Abstract
Many factors influence the rewarding effects of drugs such as cocaine. The present study was done to determine whether social and environmental factors alter behavior in adolescent male and female rats. On postnatal day (PND) 23, rats were housed in one of several same-sex conditions. Both social (number of rats per cage) and environmental (availability of toys) factors were manipulated. Socially isolated rats were housed alone (1 rat/cage) in an environment that either was impoverished (with no toys; II)) or enriched (with toys; IE). Standard housing for these studies was social and impoverished, which was 2 rats/cage with no toys (SI2). Other rats were housed 2/cage with toys (SE2), or 3/cage with (SE3) or without (SI3) toys. On PND 37, novelty-induced locomotor activity was measured for 30 minutes. On PND 44-46, locomotor activity in response to an injection of 5 mg/kg cocaine was measured for 60 minutes each day. For male rats, only social conditions altered novelty-induced activity. Males housed in groups of three had the most activity, compared to pair-housed and isolated rats. For females, social and environmental enrichment interacted to alter novelty-induced activity. In contrast to males, isolated females had increased activity, compared to group-housed females. Further, isolated females in impoverished environments had more activity than isolated females in enriched environments and group-housed females in impoverished environments. The effect of environmental enrichment on cocaine-stimulated locomotor activity was altered depending upon the number of rats living in a cage for males. For females, only social conditions altered cocaine-stimulated behavior, with activity increasing with the number of rats in the cage, regardless of environmental enrichment. These data show that social and environmental enrichment differentially alter novelty-induced and cocaine-stimulated locomotor activity in adolescent male and female rats.
Keywords: enrichment, adolescent, cocaine, locomotor activity, exploratory behavior
Introduction
Multiple factors, including age, sex, and previous drug history influence the reward associated with drugs of abuse such as cocaine. In addition, it has been shown that environmental factors alter behavior and play a role in mediating the behavioral and neurochemical effects of drugs of abuse in rats. For example, crowding conditions decreased the amount of food consumed in adult male and female rats, and increased the amount of water consumed by male rats [9], whereas environmental enrichment decreased food consumption and altered growth in adolescent male rats [47]. Adult rats living in an impoverished environment (singly housed rats with no toys) exhibited an increase in locomotor activity and rearing [8,22], while those living in an enriched environment (group housing plus toys) had decreased exploration and basal locomotor activity [6,18,28,45].
Several studies have shown that there are significant differences in the behavior of enriched versus impoverished adult male rats in response to stimulant drugs. There is an increased effect of amphetamine on locomotor activity in environmentally and socially enriched rats compared to environmentally impoverished and socially isolated rats [5,6]. Similar result are seen with methamphetamine [20], the selective dopamine uptake inhibitor GBR 12935 [50] or cocaine [8]. Most of the studies investigating the role of environmental conditions on the behavioral effects of drugs were done using adult animals, or animals housed under different conditions during adolescence but tested during adulthood [e.g., 16,38].
Adolescence is a critical period for the initiation of illicit drug use. According to recent statistics, an estimated 2.8 million people aged 12 or older reported using an illicit drug for the first time within the past 12 months in 2006. More than half of initiates (57.8 percent) were younger than age 18 when they first used, and about half of new users (53.2 percent) were female [29]. When youths aged 12 to 17 were considered, the rate of substance dependence or abuse among males was similar to the rate among females (8.0 vs. 8.1 percent, respectively) [29], suggesting that teenage girls are at the same risk at developing drug dependence as teenage boys. Adolescent rats appear to be more sensitive to the effects of drugs such as nicotine [2,19], haloperidol [42], and morphine [41]. With regard to nicotine, these effects are different in males and females [10,12], with the rapid development of sensitization to the locomotor-stimulant effects of nicotine in females, but not in males. Conversely, it has been shown that adolescent rats are less sensitive to behavioral effects of cocaine [4,40]. Therefore, it is important to understand the factors that play a role in the initiation and maintenance of drug use in adolescents. It will be interesting to see if the environment plays a more important role in influencing behavioral effects of cocaine when the conditions are implemented and testing occurs during adolescence.
Aside from the fact that adult animals were used in most studies of effects of environmental and/or social enrichment, often only males were used or data were collapsed across sexes. However, social and environmental enrichment have been shown to alter animal behavior and their responses to drugs of abuse, with some of the effects being sex- and age-dependent. For example, environmental and social enrichment during adolescence and young adulthood increased the social investigation behavior in males but not in females when tested as adults [30]. Further, it appears that adolescents may be particularly sensitive to social housing conditions and the rewarding effects of social interaction; this finding was strongest in male rats [17]. The effects of different types of enrichment on locomotor activity in the open field test also were gender-specific, with the effects being greater in males than females [18]. In our laboratory, we have found previously that social and environmental factors affect cocaine conditioned reward differently in male and female adolescent rats [48]. Clearly, drug effects can be altered by social and environmental factors, age, and sex, and these alterations can be different depending on the drug under consideration. The purpose of the present study was to investigate the effects of the environmental and social enrichment on exploratory locomotor behavior in a novel environment and the locomotor response to cocaine in periadolescent male and female rats.
