Abstract
OBJECTIVE
To evaluate cost effectiveness of a socio-culturally adapted collaborative depression care program among low-income Hispanics with diabetes.
RESEARCH DESIGN AND METHODS
A randomized controlled trial of 387 diabetes patients (96.5% Hispanic) with clinically significant depression followed over 18 months evaluated the cost-effectiveness of the Multifaceted Diabetes and Depression Program (MDDP) aimed at increasing patient exposure to evidenced-based depression psychotherapy and/or pharmacotherapy in two public safety net clinics. Patient medical care costs and utilization were captured from Los Angeles County Dept. of Health Services claims records. Patient reported outcomes included SF-12 and PHQ-9-calculated depression-free days (DFDs).
RESULTS
Intervention patients had significantly greater SF-12 utility improvement from baseline compared to controls over the 18 month evaluation period (4.8%; P<.001) and a corresponding significant improvement in DFDs (43.0; P<.001). Medical cost differences were not statistically significant in OLS and log-transformed cost regressions. The average costs of the MDDP study intervention were $515 per patient. The program cost effectiveness averaged $4,053/QALY per MDDP recipient and was more than 90% likely to fall below $12,000/QALY.
CONCLUSIONS
Socio-culturally adapted collaborative depression care improved utility and quality of life in predominantly low income Hispanic diabetes patients and was highly cost effective.
Keywords: depression, Diabetes-related complications, Direct care health costs, Cost-utility analysis, randomized clinical trial
INTRODUCTION
Diabetes is the fifth leading cause of death among Hispanics and is twice as prevalent in this population as in non-Hispanic whites [1]; with Mexican American being 1.9 times more likely to have diabetes compared to non-Hispanic white adults of similar age [2]. The comorbidity of diabetes and depression is estimated to be around 25% in the elderly Mexican American population [3] and as high as 33% in Hispanic primary care samples [4,5]. Hispanics, also have greater risk of cardiovascular illness and functional disability and difficulty with diabetes management can contribute to depression [4,6]. Compared to non-Hispanic whites, Hispanics are less likely to receive guideline congruent depression care even after controlling for clinical and economic factors [7], more likely to be served by physicians who fail to detect a mental health problem when one exists [8,9] and at higher risk to discontinue antidepressant use during the first 30 days of treatment [10,11].
A randomized clinical trial implemented a health services effectiveness collaborative care model – the Multifaceted Diabetes and Depression Program (MDDP) -- aimed at increasing exposure of low income, predominantly Hispanic diabetes patients with comorbid depression to evidenced-based depression psychotherapy and/or pharmacotherapy to examine both the quality of depression care and outcomes compared to enhanced usual care [12]. As shown by Ell and colleagues [12]. MDDP intervention patients had significantly greater depression improvement compared to usual care patients. As Ell et al. reported, although there was no statistically significant improvement in glycemic control, there were significant improvements over 18 months in reported diabetes symptoms, anxiety, SF-12 emotional, physical and pain-related functioning, Sheehan disability, financial situation and number of social stressors (P=0.04 for disability and SF-12 physical, and P<0.001 for all others).
Prior studies of predominantly non-Hispanic whites have found similar depression care interventions to be highly cost effective in older adults with multiple chronic medical illnesses [13], older adults with diabetes comorbidity [14], and adults with diabetes comorbidity visiting primary care clinics of a large health maintenance organization [15]. To our knowledge this is the first research to evaluate the cost effectiveness of a randomized controlled trial depression intervention targeted at low-income Hispanic patients with diabetes comorbidity.
