Abstract
Osteonecrosis after hematopoietic stem cell transplantation (HCT) has seldom been addressed in pediatric populations. At our institution, since January 2002, children undergoing allogeneic HCT (alloHCT) receive yearly follow-up magnetic resonance imaging (MR) of hips and knees. To estimate the prevalence, longitudinal changes and associated risk factors for osteonecrosis after alloHCT, we reviewed MRs for children who underwent single alloHCT during the study period. We analyzed 149 of 344 patients who had post HCT MRI imaging performed [84 males; median age11 years (range, 0.5–21years)], median follow-up time was 32.6 months (range, 2.8–97.2 months). Forty-four (29.5%) developed osteonecrosis of hips and/or knees; of those, 20 (45%) had at least 30% epiphyseal involvement. In 23 (52%) osteonecrosis lesions were identified in the first, and 43 (98%) by the third yearly scan. Knees were more frequently involved than hips; severity of osteonecrosis was greater in hips. Those who had pre-alloHCT osteonecrosis, two patients’ hips and six patients’ knees resolved completely; three patients’ osteonecrosis lesions regressed after alloHCT. On risk factor analysis, age at time of alloHCT (p=0.051) and osteonecrosis identified by MRs before alloHCT (p=0.001) were the primary risk factors. This analysis shows that preventive strategies for osteonecrosis in this population should focus on measures to minimize risk factors before alloHCT.
Keywords: Hematopoietic stem cell, transplantation, osteonecrosis, children, graft versus host disease
Introduction
The increased use of hematopoietic stem cell transplantation (HCT) among children, coupled with an increase in survival, has led to a rise in the number of young HCT survivors.[1,2] Osteonecrosis, a debilitating skeletal morbidity[3,4] is one complication that can compromise quality of life among these survivors. Osteonecrosis is a challenge to treat in this population because it is often identified in advanced stages, and in multiple joints, when the only available treatment is joint replacement. Surgery can be accompanied by high rates of infection because of immunosuppression, and often requires revision because of hardware failure in these young patients.[5,6]
Although published reports describe osteonecrosis in adults or mixed populations,[2,3,7–11] studies describing osteonecrosis exclusively in pediatric population are few.[12–16] A wide range of incidence rates, 3.9–44.0%, following pediatric HCT is reported. Among adults, the incidence of osteonecrosis is higher in allogeneic than in autologous HCT survivors[3,7,11] Previously reported risk factors for osteonecrosis include acute and chronic graft-versus-host disease (GVHD) requiring steroids, older age at transplantation, and a primary diagnosis of leukemia or aplastic anemia[2,3,9,11,12]. Limiting understanding of osteonecrosis in pediatric HCT patients are the diverse diagnostic criteria used in previous studies[3,8,11,12,17,18], ranging from symptom-based diagnosis and plain radiographs to magnetic resonance studies (MR).
We reviewed prospectively acquired serial MRs in children who underwent a single alloHCT at our institution from December 2000 to September 2007. We sought to assess the prevalence, associated risk factors, and longitudinal changes observed over time in the development of osteonecrosis after alloHCT. This knowledge provides an assessment of the magnitude of this problem and an understanding of the timing of onset of osteonecrosis in this vulnerable young population.
Methods
Study population
We retrospectively reviewed medical records and MRs of children who underwent a single alloHCT at St. Jude Children’s Research Hospital between December 2000 and September 2007. Only patients for whom post-HCT MRs of hips, knees, or both were available were included in the cohort. To assess the representativeness of the study sample, clinical characteristics of the cohort were compared with those of the excluded population.
Since January 2002, patients undergoing alloHCT at St. Jude have undergone baseline MR of the hips and knees at the time of alloHCT and follow-up protocol-driven imaging of hips and knees annually afterward. If bone marrow transplantation must be performed urgently, then pre-alloHCT MR imaging may be omitted. If the second follow-up MR is normal and the patient is asymptomatic, then no further imaging is done. If the study is abnormal or symptoms persist during the second annual imaging study, then the patient is followed until imaging findings peak or symptoms resolve.
Institutional review board (IRB) approval was obtained for this study, and data were managed in accordance with the Health Insurance Portability and Accountability Act of 1996. All patients underwent IRB-approved treatment for their primary disease under institutional or cooperative group protocols.
Osteonecrosis
Patients being evaluated for osteonecrosis had coronal noncontrast T1-weighted and short tau inversion recovery (STIR) imaging of the hips and/or knees and sagittal fast low angle shot MR 2-D imaging of the articular surfaces. MRs were performed with either a Siemens 1.5-T Symphony or a Vision MR unit (Siemens, Inc., Erlangen, Germany). MRs were reviewed and interpreted by an experienced pediatric radiologist (SCK) blinded to presence or absence of clinical symptoms. As described previously [5,19,20], osteonecrosis was defined as a geographic area of decreased signal on T1-weighted and increased signal on STIR images.
Osteonecrosis locations were classified as epiphyseal, metaphyseal, or diaphyseal.[19] Because the extent of epiphyseal involvement is an important predictor of joint outcome,[5] we categorized the extent of epiphyseal involvement as ≥30% or <30% of the weight-bearing surface. Thus, hip joint involvement was coded according to the involvement of the capital femoral epiphysis, and knees were coded as involved if an osteonecrosis lesion was present in either the distal femoral or the proximal tibial epiphysis. For patients who underwent multiple MRs after alloHCT (N=91), the MR revealing the most severe osteonecrosis was used for calculating prevalence and analyzing risk factors. We also evaluated the distribution and pattern of multiple joint involvement.
