75% B (2–20 min); 75% B (20–22 min); and 75–25% B (22–24 min). The column temperature was held at 30° C. The chromatograms were recorded at 277 nm. Data were analyzed with the Hewlett Packard ChemstationR software. Concentrations of flavonoids in SB extract were determined by HPLC using external calibration. Mice plasma and tissue concentrations were determined by internal calibration. Concentrations of the stock solutions were determined spectrophotometrically using Beer’s Law. Calibration standards were prepared from the stock solutions by serial dilution. Concentrations of WG and OAG were determined using the calibration curve of BG. For all calibration curves, there was a linear relationship between peak area and concentration in therange of 80 ng/mL to 50 μg/mL. The detection limit for B was 2.6 ng/mL.
Statistics
Descriptive statistics, such as mean and standard deviation, were used to summarize the results. Statistical comparisons were made using a paired two-tailed Student’s t test with a confidence level of 95%. Statistical significance was defined by p-value of 0.05.
RESULTS
Quantitation of flavonoids in SB extract
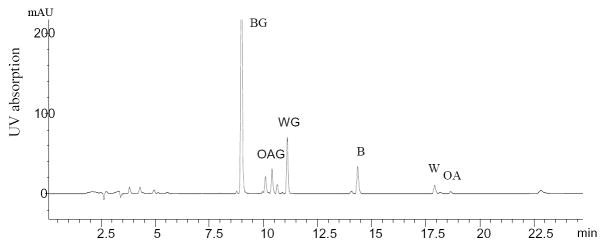
The crude extract of SB, a brownish-yellow powder from the roots of S. Baicalensis, was analyzed by HPLC. Figure 2 illustrates the HPLC profile of the SB extract, which contained several flavonoids. BG was the major flavonoid component, comprising 20.6% (wt/wt) of the extract. There were small amount of OAG (1.5%), WG (3.0%), B (1.2%), W (<1.0%), and OA (<1.0%) present in the extract. The observed UV absorption maxima of OAG (274, 312 nm) and WG (274 nm) were consistent with data reported.1
Figure 2.
HPLC/UV chromatogram of flavones in an extract of Scutellaria baicalensis monitored at 277 nm.
Plasma absorption of baicalin in mice after oral administration of SB diet
The major flavones in plasma of the tumor-bearing mice fed 1.0% SB diets for 13 weeks (calculated as 1.49 g/(kg body wt · d) SB extract intake on average) were analyzed in samples treated or not treated with glucuronidase/sulfatase. The amount of glucuronide and sulfate conjugates was determined as the amount of aglycone after enzymatic hydrolysis subtracted from the amount obtained without hydrolysis. Plasma concentrations of B, W, OA plus their conjugates were 4.37±1.69, 1.47± 0.80 and 3.41± 2.43 μM respectively, as shown in Table 1. Our data revealed that plasma contained predominantly conjugates; aglycones B, W, and OA were 2.5, 2.6 and 8.1% of total levels, respectively. Substantial amount of BG was methylated so that the concentration of OA was close to B after hydrolysis, indicating BG is methylated extensively during absorption.
Table 1.