EXPERIMENTAL PROCEDURES
Subjects
The animals used in these studies were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996 and the Institutional Animal Care and Use Committee of the University of Miami. Male and female Sprague-Dawley rats (Charles River, Wilmington, MA) were used in all studies. Rats were housed in a temperature and humidity-controlled environment under a 12 h light/dark schedule with lights on at 7 a.m. and off at 7 p.m. All behavioral testing was done during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour each day and the groups randomized over the course of the day. Food and water were available ad libitum.
Housing
Rats were received on postnatal day 23 (PND 23) and immediately housed in one of several conditions. Both social (number of rats per cage) and environmental (availability of toys) factors were manipulated (Table 1). Socially isolated/environmentally impoverished (II) rats were housed alone (1 rat/cage) and had no toys available. Socially isolated/environmentally enriched (IE) rats were housed alone (1 rat/cage) with toys available. Social/environmentally impoverished 2 (SI2) rats were housed 2/cage with no toys. Social/environmentally enriched 2 (SE2) rats were housed 2/cage with toys. Socially/environmentally impoverished 3 (SI3) rats were housed 3/cage with no toys, and socially/environmentally enriched 3 (SE3) rats were housed 3/cage with toys. For the environmentally enriched conditions, toys were placed in the cages and different toys were rotated in and out of the cages each time the cages were changed (twice/week). Plastic tunnels and hollow balls (that the animals could move in and out of or climb over), in addition to toys that the rats could chew (non-toxic dog bones and plastic toys) and scratch paper were used. All cages were the same size standard large shoebox cages (46 cm long × 29 cm wide × 20.5 cm high). For one group of animals, exploratory behavior in a novel environment was measured for 30 min after 14 days of differential housing (PND 37). Cocaine-stimulated locomotor activity measurements began on PND 43 (20 days after housing conditions began) in a separate group of animals.
Table 1.
Housing conditions
| Environmental conditions | Social conditions (# rats/cage) | ||
|---|---|---|---|
| Isolated (1 rat/cage) | Social2 (2 rats/cage) | Social3 (3 rats/cage) | |
| Impoverished (no toys) | II | SI2 | SI3 |
| Enriched (toys) | IE | SE2 | SE3 |
Body weights of males on PND 23 did not differ across groups, however, there were differences by PND 37 (F(5,42)=7.20, P≤0.0001), where SE3 rats weighed less than the other groups (P≤0.05) and this persisted in some conditions through PND 43 (F(5,42)=3.99, P≤0.005), where the SE3 rats maintained a lower rate than the impoverished rats (II and SI2) ((Table 2). This finding is consistent with our previous data showing that housing conditions altered growth rates in adolescent male rats [47]. In females, there were no differences in body weight at any of the three time periods.
Table 2.
Body weights on test days
| MALES | |||
|---|---|---|---|
| HOUSING | PND 23 | PND 38 | PND 43 |
| II | 52.0 ± 1.7 | 165.0 ± 5.9 | 205.8 ± 2.9 |
| IE | 50.0 ± 0.8 | 168.3 ± 4.6 | 200.3 ± 5.9 |
| SI2 | 51.4 ± 1.1 | 144.0 ± 3.7 | 212.6 ± 3.0 |
| SE2 | 51.1 ± 1.2 | 154.6 ± 2.2 | 202.5 ± 2.9 |
| SI3 | 47.8 ± 0.8 | 157.3 ± 2.6 | 197.4 ± 7.5 |
| SE3 | 51.4 ± 1.1 | 135.0 ± 6.4 | 186.0 ± 1.6 |
| FEMALES | |||
| HOUSING | PND 23 | PND 38 | PND 43 |
| II | 43.6 ± 1.7 | 131.9 ± 2.6 | 173.3 ± 4.5 |
| IE | 43.3 ± 1.4 | 135.2 ± 2.4 | 164.9 ± 4.0 |
| SI2 | 46.4 ± 1.6 | 135.3 ± 1.8 | 171.7 ± 3.0 |
| SE2 | 44.0 ± 1.1 | 132.7 ± 2.2 | 160.4 ± 4.2 |
| SI3 | 43.1 ± 1.8 | 131.5 ± 2.4 | 171.5 ± 2.9 |
| SE3 | 45.3 ± 2.3 | 130.1 ± 2.4 | 159.3 ± 1.8 |
Novelty--Induced Locomotor Activity
On PND 37 (fourteen days after housing), the locomotor response to a novel open field environment was measured for 30 minutes. In this study, rats were placed into a locomotor testing chamber and, starting immediately, the total distance traveled and time spent in the center of the chamber were recorded.
To determine activity levels, rats were placed in clear acrylic chambers (40.64 × 40.64 cm) inside Digiscan activity monitors (Accuscan, Columbus, OH) that were equipped with infrared light sensitive detectors mounted 2.5 cm apart along two perpendicular walls. Mounted along the opposing walls were infrared light beams that were directed at the detectors. Total distance traveled was measured based upon the number of beam breaks. Time spent in the center of the open field was measured in seconds.