Longitudinal changes in osteonecrotic lesions
Serial MRs were evaluated to assess the evolution of osteonecrosis over time. Longitudinal changes were documented separately for knees and hips by comparing annual MRs after alloHCT and by comparing pre- and post-alloHCT MRs for patients for whom pre-alloHCT MRs were available.
Any case that resolved completely over time (hip or knee) bilaterally was classified as “resolved;” any decrease in size of the osteonecrosis lesion was categorized as “regression.” Patients with negative pre-alloHCT MR who had osteonecrosis lesions after alloHCT and those identified with osteonecrosis after alloHCT for the first time were considered “new cases.” Cases with an increase in the size of existing osteonecrosis lesions were labeled as “progression.” We also collected information about surgical interventions for osteonecrosis during the follow-up period.
Transplantation
All patients underwent alloHCT on IRB-approved protocols. Grafts were obtained from human leukocyte antigen (HLA)-identical siblings, matched unrelated donors (matched for five or six HLA loci), or mismatched family members. Patients with hematologic malignancies were conditioned with myeloablative total body irradiation (TBI)-based conditioning (12 Gy in 8 fractions over 4 days in doses of 150 cGy per fraction) or with reduced-intensity fludarabine-based conditioning (40 mg/m2 for 5 days). Patients with non-malignant conditions were given busulfan-based conditioning (8–16 mg/kg). All recipients received either a calcineurin inhibitor or mycophenolate mofetil for GVHD prophylaxis.
GVHD was graded according to standard criteria.[21,22] Patients with grade 2 to 4 acute GVHD were treated with methylprednisolone (1–2 mg/kg daily). If GVHD remained at grade 2 or more for more than 7 days or progressed, secondary agents were started, such as a calcineurin inhibitor or mycophenolate mofetil that the patient was not receiving.
Risk factors
We considered associations between osteonecrosis after alloHCT and the following risk factors: sex, race, age at time of alloHCT (i.e., <10 years or ≥10 years), age at primary diagnosis, primary diagnosis (lymphoid malignancies and aplastic anemia vs. others), body mass index (BMI) at time of alloHCT (normal and underweight vs. overweight and obese), donor type (related vs. unrelated), conditioning regimen (TBI vs. non-TBI), acute GVHD (none vs. grade 1 or higher), chronic GVHD (none vs. limited, none vs. extensive), and physis status at the time of HCT (open vs. closed). BMI at the time of HCT was calculated only for patients older than 2 years, per Centers for Disease Control and Prevention (CDC) criteria (www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html).[23] Since physeal development was bilaterally similar (correlation coefficient>0.95 with p<0.0001; results not shown) and there were only minor differences between hips and knees (correlation coefficient around 0.85 with p<0.0001), we used a majority rule to combine all six physis statuses into one status value. For patients with more than half physis statuses as “closed,” we defined the physis status as “closed.”
As previously described, [19] malignant conditions were categorized as lymphoid (i.e., acute lymphoblastic leukemia, non-Hodgkin lymphoma [NHL], and Hodgkin disease) or non-lymphoid (malignant conditions other than described as lymphoid). Because statistical power for eliciting the effect of individual treatment regimens was limited and individual cumulative doses of steroids received by each patient were unavailable, primary diagnoses were categorized on the basis of contemporary treatment protocols to assess the potential effect of steroid exposure. Diagnoses were grouped as follows: lymphoid malignancies and aplastic anemia vs. other conditions (i.e. non-lymphoid malignancies and sickle-cell disease, immunodeficiency syndromes, osteopetrosis, I-cell disease, Hurler disease, thromboasthenia, and Glanzmann disease).
Statistical analyses
Descriptive statistics were used to describe the study population by sex, race, primary diagnosis, age at the time of MR; age, BMI and physis status at the time of HCT, conditioning regimen, product type, donor type, donor match type, acute and chronic GVHD grades and duration of chronic GVHD.
Osteonecrosis prevalence rates were calculated for epiphyseal involvement of any of the four lower extremity joints and for hips and knees separately. Time to development of osteonecrosis after alloHCT was calculated only for children with negative or undetected osteonecrosis before alloHCT. The pattern and distribution of joint involvement was examined by evaluating the number of lower extremity joints involved at the time when the earliest MR showed the highest grade of osteonecrosis. Cumulative incidences of osteonecrosis after alloHCT were estimated with death as the competing event.
We used a generalized estimating equation (GEE) models[24] to estimate the incidence of osteonecrosis over time. Independent variables with a significance level of 0.01 in univariate analysis were included in the multivariate GEE model. Because sickle cell anemia[25] is highly correlated with black race, the analysis was repeated with and without sickle cell patients in the model.
Data were analyzed by using SAS (Version 9.13; SAS Institute, Inc., Cary, NC). Statistical significance was set at a p-value of 0.05.