Baicalein, wogonin and oroxylin A levels in tissues of mice fed 1% SB diet for 13 weeksa
| nmol/g wet tissue | |||||||
|---|---|---|---|---|---|---|---|
| Baicalein | Wogonin | Oroxylin-A | Sumf | ||||
| Totalb | %c | Totalb | %c | Totalb | %c | ||
| Colon | 172.60±21.58 | 91.60 | 33.32±6.10 | 93.45 | 128.68±31.66 | 94.17 | 334.60 |
| Small intestine | 110.58±20.16 | 35.38 | 28.94±5.19 | 72.33 | 114.98±22.23 | 45.97 | 254.50 |
| Liver | 34.00±3.83 | 38.18 | 8.06±0.75 | 91.27 | 29.94±3.76 | 92.28 | 72.00 |
| Kidney | 6.49±0.81 | 91.00 | 1.65±0.26 | 81.26 | 4.87±0.94 | 85.85 | 13.01 |
| Prostated | 4.60±1.00 | 47.14 | 1.46±0.38 | 69.84 | 2.38±0.39 | 89.10 | 8.44 |
| Pancreas | 1.34±0.50 | 49.31 | 0.63±0.24 | 77.49 | 2.25±1.03 | 53.58 | 4.23 |
| Lung | 1.33±0.26 | 93.00 | 0.46±0.16 | 67.22 | 1.09±0.31 | 97.88 | 2.89 |
| Tumore | 0.85±0.02 | 70.51 | 0.46±0.09 | 79.89 | 1.00±0.16 | 93.46 | 2.32 |
| Plasma, μM | 4.37±1.69 | 2.52 | 1.47±0.80 | 2.59 | 3.41±2.43 | 8.09 | 9.09 |
Values are mean ± SEM (n=6)
Total: aglycone + conjugates
%: % of aglycone
n=5
n=3
sum=concentration baicalein + wogonin + oroxylin A
Tissue levels of baicalin and its metabolites in xenograft mice after oral administration of SB diet
To investigate the absorption of SB extract in pancreas and tumor as target tissues, we also evaluated concentrations of metabolites in lung and liver, as lung and liver are common sites of metastases, especially of pancreatic tumors. In addition, we also investigated metabolites in the tissues of small intestine, colon, kidney, and prostate. Generally, all tissues from mice fed with 1% of SB extract diet accumulated B, W, and OA, as well as their glucuronidates/sulfates. Table 1 shows that levels of total B, W, and OA are highest in colon, followed by small intestine, liver, kidney, prostate, pancreas, lung, and tumor. Moreover, except for in prostate, about half of the BG is metabolized to the 6-O-methylated OA compound. The percentage of OA was highest in pancreas (63%), followed by tumor (54%), small intestine (51%), liver (47%), lung (45%), colon and kidney (both 43%), and prostate (34%). Note that only 3 out of 6 mice treated with SB extract grew tumors large enough to be analyzed.
It has been known that human and rat tissues contain β-glucuronidase thatdeglycosylates and deconjugates flavonoid glycosides and glucuronide.19, 20 Similar to these flavonoids reported, deconjugated aglycones were found in various mouse tissues in our study. On average, 80% of W and OA, both of which are O-methylated but at different positions on the A ring, were found to be in aglycone forms in all tissues while only 65% of the unmethylated compound B was in aglycone form. Lung contained highest concentrations of both B and OA free forms, consistent with other study.8
Metabolic stability of BG in mouse tissue homogenates
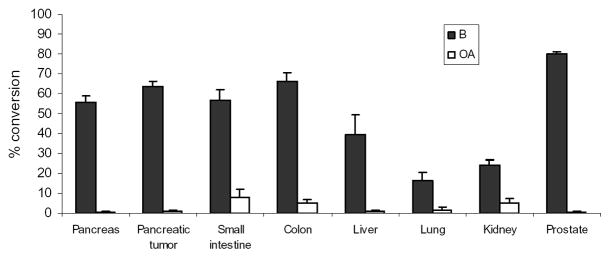
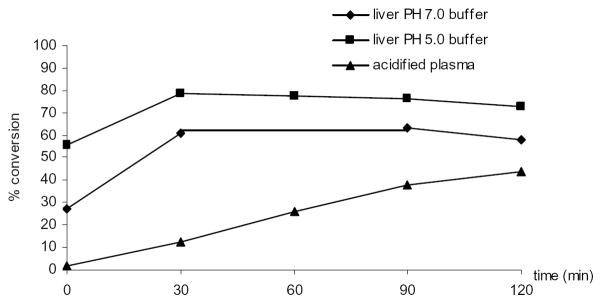