Cocaine-Stimulated Locomotor Activity
In a separate group of rats, locomotor activity in response to cocaine was measured. On PND 43, rats were habituated in the test chamber for 15 min and then injected with a 1 ml/kg i.p. injection of saline and baseline locomotor activity was measured for 60 minutes. For the next three days (PND 44-46), rats were placed into the chamber for 15 min of habituation, after which they were injected with 5 mg/kg cocaine i.p. Activity was monitored immediately after cocaine administration for a total of 60 min. We have been able to measure tolerance and sensitization to cocaine with this method [23,24,26], showing that it is possible to measure changes in both upward and downward directions. This dose of cocaine was chosen based on the data from our prior CPP studies showing that maximal place preference during adolescence was seen in response to training with 5 mg/kg of cocaine [48,49]. After the last test session, vaginal smears were taken from all female rats to determine estrous cycle. They were done at this time so as not to interfere with the behavioral studies. An overall analysis showed that there was no relationship between estrous cycle and the effects of either novelty or cocaine on locomotor activity. It is possible that the rats are not yet cycling normally, since these are young animals and vaginal opening occurred at the beginning of these experiments. There were no differences in date of opening as a function of housing conditions.
Chemicals
Cocaine HCl was obtained from NIDA, (Rockville, MD) and was dissolved in saline.
Data Analysis
Novelty-induced locomotor activity (total distance and time spent in the center of the locomotor chamber) was analyzed by a two-way (social × environmental) Analysis of Variance (ANOVA). The three days of cocaine-stimulated locomotor activity were analyzed by three-way ANOVA (social × environmental × day). Where appropriate, ANOVAs were followed by post hoc analyses with Fisher’s Protected Least Significant Difference (PLSD). P values less than 0.05 were considered significant.
Results
Novelty-Induced Locomotor Activity
Overall, there was a significant effect of sex on novelty-induced exploratory behavior (total distance traveled during a 30 minute session upon first exposure to the test chamber) on PND 37 (F(1,227) =6.599, P≤0.01). In addition, there was a significant overall effect of environment (F(2, 227) = 5.028, P≤ 0.03). There also was a social housing × sex interaction (F(2, 227) = 6.409, P≤ 0.002), with isolated females exhibiting significantly higher levels of activity than males (P≤0.0002).
Across sexes and housing conditions, there was a significant effect of both social housing conditions (F(2,227) = 3.515, P≤ 0.0314) and environmental enrichment (F(1,227) = 6.620, P≤0.01) on time spent in the center of the chamber. Overall, rats living 3/cage spent more time in the center of the test chamber than did rats living alone, and rats in enriched conditions spent less time in the center than rats without environmental enrichment. There also was a significant interaction between social housing × sex (F(2,227) = 12.25, P≤0.0001). Post hoc tests showed that in rats living 3/cage males spent significantly more time in the center of the chamber than females.
Males
Analysis of the amount of novelty-induced exploratory behavior (total distance traveled during a 30 minute session upon first exposure to the test chamber) on PND 37 revealed a significant main effect of social enrichment across the groups (F(2,105)=6.574, P≤0.002; Fig. 1A). The rats housed three per cage (SI3 and SE3) exhibited significantly higher levels of locomotor activity in the novel environment than any other group (P<0.05). No differences in activity on PND 37 were found between the isolated animals and rats housed two per cage suggesting that social isolation did not affect the behavior in the novel environment compared to our standard housing conditions (two rats per cage). There was no effect of environmental enrichment, shown by the lack of significant differences between impoverished and enriched groups in any of the social conditions.
Fig. 1.
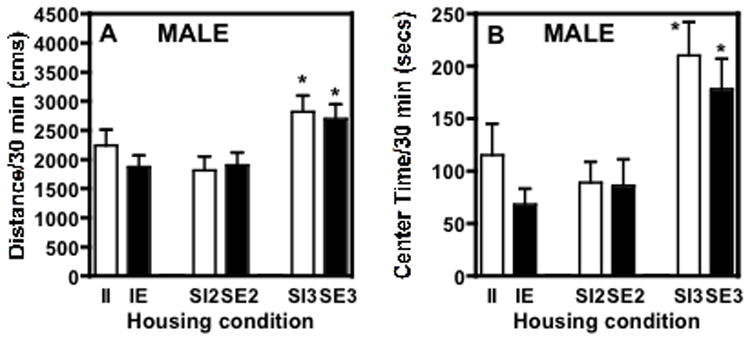
Response to a novel environment on PND 37 (two weeks after housing) of male adolescent rats housed under different conditions, expressed as mean ± SEM. (A) Distance traveled in 30 min and (B) time (secs) spent in the center of the chamber/30 min. The rats were not habituated to the locomotor chambers (i.e. testing began immediately after the rats were placed in the chambers for the first time and the sessions lasted 30 min). The SE3 and SI3 groups (3 rats/cage with or without toys, n=26 and 23, respectively) exhibited significantly higher levels of activity than any of the other groups tested. II = isolated impoverished (n=22; 1 rat/cage, no toys); SI2 = social impoverished (n=16; 2 rats/cage with no toys – this is our standard housing condition); IE = isolated enriched (n=16; 1 rat/cage with toys); SE2 = social enriched 2 (n=8; 2/cage with toys). *significant difference from isolated rats or rats housed 2/cage (p ≤ 0.05).