Results
Demographic characteristics of the study population
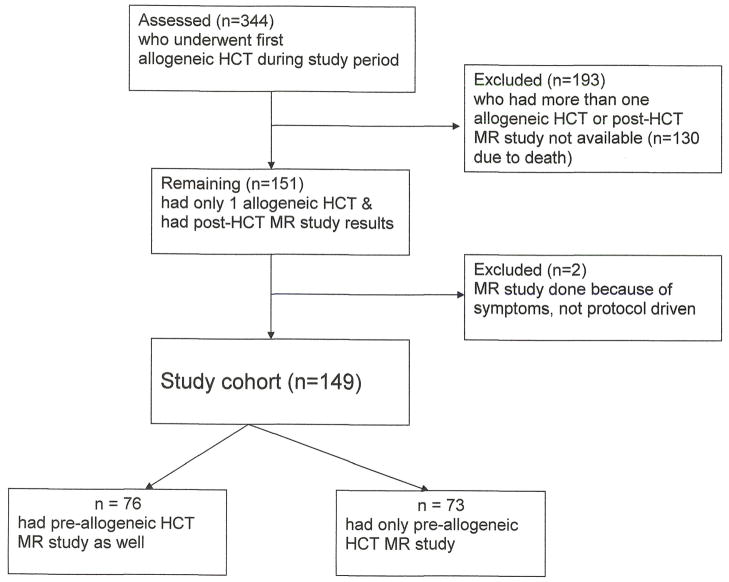
Of the 344 consecutive patients who underwent allogeneic HCT at St. Jude Children’s Research Hospital from December 2000 to September 2007, 149 (84 males) children (median age at time of alloHCT 11 years (range, 0.5–21 years)) underwent at least one post-alloHCT MR of hips and/or knees irrespective of symptoms, and had undergone a single alloHCT (Figure 1). Demographic characteristics of the cohort are shown in Table 1. 116 patients had alloHCT for malignant diseases and 33 for non-malignant conditions. Median time to follow-up from the date of alloHCT was 32.6 months (range, 2.8–97.2 months). When compared to excluded patients, the study population was older at the time of alloHCT, had fewer males and more often received total body irradiation (TBI). Patients older than 10 years of age were more likely to have undergone pre-alloHCT MR than those younger than 10 years (p<0.0001) at time of bone marrow transplantation.
Figure 1. Study cohort description.
Table 1.
Demographic data for patients who received magnetic resonance imaging after allogeneic HCT
| Demographic variable | Frequency (n=149) | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 84 | 56 |
| Female | 65 | 44 |
| Race | ||
| Other | 29 | 20 |
| Black | 28 | 19 |
| White | 92 | 62 |
| Diagnosis | ||
| Nonmalignant | 33 | 22 |
| Malignant | 116 | 78 |
| Primary diagnosis | ||
| Others | 87 | 58 |
| Lymphoid malignancies & aplastic anemia | 62 | 43 |
| Survival status | ||
| Dead | 28 | 19 |
| Alive | 121 | 81 |
| BMI at alloHCT* | ||
| Normal & underweight | 96 | 67 |
| Overweight & obese | 47 | 33 |
| Age at MR** | ||
| <10 | 51 | 34 |
| ≥10 | 98 | 66 |
| Age at alloHCT | ||
| <10 | 65 | 44 |
| ≥10 | 84 | 56 |
| Conditioning regimen | ||
| Total body irradiation | 101 | 68 |
| No-total body irradiation | 48 | 32 |
| Physis status at allo HCT | ||
| Closed | 42 | 28 |
| Open | 107 | 72 |
| Acute GVHD maximum Overall grade | ||
| No acute GVHD | 30 | 20 |
| Grade 1, 2 | 81 | 54 |
| Grade 3, 4 | 38 | 26 |
| Chronic GVHD grade | ||
| No chronic GVHD | 92 | 62 |
| Limited | 26 | 18 |
| Extensive | 31 | 21 |
| Donor type | ||
| Unrelated | 57 | 381 |
| Related | 92 | 62 |
| Donor match type | ||
| HLA-matched siblings | 49 | 33 |
| Matched unrelated donors | 57 | 38 |
| Mismatched family members | 43 | 29 |
| Product type | ||
| HPC-A | 47 | 32 |
| HPC-C | 1 | 1 |
| HPC-M | 101 | 68 |
| Duration of chronic GVHD | ||
| No prior chronic GVHD | 92 | 62 |
| Duration ≤ 6 months | 39 | 26. |
| Duration > 6 months | 18 | 12 |
BMI calculated only for children more than 2 years old.
Age at the first peak osteonecrosis, if a patient did not have any positive imaging result; age at the last imaging date was used.
Abbreviations: alloHCT, allogeneic hematopoietic stem cell transplantation; BMI, Body mass index; GVHD, Graft vs. host disease;; HPC-A, Hematopoietic stem cell from peripheral blood; HPC-M, Hematopoietic stem cell from Marrow; HPC-C, Hematopoietic stem cell from cord; MR, magnetic resonance imaging
Prevalence, severity and time to development of osteonecrosis
When considering all four joints, the prevalence of osteonecrosis was 29.5% (44/149); 45.5% (20/44) had ≥30% epiphyseal involvement. The prevalence of osteonecrosis in the knees was 28.2% (40/142), greater than the prevalence of 9% (13/143) in hips. Fourteen of the 40 patients with osteonecrosis of the knees, 14 had ≥30% epiphyseal involvement. Twelve of the 13 patients with hip osteonecrosis had ≥30% epiphyseal involvement Cumulative incidence plots (Figure 2) show that 52.3% (23/44) of osteonecrosis cases were identified in the first and 97.8% (43/44) by the third annual scans.
Figure 2. Cumulative incidence of osteonecrosis.