BG has been reported to be efficiently converted to B in rat tissue homogenates.18 To examine if BG converts ex vivo to B during the extraction procedure, we spiked 2.24 (low, n=2) and 22.40 (high, n=3) nmol/g tissue of BG in various control mouse tissue homogenates in pH 7 buffer and incubated at 37°C for 2 hr under anaerobic and dark conditions. HPLC analysis of homogenates following extraction showed the conversion of BG to B occurred in all tissues investigated. Among them, pancreas, tumor, colon, small intestine, and prostate displayed higher metabolic activity compared to liver, kidney, and lung (Figure 3). Percent of molar conversion after addition of low and high concentration of BG were comparable in most tissues with the exception of liver and lung, where low concentration of BG yielded low conversion to B (15.9% vs. 55.0% for liver, 6.10% vs. 22.9% for lung), therefore data were combined (n=5) and averaged in the figure. Small but detectable amount of O-Methylated OA was observed in all organs but level was higher in kidney, small intestine and colon. Figure 4 shows that in liver homogenates, enzymatic hydrolysis at pH 7 occurred almost immediately after addition of BG (t=0 min) and reached a maximum with 60% molar conversion at 30 min, whereas at pH 5, the optimal pH for β-glucuronidase enzyme activity, 80% of BG was hydrolyzed to B. The level of B remained the same from 30 min until 120 min in both pH conditions. Our results indicated that in buffers (pH 5 and 7) BG was stable at least for 2 hr (data not shown). In acidified plasma (pH 5), the rate of hydrolysis of BG to B was nearly linear with time (Figure 4). These data suggest that BG undergoes ex vivo hydrolysis and phase II metabolic pathways in various tissues.
Figure 3.
Percentage conversion of baicalin to baicalein in mouse tissue and tumor homogenates after addition of known concentrations of baicalin (2.24 or 22.4 nmol/g tissue) and incubation at 37°C at pH 7. % conversion was determined as moles of baicalein determined by HPLC divided by moles of baicalin added. Data represents mean ± SEM (n=5; for tumor and prostate, n=4).
Figure 4.
Metabolic stability of baicalin in liver homogenates and acidified plasma. Baicalin was added to mouse control liver or acidified plasma to a final concentration of 2.24 nmol/g tissue or 2.24 nmol/ml plasma; the resulting mixtures were incubated at 37°C at pH 5 or 7 and sampled at times indicated. Samples were then extracted and analyzed by HPLC. % conversion was determined as moles of baicalein determined by HPLC divided by moles of baicalin added.
DISCUSSION
Oral bioavailability of SB has been a subject of research interests due to its potential multiple therapeutic benefits.21, 22 However, accumulation of the SB flavones in plasma and tissues of animals fed SB diet for a relatively long term has not been reported. In this study, we reported that flavones from SB and their metabolites accumulate in tumor xenograft, pancreas, and other tissues in mice after administered with 1% SB diet for 13 weeks. B, W, OA, and their conjugates were found in all organs investigated and in tumor, but levels varied on the order of 100-fold from organ to organ. Generally, tissues from the GI tract accumulated highest levels of B, W, OR, and their conjugates with the highest level observed in colon and small intestine, followed by liver, kidney, prostate, pancreas, lung, and tumor. Levels of W and OA in tissues followed the same order. Furthermore, we found that all investigated organs contained a considerable proportion of deconjugated B, W, and OA, ranging from 38–97% of the total concentration. In general, higher proportions of O-methylated aglycones W and OA were found in tissues compared with non-methylated B. In tumor, 71% of B, 79% of W and 93% of OA are in aglycone forms.