Analysis of the amount of time the males spent in the center of the locomotor chamber on PND 37 revealed a significant effect of the social enrichment across the groups (F(2,105)=9.094, P≤0.0002; Fig. 1B). Social groups with rats housed three per cage spent significantly more time exploring the center of the test chamber during the session compared to all other groups. As with the total activity, there was no significant effect of environmental enrichment on center time regardless of the social conditions.
Females
Statistical analysis revealed significant effects of social (F(2,122) = 3.144, P≤ 0.05) and environmental (F(1,122) = 6.207, P≤ 0.05) enrichment on novelty-induced locomotor activity (Figure 2A). There was no interaction between these factors. Post hoc analyses showed that isolated rats exhibited significantly greater levels of exploratory behavior in the novel environment than rats housed two or three per cage (P≤ 0.05). In addition, the II group was significantly more active than IE (P≤ 0.01), SI2 (p ≤ 0.05) and SI3 (P≤ 0.01) groups (Figure 2A).
Fig. 2.
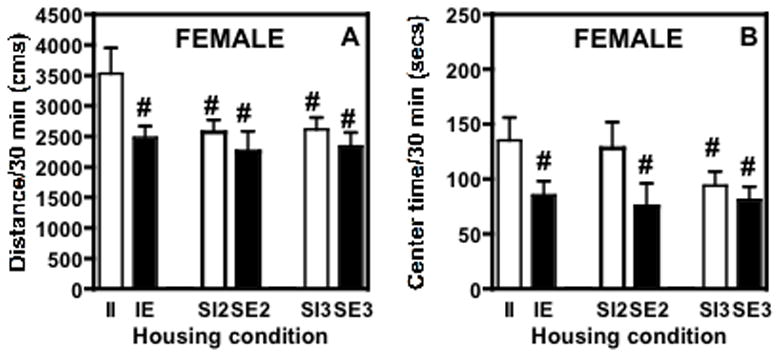
Response to a novel environment on PND 37 (two weeks after housing) of female adolescent rats housed under different conditions, expressed as mean ± SEM. (A) Distance traveled in 30 min and (B) time (secs) spent in the center of the chamber/30 min. The rats were not habituated to the locomotor chambers (i.e. testing began immediately after the rats were placed in the chambers for the first time and the sessions lasted 30 min). (A) The II group exhibited significantly higher levels of activity than any of the other groups tested. (B) Both the II and SI2 rats spent more time exploring the center of the test chamber than any of the other groups. II = isolated impoverished (one rat/cage without toys, n=24) IE = isolated enriched (n=24; one rat/cage with toys); SI2 = social impoverished 2 (n=16; 2/cage without toys); SE2 = social enriched 2 (n=16; 2/cage with toys); SI3 = social impoverished 3 (n=24; 3 rats/cage, no toys); SE3 = social enriched 3 (n=24; 3 rats/cage, with toys). #significant difference from isolated impoverished rats (p ≤ 0.05).
For time spent in the center of the apparatus, there was a significant overall effect of environment (F(1,122) = 5.1557, P≤ 0.05; Fig. 2B). In isolated animals, those in an impoverished environment (II) spent significantly more time in the center than those living in an enriched environment (IE) (P≤ 0.05). When the social enrichment was considered, impoverished pair-housed (SI2) rats spent significantly more time in the center than SE2, SI3, and SE3 rats (P≤ 0.05).
Cocaine-Stimulated Locomotor Activity
Baseline locomotor activity (after habituation) was significantly affected by sex (F(1,120)=27.532, P≤0.0001), by environment (F(1,120)=12.770, P≤0.0005), and there was a sex × environment interaction (F(1,120)=8.321, P≤0.005). Fig. 3 shows baseline locomotor activity in (A) males and (B) females. Post hoc analyses showed that there were significant differences between baselines of impoverished females and males, and impoverished and enriched females (P≤0.0001). Enriched males did not differ significantly from impoverished males or from enriched females.
Fig. 3.
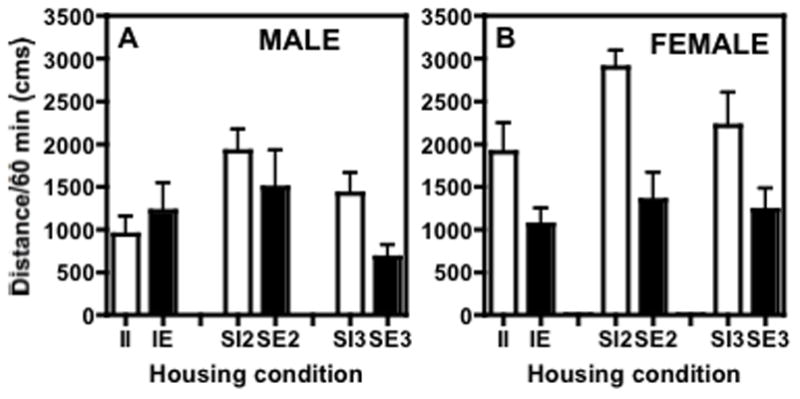
Baseline locomotor activity on PND 43 in (A) male rats and (B) female rats. In males, there was a significant interaction between social and environmental housing conditions. Post-hoc tests showed that environmental enrichment decreased activity only in the rats housed 3/cage, but did not have a significant effect in rats housed alone or in pairs. In females, there was a significant effect of environment in that impoverished rats had higher levels of activity overall compared to enriched rats, but no effect of social housing and no social × environmental interaction.