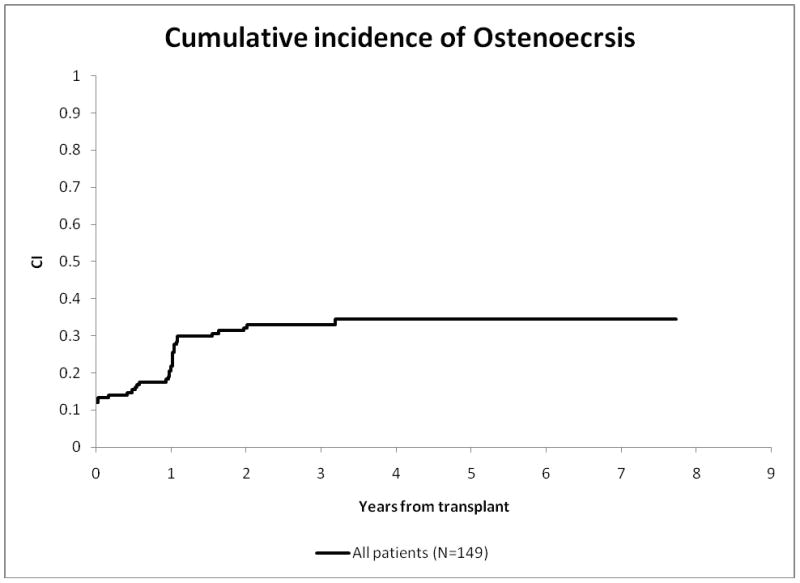
Note: The intercept (in red) has been shown to be 12% (SE 2.68%), to emphasize that 14 patients were already known to have osteonecrosis at the time of alloHCT because of magnetic resonance imaging done prior to alloHCT.
Abbreviations: CI, cumulative incidence; ON, osteonecrosis
Among the 44 children with osteonecrosis after alloHCT, 14 had evidence of osteonecrosis on pre-alloHCT MR. The median times to development of osteonecrosis in any joint in children with unknown pre-alloHCT osteonecrosis status (n=16), and in children with negative pre-alloHCT status (n=14), were 12.4 months (0.2–38.7 months) and 12.2 months (0.2–24.4 months), respectively.
Pattern of joint involvement
Of the 44 patients with osteonecrosis, 30 (68.2%) had multiarticular involvement; most commonly, bilateral knee involvement (21/44). Three of these patients had bilateral hip coupled with uni- or bilateral knee involvement. Of the 14 of 76 children who had both pre- and post-alloHCT MR, nine were found to have multiarticular involvment.
Longitudinal changes
Among the 149 patients, 58 had only one post-HCT imaging study available for review, 32 had two, and 59 had more than two. Pre-HCT imaging studies were available for 76 children (Figure 1).[19]
During the first year after alloHCT, hip MRs were available for 124 and knee MRs for 129 patients (Table 3). Of the 129 patients with first annual hip MRs, 58 also had pre-alloHCT MRs; two showed complete resolution, and seven new cases of osteonecrosis were identified. For knees, 57 of 129 had pre-alloHCT MRs; two showed complete resolution, one regressed, and 25 new cases were identified.
Table 3.
Longitudinal changes in the osteonecrotic lesions in children observed with magnetic resonance imaging during the first year after alloHCT.
| MR results before alloHCT | MR results one year after alloHCT | Total | |||
|---|---|---|---|---|---|
| Not Involved | ON; < 30% Epiphyseal involvement | ON; ≥ 30% Epiphyseal involvement | |||
| Hips: | Not done/Missing | 63 | 0 | 3∞ | 66 |
| Not Involved | 49 | 0 | 4∞ | 53 | |
| ON; < 30% Epiphyseal involvement | 1• | 1 | 0 | 2 | |
| ON; ≥ 30% Epiphyseal involvement | 1• | 0 | 2 | 3 | |
| Total | 114 | 1 | 9 | 124 | |
|
| |||||
| Knees: | Not done/Missing | 59 | 8∞ | 5∞ | 72 |
| Not Involved | 34 | 7∞ | 5∞ | 46 | |
| ON; < 30% Epiphyseal involvement | 2• | 7 | 1 | 10 | |
| ON; ≥ 30% Epiphyseal involvement | 0 | 1± | 0 | 1 | |
| Total | 95 | 23 | 11 | 129 | |
Abbreviations: alloHCT, allogeneic hematopoietic stem cell transplantation; MR, magnetic resonance imaging; ON, osteonecrosis
Cases that showed complete resolution;
New lesions identified during first year
Cases that showed regression of the osteonecrosis lesion
During the second year after alloHCT, 124 patients had hip MRs for both the first and second year, and 129 had knee MRs for both years. On comparing serial MRs, regression was observed in one hip and one knee and complete resolution in four knees (Table 4). The four patients with complete resolution of lesions ranged in age from 2 years 8 months to 14 years 8 months at primary diagnosis and 3 years 2 months to 6 years 6 months at time of alloHCT. Three of the four were skeletally immature as evidenced by physeal patency at time of alloHCT and were shown to have epiphyseal involvement of < 30%. The patient with closed physes had epiphyseal involvement of at least 30%. Three of the four patients had a primary diagnosis of acute lymphoblastic leukemia; one had acute myelogeneous leukemia. All four patients were of healthy body weight; all had received total body irradiation. Two received 6/6 HLA matched sibling and two HLA 6/6 matched unrelated donor cells. Three patients developed acute grade 1 GVHD; one developed no acute GVHD. All four developed chronic GVHD (two limited, two extensive and all four with duration of less than 6 months).
Table 4.