Plasma samples in the enzyme-untreated samples showed B and W aglycones present at very low levels, which is in agreement with other studies reporting that aglycones are generally either absent in blood or present at low concentrations. We found SB extract contained less than 1% OA, however 45% and 61% of BG were methylated in plasma and small intestine, respectively. Flavonoids bearing ortho-hydroxy functional groups, such as quercetin and tea catechins, are predictably prone to O-methylation by soluble catechol-O-methltransferase (COMT). This reaction apparently occurs efficiently with BG. Methylated flavonoids have been reported to have greatly improved intestinal absorption and metabolic stability.23
Most of the flavonoids from plant origin are present in the form of β-glycosides. The flavones are mainly glycosylated in the 7 position. It is generally accepted that the mechanism of absorption of these glycosides involves hydrolysis of the glucosides in the intestinal lumen by lactase phlorizin hydrolase followed by the diffusion of the released aglycones and/or active transport by the sodium-dependant glucose transporter into enterocytes, with subsequent deglycosylation within the enterocyte by cytosolic β-glucosidase. It has been reported that human and rat tissues possess β-glucuronidase enzymes, which can be released under certain physiological conditions such as inflammation.24 Mouse tumor 25 and human pancreatic cancer tissue26 contain large amounts of β-glucuronidase as well. The deglycosylation of flavonoids and isoflavonoids by β-glucuronidase is an important first step in their uptake, metabolism, excretion, and biological activity.19 A study by Day et al revealed that the rate and extent of deglycosylation depends on the structure of the flavonoids and the position/nature of the sugar substitutions. Genistein and apigenin 7-glucosides were all substrates for the human hepatic and intestinal β-glucuronidase.19 BG, a glucuronide of B, has been reported to be a major glucuronide present in rat tissues and plasma, and is deconjugated readily by rat liver β-glucuronidase.1 A similar metabolite pattern has been observed in rats after repeated dosing of SB decotion.8 Rat tissues of liver, lung, and kidney were shown to possess a high deconjugation activity due to the presence of enzymes with β-glucuronidase activity. In line with these findings, we have demonstrated that homogenates from various tissues of the nude mouse possess a high deconjugation activity that efficiently converts BG to aglycones B. High enzyme activity was seen in pancreas, liver, colon, small intestine, prostate, as well as in pancreatic tumor tissue. However, there was no correlation between the tissue concentration of aglycone B in selected tissues and the tissue-specific β-glucuronidase activity. The lung and kidney tissues had the highest aglycone B (91% and 90% of the conjugated plus free form respectively), but had lowest enzyme activity. The rate of deconjugation occurred rapidly in liver homogenates, with 28% of BG being hydrolyzed to B initially at pH 7. Within 30 min, conversion reached 61% and remained almost the same until 120 min. In liver homogenate at pH 5, 57% of BG was initially converted. Xing et al reported that half of the amount of BG added to rat liver homogenate was degraded after only 7 min. The main degradation products were identified as B, OA, and 3 phase II metabolites, 6-O-β-glucopyranuronoside, baicalein 6,7-di-O-β-glucopyranuronoside, and 6-O-β-methyl-baicalein-7-O-β-glucopyranuronoside, as detected by LC/MSn.18 The high enzyme activity also resulted in an ex vivo conversion of BG and other glucuronides to aglycones during the extraction, and this may also happen in tissues in situ. Hence, the reported tissue levels of aglycones, B, W, and OA are likely to be higher than the actual in vivo concentrations.
In summary, this study showed that BG can accumulate in plasma, tumor xenografts, and pancreas, liver, lung, and other tissues in vivo. Substantial amount of BG (34–63%) is methylated to OA in various organs during absorption. While plasma contains predominantly conjugates of B, W, and OA, in all other organs investigated and in tumor, both aglycones and conjugates were found. Enzymes such as glucuronidase and sulfatase present in the cell membrane in mouse organs contribute to the aglycones found in tissues. Our data can be used to predict the organ site(s) most likely to benefit from SB treatment, and to provide important information on predicting chemopreventive or therapeutic efficacy of SB.
Acknowledgments
This work was supported by the National Institutes of Health (P01AT003960) and the Hirshberg Foundation for Pancreatic Cancer Research.