METHODS
As described by Ell and colleagues [12], the randomized controlled trial, approved by the University of Southern California Institutional Review Board, was conducted in Los Angeles County public community clinics. Trained bilingual study recruiters identified diabetes patients from medical charts. Study-eligible patients were ≥18 years, reported at least one of two cardinal depression symptoms (items 1 or 2 of the PHQ-9 survey) more than half the days in a two week pre-study period, and also scored ≥10 on the PHQ-9 indicating a high likelihood of clinically significant depression. Patients meeting study criteria were randomized to either enhanced usual care (EUC) or the Multifaceted Diabetes and Depression Program (MDDP). Key elements in the MDDP are based on evidence-based depression practice guidelines for primary care and are responsive to known barriers to treatment among patients in public safety net clinics. The structured stepped care algorithm 12-month intervention included: 1) Problem-Solving Therapy (PST) provided by bilingual graduate social work diabetes depression clinical specialists (DDCS) and/or antidepressant medications (AMs) prescribed by the treating primary care provider (PCP); 2) DDCS monthly telephone follow-up symptom monitoring, treatment maintenance and relapse prevention; and 3) care and service system navigation by the DDCS and an assistant patient navigator. A psychiatrist and PI (Ell) provided weekly telephone DDCS supervision and, if requested, the psychiatrist provided PCP AM telephone consultation.
EUC patients received standard clinic care and in addition were given patient and family focused depression educational pamphlets (Spanish or English) and a community, financial, social services, transportation, and child care resource list. EUC PCPs were informed of patient depression diagnoses and their study participation and could prescribe antidepressants or refer patients to community mental health care. Patients could also independently seek mental health treatment.
Data Collection
The complete set of data collection instruments are described in detail elsewhere [12]. Patients were surveyed at baseline and outcomes were reported at 6-month intervals thereafter (out to 24 months). Consistent with the prior assessment of study outcomes [12], we evaluated the cost and cost effectiveness outcomes within the 18 month follow-up evaluation period. Cost and cost effectiveness results measured out to 24 months were similar.
Depression-free days (DFD) were calculated from the Patient Health Questionnaire-9 (PHQ-9). A PHQ-9 score less than 5 meant that the patient has 1 full depression-free day and PHQ-9 score >14 meant 0 DFDs. Scores between 5 and 14 reflected linearly interpolated (0–1) depression scores between remission and major depression [16]. The PHQ-9 was used because it provides both a dichotomous diagnosis of major depression, a continuous severity score and has been found to have high sensitivity and specificity for a diagnosis of Major Depressive Disorder (MDD) based on structured psychiatric interview [17,18]. Health-related quality of life (QOL) was assessed using the MOS Short-Form Health Survey (SF-12) Physical and Mental Component Summary (PCS and MCS) fitted to the Brazier and Roberts SF-6D utility scale [19]. These utility scores were used to estimate quality-adjusted life years (QALYs) gained during the evaluation period relative to baseline.
Medical care costs and utilization were obtained from Los Angeles County Department of Health Service electronic medical services records for all study patients, based on Medicare International Classification of Diseases (ICD-9), Diagnosis Related Groups (DRG), National Drug Code (NDC) and Current Procedures Terminology (CPT-4) coding. Because County payments are confidential and also so as to make the cost analysis generalizable beyond southern California, we used 2009 Medicare prices to measure medical service costs per unit. Medicare prices (payment amounts allowed by Medicare) were attached to these medical services based on the RBRVS EZ-Fees software program that creates and analyzes physician payments using Medicare's RBRVS (Resource Based Relative Value Scale) for all services except pharmaceuticals [20]. Since the Medicare outpatient drug program (Medicare Part D) wasn’t implemented until after the study was initiated, drug prices were obtained from the 2009 Federal Supply Schedule price list [21]. Since the same 2009 prices were assigned to all medical services, regardless of time period, medical cost inflation was not relevant to the cost estimates.
Intervention costs were measured as actual budget-based cost (not charges) for all diabetes depression clinical specialists (DDCS) and patient navigator services, using actual salary plus a 32% fringe benefit. Resulting unit costs were $71 per patient visit (90 minutes), $35 per DDCS telephone follow-up (45 minutes), and $10 for each patient navigation call (10–15 minutes). Estimates included record keeping time. Additional costs included $10 for relaxation videotape, $136 per patient for DDCS communication with PCP, and $21 per patient for clinical supervision.