Longitudinal changes in the osteonecrotic lesions in children observed with magnetic resonance imaging during the second year after alloHCT.
| One year post alloHCT MR imaging results | Two year post alloHCT MR imaging results | Total | ||||
|---|---|---|---|---|---|---|
| Not done/Missing | Not Involved | ON; < 30% Epiphyseal involvement | ON; ≥ 30% Epiphyseal involvement | |||
| Hips: | Not done/Missing | 12 | 2 | 0 | 0 | 14 |
| Not Involved | 58 | 55 | 0 | 1∞ | 114 | |
| ON; < 30% Epiphyseal involvement | 0 | 0 | 1 | 0 | 1 | |
| ON; ≥ 30% Epiphyseal involvement | 3 | 0 | 1± | 5 | 9 | |
| Total | 73 | 57 | 2 | 6 | 138 | |
|
| ||||||
| Knees: | Not done/Missing | 6 | 2 | 0 | 1∞ | 9 |
| Not Involved | 53 | 38 | 4∞ | 0 | 95 | |
| ON; < 30% Epiphyseal involvement | 7 | 4• | 11 | 1 | 23 | |
| ON; ≥ 30% Epiphyseal involvement | 2 | 0 | 1± | 8 | 11 | |
| Total | 68 | 44 | 16 | 10 | 138 | |
Abbreviations: MR, magnetic resonance imaging; ON, osteonecrosis
New cases identified with osteonecrosis during second year after allogeneic HCT
Case that showed regression in the size of the osteonecrosis lesions after allogeneic HCT
Cases that showed complete resolution
New cases of osteonecrosis were identified in one hip and five knees as (Table 4). Among the 13 involved hips identified during follow-up, four have undergone total hip arthroplasty (THA). Two of these had THA before alloHCT and two afterward. In one patient, transient improvement in symptoms was documented after alloHCT, but subsequent progression required THA. All of these patients underwent core decompressions of the involved joints before THA.
Risk factors
Among the various risk factors studied, only pre-alloHCT osteonecrosis status (OR 13.50; 95% CI 2.64–68.92) and age ≥ 10 years at the time of alloHCT (OR 4.00; 95% CI 1.00–16.67) were associated with greater odds of osteonecrosis in the 3 years following alloHCT (Table 5). Black race was found to be significant on univariate analysis p=0.0431, but was not significant in models when patients with sickle cell disease were excluded. Other risk factors studied were not associated with an increased risk of osteonecrosis.
Table 5.
Risk factor analysis for osteonecrosis after alloHCT
| Univariate analysis using GEE model
| |||
|---|---|---|---|
| Clinical Variables | Clinical Level | 95% CI of OR | P Values |
| BMI at alloHCT | Normal Underweight | 0.86(0.40 to 1.83) | 0.6925 |
| Overweight Obese | |||
| Gender | Female | 1.81(0.87 to 3.74) | 0.1108 |
| Male | |||
| Race | Black | 2.09(0.87 to 5.05) | 0.1011 |
| Other | 0.49(0.06 to 4.25) | 0.5138 | |
| White | |||
| Diagnosis type | Malignant | 1.04(0.43 to 2.50) | 0.9283 |
| Non-malignant | |||
| Primary Diagnosis | Lymphoid Malignancies + Aplastic Anemia | 1.40(0.68 to 2.88) | 0.3662 |
| Others | |||
| Age at alloHCT | Age at alloHCT <10 | 0.15(0.06 to 0.37) | <.0001 |
| Age at alloHCT >=10 | |||
| Chronic GVHD grade | Extensive | 1.33(0.55 to 3.21) | 0.5302 |
| Limited | 1.33 (0.48 to 3.64) | 0.5819 | |
| No chronic GVHD | |||
| Acute GVHD Maximum Overall Grade | No acute GVHD grade ≥ 1 | 0.82(0.32 to 2.12) | 0.6834 |
| Physis Status | Closed | 3.33(1.55 to 7.15) | 0.0020 |
| Open | |||
| Donor Type | Related | 1.78(0.82 to 3.89) | 0.1465 |
| Unrelated | |||
| Conditioning regimen | NO TBI | 1.16(0.54 to 2.51) | 0.7020 |
| TBI | |||
| Duration of Chronic GVHD, 6 months | No prior chronic GVHD | ||
| Duration <= 6 month | 1.29(0.56 to 2.95) | 0.5455 | |
| Duration > 6 month | 1.42(0.44 to 4.58) | 0.5546 | |
| Pre-alloHCT MR | Involved | 14.19(3.52 to 57.27) | 0.0002 |
| Not done/Missing | 0.75(0.33 to 1.74) | 0.5074 | |
| Not involved | |||
|
| |||
|
Multiple GEE model analysis⫲
| |||
| Variable | Clinical Level | 95% CI of OR | P Values |
| Pre-alloHCT MR | Involved | 13.50(2.64 to 68.92) | 0.0018 |
| Not Involved | 0.86(0.30 to 2.51) | 0.7867 | |
| Not done/Missing | |||
| Physis | Closed | 1.42(0.41 to 4.94) | 0.5827 |
| Open | |||
| Age at alloHCT | Age at HCT <10 | 0.25(0.06 to 1.01) | |
| Age at HCT >=10 | 4.00 (1.00 to 16.7) | 0.0518 | |
| Race | Black | 2.64(0.82 to 8.52) | 0.1046# |
| Other | 0.53(0.05 to 5.44) | 0.5925# | |
| White | |||
| Gender | Female | 1.10(0.42 to 2.86) | 0.8462 |
| Male | |||
Abbreviations: alloHCT, allogeneic hematopoietic stem cell transplantation; BMI, body mass index; GVHD, graft vs. host disease; TBI, total body irradiation; MR, magnetic resonance imaging
Only factors significant (0.1 significant level) from univariate GEE model were included in this model
For race analysis sickle cell patients were excluded.