References
- 1.Li C, Zhang L, Lin G, Zuo Z. Identification and quantification of baicalein, wogonin, oroxylin A and their major glucuronide conjugated metabolites in rat plasma after oral administration of Radix scutellariae product. J Pharm Biomed Anal. 2011;54:750–758. doi: 10.1016/j.jpba.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XP, Zhang L, He JX, et al. Experimental study of therapeutic efficacy of baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:717–724. doi: 10.3748/wjg.v13.i5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H, Kang R, Hahn Y, et al. Antiobesity effect of baicalin involves the modulations of proadipogenic and antiadipogenic regulators of the adipogenesis pathway. Phytother Res. 2009;23:1615–1623. doi: 10.1002/ptr.2937. [DOI] [PubMed] [Google Scholar]
- 4.Guo HX, Liu DH, Ma Y, et al. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30:1505–1512. doi: 10.1038/aps.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang DY, Wu J, Ye F, et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003;63:4037–4043. [PubMed] [Google Scholar]
- 6.Bonham M, Posakony J, Coleman I, et al. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 2005;11:3905–3914. doi: 10.1158/1078-0432.CCR-04-1974. [DOI] [PubMed] [Google Scholar]
- 7.Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou YC, Lin SP, Tsai SY, et al. Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats. Planta Med. 2011;77:455–460. doi: 10.1055/s-0030-1250433. [DOI] [PubMed] [Google Scholar]
- 9.Lai MY, Hsiu SL, Tsai SY, et al. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol. 2003;55:205–209. doi: 10.1211/002235702522. [DOI] [PubMed] [Google Scholar]
- 10.Lu T, Song J, Huang F, et al. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol. 2007;110:412–418. doi: 10.1016/j.jep.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Akao T, Kawabata K, Yanagisawa E, et al. Baicalin, the predominant flavone glucuronide of Scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J Pharm Pharmacol. 2000;52:1563–1568. doi: 10.1211/0022357001777621. [DOI] [PubMed] [Google Scholar]
- 12.Xing J, Chen X, Zhong D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005;78:140–146. doi: 10.1016/j.lfs.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Lin G, Kovacs B, et al. Mechanistic study on the intestinal absorption and disposition of baicalein. Eur J Pharm Sci. 2007;31:221–231. doi: 10.1016/j.ejps.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Dai JY, Yang JL, Li C. Transport and metabolism of flavonoids from Chinese herbal remedy Xiaochaihu- tang across human intestinal Caco-2 cell monolayers. Acta Pharmacol Sin. 2008;29:1086–1093. doi: 10.1111/j.1745-7254.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Jeong DW, Kim YC, et al. Pharmacokinetics of baicalein, baicalin and wogonin after oral administration of a standardized extract of Scutellaria baicalensis, PF-2405 in rats. Arch Pharm Res. 2007;30:260–265. doi: 10.1007/BF02977703. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Chen MC, Harris DM, et al. Down-regulation of Mcl-1 by baicalein induces apoptosis through the mitochondrial pathway in human pancreatic cancer cells. Pancreas; 41st Annual Meeting of the American Pancreatic Association; November 3–6, 2010; Chicago, IL. 2010. p. 1350. [Google Scholar]
- 17.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 18.Xing J, Chen X, Zhong D. Stability of baicalin in biological fluids in vitro. J Pharm Biomed Anal. 2005;39:593–600. doi: 10.1016/j.jpba.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Day AJ, DuPont MS, Ridley S, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–75. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 20.de Boer VC, Dihal AA, van der Woude H, et al. Tissue distribution of quercetin in rats and pigs. J Nutr. 2005;135:1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- 21.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas NR. Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica. 2010;40:357–367. doi: 10.3109/00498251003663724. [DOI] [PubMed] [Google Scholar]
- 23.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 24.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–272. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L, Wagatsuma C, Yoshida M, et al. Inhibition of human breast cancer growth by GCP (genistein combined polysaccharide) in xenogeneic athymic mice: involvement of genistein biotransformation by beta-glucuronidase from tumor tissues. Mutat Res. 2003;523–524:55–62. doi: 10.1016/s0027-5107(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 26.Yue H, Yang B, Zhang H, et al. Clinical significance of TGF- beta1 and beta-glucuronidase synchronous detection in human pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2002;1:309–311. [PubMed] [Google Scholar]