In addition, an overall analysis of cocaine-stimulated activity showed that there was a significant effect of sex (F(1,383)=8.438, P≤0.004), of social conditions (F(2,383)=8.570, P≤0.0002) and an interaction between social and environmental housing (F(2,383)=7.389, P≤0.001).
Males
Because of the significant differences in the effects of social conditions on baseline locomotor activity (after 15 minutes of habituation) on PND 43 (F(2;42)=3.823; P≤0.0298), activity on subsequent days was evaluated as percent of baseline (PND 43) distance traveled for each group. Fig. 3 shows the baseline activity, where male rats housed two per cage had significantly higher baselines than those housed either one per cage or three per cage, irrespective of environmental enrichment.
An overall analysis revealed a significant effect of social enrichment on cocaine-induced locomotor activity (F(2;126)=4.297, P≤0.0157) and a significant interaction of social and environmental enrichment (F(2;126)=8.352, P≤0.0004). Post hoc analyses showed that the II rats were more active in response to cocaine than IE rats (P≤0.0388; Fig. 3A). There were no significant differences between the SI2 and SE2 rats (Fig. 3B). SE3 rats showed significantly higher level of cocaine-induced locomotor activity than did the animals housed SI3 (P≤0.012; Fig. 3C). In addition, the SE3 group had higher activity in response to cocaine than did environmentally enriched rats housed in either the IE (P≤0.0003) or the SE2 (P≤0.0001) condition. In contrast, there were no significant differences in the impoverished rats across all three days of testing in that the II, SI2, and SI3 rats exhibited similar amounts of activity. Environment alone (enriched vs. impoverished conditions) did not significantly alter the response to cocaine across groups. Similarly, there was no effect of test day in that there were no significant within-group changes across the three days of testing. Thus, sensitization to cocaine did not occur in any group over the three days of cocaine administration.
Females
Because of small but significant differences in effects of environmental conditions on baseline locomotor activity (after 15 minutes of habituation) in female rats on PND 43 (F(1;42) = 9.813; P≤ 0.003), cocaine-induced locomotor activity was evaluated as percent of baseline total distance each group. Overall, the rats housed with toys had significantly lower baselines than the groups housed in impoverished conditions, irrespective of number of the rats per cage (Fig. 3B).
There was a significant effect of social enrichment on cocaine-induced locomotor activity (F(1;84) = 20.639; P≤ 0.0001). Rats housed three per cage had significantly higher levels of locomotor activity in response to 5 mg/kg cocaine compared to the isolated rats for three days of treatment, irrespective of environmental enrichment. Rats housed in groups of two or groups of three did not show any differences in cocaine-induced locomotor activity and there was not a significant effect of environmental enrichment, although in the rats housed two or three per cage, there did appear to be a trend towards an increase in activity in the environmentally enriched female rats. Sensitization did not occur in any group across the three days of cocaine administration.
Discussion
The present study shows that, like adult rats, adolescent rats are affected by social and environmental housing conditions. These data extend the existing literature of studies done primarily in adult rats by showing that social and environmental enrichment act independently and interact to alter behavior and the behavioral response to cocaine. These alterations can be observed as soon as two weeks after housing the rats in different conditions.
Novelty-Induced Locomotor Activity
Social enrichment altered exploratory behavior of a novel environment for both male and female adolescent rats. For male adolescent rats, novelty-induced locomotor activity was increased in rats housed three per cage compared to rats that were pair-housed or housed alone. This pattern was true for both total distance traveled and time spent in the center of the chamber. Greater exploration of the center of the chamber has been suggested to indicate lower anxiety [13,37,44], thus, this finding suggests that adolescent male rats housed three per cage were less anxious in the novel environment than were those housed one or two per cage. Interestingly, physical enrichment had no effect on novelty-induced locomotor activity alone or in combination with social enrichment in males.
Like adolescent males, social enrichment altered novelty-induced locomotor activity in adolescent female rats, although the effect was in the opposite direction. Whereas adolescent males housed in groups of three had greater activity than those that were pair-housed or housed alone, adolescent females housed alone had greater activity than those housed in groups of two or three. In fact, female rats housed in either of the social conditions (two or three rats/cage) did not differ from one another. In addition to the social environment altering novelty-induced locomotor activity, there was an interaction between the social and physical enrichment in females. In rats housed alone, environmentally impoverished female rats had greater novelty-induced activity than environmentally enriched animals. This difference in the effects of environmental enrichment was not observed in the socially housed rats. For time spent away from the walls in the center of the chamber, in both the singly and doubly housed rats, environmental enrichment decreased exploration, suggesting that environmentally impoverished female rats are less anxious than enriched rats. It generally has been believed that single housing is stressful for rats, yet these data suggest that this may be true only for male rats and not for female rats, especially during adolescence. Social housing for laboratory animals is a widely accepted practice for many species and is mandated for nonhuman primates. These findings suggest that males and females should be considered separately when determining optimal housing conditions.