Discussion
Our analysis of prospectively acquired longitudinal MRs of hips and knees revealed a prevalence of osteonecrosis in almost 30% of the children in the first 3 years after a single alloHCT. Although knees were more often involved, hip involvement was more extensive. We observed complete resolution of osteonecrosis after alloHCT in two patients’ hips and six patients’ knees. Of those, one had osteonecrosis lesion involving more than 30% of the epiphyseal surface before alloHCT.
The prevalence of 30% is higher than previously reported rates that have been based on symptoms or less sensitive diagnostic techniques, but approach rates reported in MR-based studies;[12–16,26–30] symptom-based studies may underreport early-stage osteonecrosis. As we focused only on epiphyseal lesions of hips and knees[3], our findings may underestimate osteonecrosis-associated skeletal morbidity.
Aplastic anemia and ALL are well recognized risk factors for osteonecrosis following HCT[2,9]; we found no association in our post-alloHCT cohort. Our results conflict with those of our previous study, [19] where we evaluated risk factors for osteonecrosis before alloHCT and found some indication that a primary diagnosis of aplastic anemia or lymphoid malignancies may be a risk factor. As none of the published studies considered pre-alloHCT status, this discrepancy may be attributable to our risk factor analysis model adjusted for pre-alloHCT status.
The median time to development of osteonecrosis in our MR-based study was 12.3 months, a much shorter time period than reported in previous studies[3,7,8,10,12,31]. We found that only age older than 10 years and pre-existing osteonecrosis were predictive of an osteonecrosis in the 3 years following alloHCT. When future interventions that target early detection and prevention of osteonecrosis in the first 3 years following transplant are available, older children and children with existing lesions will be prime candidates for screening.
Older age has been consistently recognized as a risk factor for osteonecrosis [4,8]. White race has also been implicated as a risk factor for osteonecrosis [32]. We found no such association in our study population. Race was found to be statistically significant on univariate analysis but as sickle cell disease is strongly associated with black race, [25] we repeated analysis after excluding sickle cell patients and found no such association. Our cohort included 16 sickle cell patients, of whom eight were found to have osteonecrosis. Osteonecrosis is a well-known morbidity of sickle cell disease that has been positively associated with the frequency of vaso-occlusive pain crises and elevated hematocrit [33]. This morbidity has been reported in 10–16% of symptomatic patients assessed radiographically [30,34,35] and in up to 40% of patients radiographically examined regardless of symptoms [29]. The high 50% prevalence of osteonecrosis we found in our sickle cell patients is similar to the MR-detected incidence of 65% reported after a follow-up period of 4 to 5 years [36].
Unlike other investigators, [10,13], we found no significant increase in risk for osteonecrosis among those who received TBI, possibly because our patients received very small doses of radiation per fraction (150 cGy/fraction twice daily).
The findings that pre-alloHCT osteonecrosis status was a risk factor for post-alloHCT osteonecrosis, and that GVHD was not an important risk factor during 3 years of follow-up supports the multiple hit theory of osteonecrosis; [37,38] steroid exposure imparts secondary rather than primary insult on the osteonecrosis pathway. Previous investigations, primarily conducted in adult populations, have identified an association between higher grade or longer duration of GVHD (acute or chronic) and osteonecrosis [2,3,7,8,11,39,40], attributing risk to prolonged exposure to steroids. We found no such association. Prolonged exposure to steroids was not a prominent characteristic of our population. In our study, only 12% of children developed GVHD that required steroid therapy treatment for longer than 6 months. As our population was followed for only 3 years post-alloHCT, it is possible that children in our cohort with chronic GVHD who eventually experience prolonged steroid exposure will develop osteonecrosis. Our analysis underscores the importance of pre-alloHCT therapeutic exposures, and the likelihood of osteonecrosis following alloHCT, and encourages us to explore novel options to curtail the compounding osteotoxic effect of glucocorticoids[41].
Another compelling finding in our study was regression or resolution of post-alloHCT osteonecrosis in several children. Of the patients whose osteonecrosis resolved completely, one initially had more than 30% epiphyseal involvement. As a standard of care at our institution, all children with osteonecrosis are managed conservatively until pain or restricted movement develops. At that time, analgesics and/or surgical options are offered. Whether these osteonecrosis regressions were the natural course of disease, a response to joint protection measures instituted in physical therapy[42], or a result of a protective effect of alloHCT is unknown.
Our data preclude us from making definitive conclusions about osteonecrosis recovery due to alloHCT. However, limited evidence[43–46] suggests that stem cells have a role in regenerative medicine. This reparative phenomenon may in part be attributed to mesenchymal stem cells or primitive hematopoietic cells with osteoblastic potential[43] engrafted during alloHCT. Increased chemotaxis by cytokines released from the necrosed bone may also facilitate mesenchymal stem cell homing and vascular proliferation[47,48].
Our findings should be interpreted with caution since the diverse primary diagnoses limited our ability to estimate the effect of disease pathophysiology or its treatment on the evolution of osteonecrosis. Because our study population was slightly older than patients who did not receive imaging, and because older age has been consistently associated with development of osteonecrosis, our estimated incidence for osteonecrosis in the first 3 years following alloHCT may be inflated. Finally, although the lesions we detected in this cohort developed during the first 3 years after alloHCT, our study results do not provide information needed to ascertain delayed onset osteonecrosis in this population. Despite these limitations, we provide information about the prevalence and risk factors for development of osteonecrosis during the first 3 years after alloHCT among children.
Our results lead us to conclude that future screening and preventive strategies for osteonecrosis in children undergoing alloHCT should focus on pre-alloHCT risk factors among children who are older than 10 years of age. We recommend prospective controlled trials to determine the roles of physical therapy, mesenchymal stem cell therapy, and other novel interventions for treating early osteonecrotic lesions.
Supplementary Material
Table 2.
Characteristics of study cohort (N =149) versus those who were excluded (N = 195)
| Characteristics | Total n (%) | Excluded n (%) | Study Cohort n (%) | P value | |
|---|---|---|---|---|---|
| Gender | Male | 216 (63) | 132 (68) | 84 (56) | 0.0332• |
| Female | 128 (37) | 63 (32) | 65 (44) | ||
| Race | White | 213 (62) | 121 (62) | 92 (62) | 0.8501 |
| Black | 68 (20) | 40 (21) | 28 (19) | ||
| Other | 63 (18) | 34 (17) | 29 (20) | ||
| Diagnosis | Non-malignant | 68 (20) | 35 (18) | 33 (22) | 0.3422 |
| Malignant | 276 (80) | 160 (82) | 116 (79) | ||
| Primary Disease | Others | 212 (62) | 125 (64) | 87 (58) | 0.3143 |
| Lymphoid Malignancies + Aplastic Anemia | 132 (38) | 70 (36) | 62 (42) | ||
| Age at alloHCT | Age at alloHCT <10 | 174 (52) | 109 (56) | 65 (44) | 0.0294• |
| Age at alloHCT >=10 | 170 (49) | 86 (44) | 84 (56) | ||
| Donor Type | Unrelated | 113 (33) | 56 (29) | 57 (38) | 0.0650 |
| Related | 231 (67) | 139 (71) | 92 (62) | ||
| Conditioning regimen | Total Body Radiation | 204 (59) | 103 (54) | 101 (68) | 0.0103• |
| No TBI | 137 (40) | 89 (46) | 48 (32) | ||
Values depicted in bold were statistically significant
Abbreviations: alloHCT, allogeneic hematopoietic stem cell transplantation; TBI, Total body irradiation
Acknowledgments
This work was supported in part by grant number P30 CA-21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
Reference List
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socie G, Cahn JY, Carmelo J, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) Br J Haematol. 1997;97(4):865–870. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- 3.Enright H, Haake R, Weisdorf D. Avascular necrosis of bone: a common serious complication of allogeneic bone marrow transplantation. Am J Med. 1990;89(6):733–738. doi: 10.1016/0002-9343(90)90214-x. [DOI] [PubMed] [Google Scholar]
- 4.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(18):3038–3045. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimova EJ, Rai SN, Howard SC, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25(12):1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 6.Hungerford MW, Hungerford DS, Khanuja HS, et al. Survivorship of femoral revision hip arthroplasty in patients with osteonecrosis. J Bone Joint Surg Am. 2006;88(Suppl):3126–130. doi: 10.2106/JBJS.F.00777. [DOI] [PubMed] [Google Scholar]
- 7.Tauchmanova L, De Rosa G, Serio B, et al. Avascular necrosis in long-term survivors after allogeneic or autologous stem cell transplantation - A single center experience and a review. Cancer. 2003;97(10):2453–2461. doi: 10.1002/cncr.11373. [DOI] [PubMed] [Google Scholar]
- 8.Socie G, Selimi F, Sedel L, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: clinical findings, incidence and risk factors. Br J Haematol. 1994;86(3):624–628. doi: 10.1111/j.1365-2141.1994.tb04795.x. [DOI] [PubMed] [Google Scholar]
- 9.Schulte CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic stem-cell transplantation: diagnosis and gender matter. Transplantation. 2004;78(7):1055–1063. doi: 10.1097/01.tp.0000138026.40907.38. [DOI] [PubMed] [Google Scholar]
- 10.Fink JC, Leisenring WM, Sullivan KM, et al. Avascular necrosis following bone marrow transplantation: a case-control study. Bone. 1998;22(1):67–71. doi: 10.1016/s8756-3282(97)00219-6. [DOI] [PubMed] [Google Scholar]
- 11.Campbell S, Sun CL, Kurian S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009;115(18):4127–4135. doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balduzzi A, Gooley T, Anasetti C, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86(8):3247–3256. [PubMed] [Google Scholar]
- 13.Faraci M, Calevo MG, Lanino E, et al. Osteonecrosis after allogeneic stem cell transplantation in childhood. A case-control study in Italy. Haematologica. 2006;91(8):1096–1099. [PubMed] [Google Scholar]
- 14.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33(4):435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 15.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86(4):215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 16.Mascarin M, Giavitto M, Zanazzo GA, et al. Avascular necrosis of bone in children undergoing allogeneic bone marrow transplantation. Cancer. 1991;68(3):655–659. doi: 10.1002/1097-0142(19910801)68:3<655::aid-cncr2820680336>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Wagener P, Schulte D, Link H, et al. Musculoskeletal manifestations in patients after bone marrow transplantation. Initial clinical rheumatologic observations. Z Rheumatol. 1991;50(4):199–203. [PubMed] [Google Scholar]
- 18.Atkinson K, Cohen M, Biggs J. Avascular necrosis of the femoral head secondary to corticosteroid therapy for graft-versus-host disease after marrow transplantation: effective therapy with hip arthroplasty. Bone Marrow Transplant. 1987;2(4):421–426. [PubMed] [Google Scholar]