These results in adolescent male are in contrast to studies in adult rats that showed that isolated rats are more active than grouped housed animals [18,22,45]. In those studies, testing took place when the rats were adults, and after an acclimation trial or habituation to the test environment. Thus, while both studies were measuring activity levels, in the current study activity in a novel environment was measured, while the earlier studies focused on activity in an environment to which the rats had habituated. In fact, an earlier study in our lab showed that after habituation to the locomotor chambers, adolescent male rats housed in an impoverished, isolated environment (II) had greater activity than those housed in groups of three in an enriched environment (SE3) [47]. Taken together, it appears that the difference between the present findings and previous findings may be the result, at least in part, of habituation to the test arena in other studies. These findings do suggest that the novel environment may be more stressful to animals housed in certain conditions than in others. In the novel environment, male rats in both isolated and pair-housed conditions spent less time in the center than did those housed in groups of three. It is possible that male adolescent rats housed alone or in pairs are more anxious, and perhaps more stressed, than those housed in groups of three, whereas social housing does not affect anxiety in female adolescents.
In contrast to the males, environmental enrichment had an effect on novelty-induced activity of female rats. Environmental enrichment interacted with social enrichment in that the isolated rats with environmental enrichment exhibited decreased exploration of a novel environment than did isolated impoverished females, whereas there was no effect of environment in the rats housed two or three per cage. In adult female rats habituated to the test chamber, social enrichment decreased activity [18]. Thus, the effect of housing on activity may be different depending both upon age and upon the testing conditions, with novelty, and perhaps the stress of a novel environment playing a unique role.
Cocaine-Stimulated Locomotor Activity
In male adolescent rats, cocaine-stimulated activity was altered depending on social and environmental enrichment. Environmental enrichment (toys) decreased activity in the isolated rats (II > IE), but increased activity in the socially housed animals (SE3 > SI3). Further, SE3 rats showed the greatest locomotor response to cocaine compared to the other groups. No differences were seen between pair-housed animals that were environmentally impoverished or enriched. In female adolescent rats, social enrichment (either pair-housing or triple-housing) increased cocaine-stimulated locomotor activity compared to those in the isolated condition, irrespective of environmental enrichment. Unlike in males, where environmental enrichment interacted with social enrichment to alter cocaine-stimulated locomotor activity, environmental enrichment had no significant effect on activity in female rats, although there was a trend towards increased effects in the socially housed rats with, compared to without environmental enrichment. Thus, social and environmental factors altered cocaine-stimulated locomotor activity in adolescent male rats, but only social environment altered cocaine-stimulated activity in adolescent females.
Locomotor activity after habituation to the test chamber has been examined in adult male and female animals living in different environments [18,22; described above], but few investigators have measured the effects of enrichment on cocaine-stimulated locomotor activity. In one report, adult male rats that had been group-housed in an enriched environment post-weaning had greater locomotor activity in a familiar environment in response to 10 and 20 mg/kg of cocaine at PND 63 compared to isolated animals [8]. Based on this report, it appears that the enhanced cocaine-stimulated activity observed in socially and environmentally enriched adolescents in the present study likely persists into adulthood. Similarly, adult female Long-Evans rats housed with social and environmental enrichment during adolescence and tested as adults were more sensitive to the locomotor-activating effects of cocaine than were animals housed in isolation [39]. These findings suggest that the present results not only persist into adulthood, but may also be consistent across other rat strains. The current data show that these effects are evident soon after housing and that the effects seen in adults are not the result of long-term housing conditions.
Interestingly, the enhancement of cocaine-stimulated locomotor activity in enriched animals is not consistent across all psychostimulant drugs. It has been reported that adult rats raised in enriched environments were more sensitive to the effects of amphetamine [5,6], but less sensitive to effects of nicotine [21] on locomotor activity than rats raised in impoverished environments. However, it is important to consider that although the rats were housed under different conditions during adolescence in these studies, the locomotor measures were done after PND 51, which is somewhat older than the rats (PND 44-46) in the present study. It is possible that locomotor activity, drug sensitivity, or drug-induced locomotor activity may be different between these two time periods.
The present data show that neither social nor environmental enrichment affected whether or not cocaine behavioral sensitization occurred in male and female adolescent rats. In fact, none of the rats developed sensitization to the locomotor-activating effects of 5 mg/kg cocaine over the three day administration period. This short administration period and low dose of cocaine was chosen to coincide with our cocaine conditioned place preference study [48]. However, the present results are consistent with our previous study showing that adolescent male rats did not develop sensitization to 20 mg/kg cocaine over a 7 day period, whereas adult male rats did become sensitized [11]. This three-day measurement period is shorter than most drug behavioral sensitization studies, where drug-stimulated activity often is measured for 1–2 weeks. It appears that adolescent male rats do not become sensitized to low or high doses of cocaine, but perhaps higher doses of cocaine, or a longer administration period would have induced sensitization in adolescent females.
Relationship between Novelty-Induced and Cocaine-Stimulated Activity
Individual differences in response to novelty may predict vulnerability to drug addiction, such that enhanced responses suggest greater vulnerability [3,34–36]. There are several ways to interpret the locomotor response to novelty, but some studies have suggested that it is indicative of an impulsive phenotype. For example, there was a positive correlation between response to novelty and behavioral disinhibition (a characteristic of impulsivity) [43] suggesting that high responders to novelty had increased behavioral inhibition, and therefore, were more impulsive. In fact, impulsivity can enhance the vulnerability to drug addiction as well [31]. Higher levels of impulsivity were found in users of many drugs, including users of cocaine [27]. In animal models, impulsivity influences progression through the addiction cycle [14,15,31] and predicts the transition from non-escalated drug self-administration to compulsive, escalated drug self-administration and addiction in rats [1]. With regard to cocaine, impulsivity predicts acquisition of cocaine self-administration and reinstatement of cocaine-seeking in rats [32]. If a greater response to novelty is correlated with greater impulsivity, and greater impulsivity can enhance vulnerability to drug use (including cocaine), then it follows that those with an increased response to novelty would be more sensitive to effects of cocaine thereby increasing vulnerability to cocaine use. This relationship is intriguing in light of the present findings.
In male adolescent rats, those that were triple-housed had the greatest response to novelty and the highest cocaine-stimulated activity compared to isolated rats and those housed in groups of three. This was especially true for animals housed in the enriched, rather than impoverished environments. Perhaps housing adolescent male rats in groups of three increases the response to novelty (and impulsivity), which makes them more sensitive to the locomotor activating effects of cocaine. This relationship was not apparent in the adolescent females. Females housed in groups of three had the highest levels of cocaine-stimulated locomotor activity regardless of environmental conditions, but isolated rats had the greatest response to novelty in the absence of environmental enrichment. Thus, it is possible that in females, the relationship between impulsivity and cocaine sensitivity is different, or novelty-induced locomotor activity and cocaine-stimulated locomotor activity are unrelated in female adolescents. It seems that the relationship between response to novelty, impulsivity, and sensitivity to cocaine may be specific to adolescent male rats. In adults, a previous study showed that rats living in an enriched environment (10 rats/cage with toys) were less impulsive than rats in an isolated condition (1 rat with no toys) when measured using a mean adjusted delay task for food delivery [33]. While it may be that this represents a difference between adults and adolescents, our findings that adding a single rat to the cage, even when the cage is the same size, can drastically alter the behavioral outcomes, suggest that the different results also could be due to the drastically different housing conditions, as well, of course to the difference in the task. Regardless for the reasons that the findings are different, it does appear that individual differences (e.g., sex, impulsivity, or housing conditions) can alter both the response to novelty and the effects of drugs. Whether housing conditions could modify impulsivity (on tasks of impulsive choice or impulsive action) in adolescents and alter vulnerability to drug addiction remains to be seen.
Summary
In summary, there appear to be significant sex-dependent effects of both social and environmental conditions on novelty-induced locomotor activity and cocaine-stimulated locomotor activity in adolescent rats. The behavioral effects of cocaine were altered depending on housing condition and this was different in male and female adolescents. Of course, differential hormonal regulation in male and female rats likely plays an important role in mediating these behavioral differences, as has been shown in numerous studies [e.g. 7,25,46]. Additional studies would need to be done to determine what role sex hormones play in mediating the behavioral effects of housing conditions in male and female adolescent rats. These data suggest that sex-dependent treatment strategies and prevention measures should be developed and that the types of activities that would positively influence whether or not drugs are rewarding for males may not be the same as for females. Further, these data show that care must be taken when implementing rules for the housing of rodents for behavioral and/or pharmacological studies, since housing conditions clearly will have differential effects on developing male and female rats.
Fig. 4.
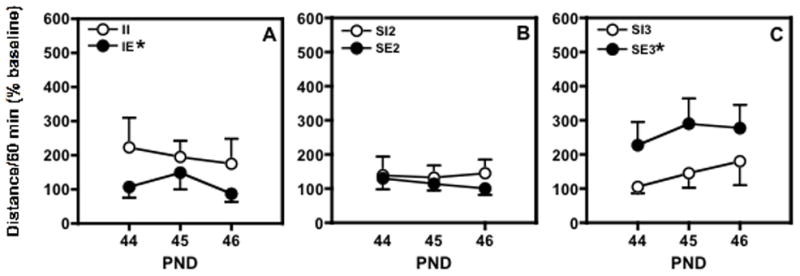
The effect of 5 mg/kg of cocaine on locomotor activity in male adolescent rats (PND 44-46) habituated to the test chamber after housing in different social/environmental conditions. Rats were housed (A) one/cage (II vs IE); (B) two/cage (SI2 vs SE2); and (C) three/cage (SI3 vs SE3). Data are presented as mean ± SEM total distance traveled during a 60 min session after habituation as a percent of baseline distance traveled on PND 43. *significant difference vs impoverished rats over 3 days of testing (p<0.05).