- 19.Sharma S, Yang S, Rochester R, et al. Prevalence of osteonecrosis and associated risk factors in children before allogeneic BMT. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimova EJ, Rai SN, Deng X, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma: Part 1, observer agreement. AJR Am J Roentgenol. 2006;186(2):470–476. doi: 10.2214/AJR.04.1598. [DOI] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC Criteria. 2011 Available from: www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 25.Flint J, Harding RM, Boyce AJ, et al. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11(1):1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 26.Marchese VG, Connolly BH, Able C, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88(3):341–350. doi: 10.2522/ptj.20070108. [DOI] [PubMed] [Google Scholar]
- 27.Niinimaki RA, Harila-Saari AH, Jartti AE, et al. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25(12):1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 28.Ojala AE, Paakko E, Lanning FP, et al. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Med Pediatr Oncol. 1999;32(1):11–17. doi: 10.1002/(sici)1096-911x(199901)32:1<11::aid-mpo4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Ware HE, Brooks AP, Toye R, et al. Sickle cell disease and silent avascular necrosis of the hip. J Bone Joint Surg Br. 1991;73(6):947–949. doi: 10.1302/0301-620X.73B6.1955442. [DOI] [PubMed] [Google Scholar]
- 30.Akinyoola AL, Adediran IA, Asaleye CM. Avascular necrosis of the femoral head in sickle cell disease in Nigeria: a retrospective study. Niger Postgrad Med J. 2007;14(3):217–220. [PubMed] [Google Scholar]
- 31.Mattano L. The skeletal remains: porosis and necrosis of bone in the marrow transplantation setting. Pediatr Transplant. 2003;7(Suppl):371–75. doi: 10.1034/j.1399-3046.7.s3.11.x. [DOI] [PubMed] [Google Scholar]
- 32.Mattano LA, Jr, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18(18):3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 33.Milner PF, Kraus AP, Sebes JI, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325(21):1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 34.Hernigou P, Habibi A, Bachir D, et al. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88(12):2565–2572. doi: 10.2106/JBJS.E.01455. [DOI] [PubMed] [Google Scholar]
- 35.Mahadeo KM, Oyeku S, Taragin B, et al. Increased prevalence of osteonecrosis of the femoral head in children and adolescents with sickle-cell disease. Am J Hematol. 2011;86(9):806–808. doi: 10.1002/ajh.22103. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R, Adekile AD. MRI follow-up and natural history of avascular necrosis of the femoral head in Kuwaiti children with sickle cell disease. J Pediatr Hematol Oncol. 2004;26(6):351–353. doi: 10.1097/00043426-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: A new understanding of the mechanisms of action. The Journal of Steroid Biochemistry and Molecular Biology. 2009;114(3–5):121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenzora JE, Glimcher MJ. Accumulative cell stress: the multifactorial etiology of idiopathic osteonecrosis. Orthop Clin North Am. 1985;16(4):669–679. [PubMed] [Google Scholar]
- 39.Jacobsohn DA. Acute graft-versus-host disease in children. Bone Marrow Transplant. 2008;41(2):215–221. doi: 10.1038/sj.bmt.1705885. [DOI] [PubMed] [Google Scholar]
- 40.Liu YM, Hockenberry M. Review of chronic graft-versus-host disease in children after allogeneic stem cell transplantation: nursing perspective. J Pediatr Oncol Nurs. 2011;28(1):6–15. doi: 10.1177/1043454210377177. [DOI] [PubMed] [Google Scholar]
- 41.Lu BB, Li KH. Lipoic acid prevents steroid-induced osteonecrosis in rabbits. Rheumatol Int. 2011 doi: 10.1007/s00296-011-1846-6. [DOI] [PubMed] [Google Scholar]
- 42.Neumayr LD, Aguilar C, Earles AN, et al. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J Bone Joint Surg Am. 2006;88(12):2573–2582. doi: 10.2106/JBJS.E.01454. [DOI] [PubMed] [Google Scholar]
- 43.Pontikoglou C, Deschaseaux F, Sensebe L, et al. Bone Marrow Mesenchymal Stem Cells: Biological Properties and Their Role in Hematopoiesis and Hematopoietic Stem Cell Transplantation. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 44.Hauzeur JP, Gangji V. Phases 1–3 clinical trials using adult stem cells in osteonecrosis and nonunion fractures. Stem Cells Int. 2010:2010410170. doi: 10.4061/2010/410170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirai K, Sera Y, Bulkeley W, et al. Hematopoietic stem cell origin of human fibroblasts: cell culture studies of female recipients of gender-mismatched stem cell transplantation and patients with chronic myelogenous leukemia. Exp Hematol. 2009;37(12):1464–1471. doi: 10.1016/j.exphem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005;5(4):437–442. doi: 10.1517/14712598.5.4.437. [DOI] [PubMed] [Google Scholar]
- 47.Cui Q, Botchwey EA. Emerging Ideas: Treatment of Precollapse Osteonecrosis Using Stem Cells and Growth Factors. Clin Orthop Relat Res. 2010 doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song HJ, Lan BS, Cheng B, et al. Peripheral blood stem cell transplantation for ischemic femoral head necrosis. Transplant Proc. 2010;42(5):1862–1864. doi: 10.1016/j.transproceed.2010.02.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.