Fig. 5.
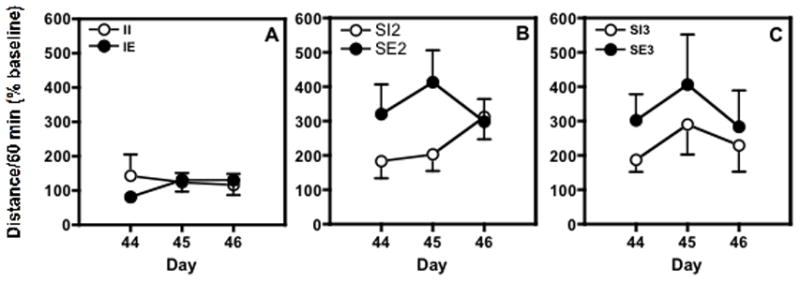
The effect of 5 mg/kg of cocaine on locomotor activity in female adolescent rats (PND 44-46) habituated to the test chamber after housing in different social/environmental conditions. Rats were housed (A) one/cage (II vs IE); (B) two/cage (SI2 vs SE2); and (C) three/cage (SI3 vs SE3). Data are presented as mean ± SEM total distance traveled during a 60 min session after habituation as a percent of baseline distance traveled on PND 43.
Research Highlights.
Enrichment differentially altered response to novelty in male and female adolescent rats
Social and environmental enrichment altered cocaine-stimulated activity
Male and female adolescents react differently to social and environmental enrichment
Acknowledgments
The project described was supported by Grant Number (grants R01DA015119 and P50DA024584-02) from the National Institute On Drug Abuse and the NIH Office of Research on Women’s Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health.
Footnotes
Disclosure/Conflict of Interest
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Developmental Brain Research. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 5.Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 6.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 7.Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. Journal of Pharmacology and Experimental Therapeutics. 1999;290:1315–1323. [PubMed] [Google Scholar]
- 8.Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown KJ, Grunberg NE. Effects of environmental conditions on food consumption in female and male rats. Physiol Behav. 1996;60:293–297. doi: 10.1016/0031-9384(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Developmental Brain Research. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 12.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Developmental Brain Research. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 14.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Kang L, Li B, Ma L. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacology Biochemistry & Behavior. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 18.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacology Biochemistry and Behavior. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 20.Gehrke BJ, Cass WA, Bardo MT. Monoamine-depleting doses of methamphetamine in enriched and isolated rats: consequences for subsequent methamphetamine-induced hyperactivity and reward. Behav Pharmacol. 2006;17:499–508. doi: 10.1097/00008877-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170:235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 22.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 23.Izenwasser S, Coy A, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. European Journal of Pharmacology. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- 24.Izenwasser S, French D, Carroll FI, Kunko PM. Continuous infusion of selective dopamine uptake inhibitors or cocaine produces time-dependent changes in rat locomotor activity. Behavioural Brain Research. 1999;99:201–208. doi: 10.1016/s0166-4328(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn C, Francis R. Gender differences in cocaine-induced HPA axis activation. Neuropsychopharmacology. 1997;16:399–407. doi: 10.1016/S0893-133X(96)00278-3. [DOI] [PubMed] [Google Scholar]
- 26.Kunko P, French D, Izenwasser S. Alterations in locomotor activity during chronic cocaine administration: effect on dopamine receptors and interaction with opioids. Journal of Pharmacology and Experimental Therapeutics. 1998;285:277–284. [PubMed] [Google Scholar]
- 27.Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 28.Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res. 2004;153:213–223. doi: 10.1016/j.devbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 29.NSDUH. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06–4194) Rockville, MD: 2007. [Google Scholar]
- 30.Pena Y, Prunell M, Dimitsantos V, Nadal R, Escorihuela RM. Environmental enrichment effects in social investigation in rats are gender dependent. Behav Brain Res. 2006;174:181–187. doi: 10.1016/j.bbr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 32.Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- 33.Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piazza PV, Deroche-Gammonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response function predict a drug-vulnerable phenotype predisposed to addiction. Journal of Neuroscience. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 36.Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Moal ML, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- 37.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 38.Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- 39.Smith MA, Iordanou JC, Cohen BM, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates. Behav Pharmacol. 2009;20:312–321. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Biol. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- 41.Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- 42.Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology. 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- 43.Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Valle F. Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol. 1970;83:103–111. [PubMed] [Google Scholar]
- 45.Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biological Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- 46.Walker QD, Cabassa J, Kaplan KA, Li S-T, Haroon J, spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate efects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 47.Zaias J, Queeney TJ, Kelley JB, Zakharova ES, Izenwasser S. Social and physical environmental enrichment differentially affect growth and activity of preadolescent and adolescent male rats. J Am Assoc Lab Anim Sci. 2008;47:30–34. [PMC free article] [PubMed] [Google Scholar]
- 48.Zakharova E, Miller JS, Unterwald EM, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacology Biochemistry & Behavior. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]