Statistical Methods
The key outcomes of interest for the cost effectiveness analysis were medical and intervention costs, depression free days and SF-12 utilities. We conducted the primary cost effectiveness analysis in terms of cost per QALY from a payer perspective, with additional consideration of the overall impacts on medical costs, QOL and DFDs.
Intent-to-treat analysis was conducted to evaluate all intervention effects. Differences-in-differences regression models were estimated to evaluate systematic cost and utilization differences between EUC and MDDP at 6-, 12- and 18-month follow-up [22,23]. The differences-in-differences regression analysis method is a powerful method for adjusting for any individual-specific unobservable factors that are time-invariant and account for variation in the outcomes.
This is demonstrated in the following equation specification. Suppose that we are interested in the regression specification for an outcome Oit, where i is the subscript for individual i and t is the subscript for time period t (Oi0 represents outcomes measured at the pre-intervention baseline for individual i).
Suppose that we have a (1 × J) vector of J observable exogenous characteristics Xit, with the j’th characteristic Xijt for individual i at time t. Supposed that there is an additional (1 × K) vector of K time-invariant unobservable individual-level exogenous characteristics Ii (e.g., underlying health, personal attitudes and/or behaviors, personality traits, aptitudes, background, etc.) with the k’th unobservable characteristic Iik. Let eit represent the residual random error for each individual i at each time point t.
Then we can write the panel data regression specification for Oit as:
| [1] |
| [2] |
γ is the treatment effect parameter and ξ1, ξ2 capture a (quadratic) time trend.
We can combine equations 1 and 2 into the differencing estimation equation:
| (3) |
Where e*it = (eit - ei0). Using this differencing specification in equation [3], the β0 and δ parameters for all baseline exogenous characteristics (both observed and unobserved), which are unnecessary for estimating treatment effects, are netted out of the final estimation equation.
Because the cost distribution was skewed, in addition to a standard cost regression specification we also used a log-normal cost distribution estimation equation. Tests of heteroskedasticity across treatment groups were not significant, implying that Duan smearing estimates for retransformation bias were not necessary [24]. A Park Test for alternative generalized linear model specifications failed to reject the log-normal error specification for the cost regression [25].
As previously shown by Ell and colleagues [12] there was a lack of balance in treatment assignment between the EUC and MDDP treatment groups, with many baseline characteristics being statistically significantly different across treatment assignment despite randomization. In order to adjust for any potential observable variable confounding between treatment assignment and cost or other outcome variables we present both ordinary least squares (OLS) and propensity score-adjusted regression estimates [26]. The propensity score used was the predicted probability of treatment assignment from a logistic regression of actual treatment assignment on all available observed patient baseline characteristics. These baseline values of exogenous factors that were significantly associated with treatment assignment included age, gender, Latino ethnicity, foreign born, residing in the country more than ten years, married, primarily Spanish speaking, less than high school education, unemployed, # economic stressors, history of major depressive disorder, dysthymia, chronic pain, taking medications for pain, chronic disease score, # diabetes complications, treatment clinic site. Since treatment assignment in the trial was not balanced on observable baseline factors, it is appropriate to use a regression method that captures and adjusts for variation in baseline observable factors.
RESULTS
Table 1 provides the average 6–18 month difference-from-baseline cost comparisons between the control and MDDP intervention patients relative to the six month baseline period prior to study implementation for total medical costs along with the cost subcategories of medications, laboratory tests, emergency department, outpatient, inpatient and miscellaneous/other. While there is a trend for many of the cost category savings from baseline to be greater in the MDDP intervention group, this result was only statistically significant for the medication cost category (P = 0.007; 95% CI −$64.44 to −$410.39) and was offset by higher miscellaneous/other costs in the intervention group. Antidepressant medication use was significantly higher in the MDDP intervention group.
Table 1.
Medical Cost Category Average Differences from Baseline†
| Mean 6-Month Unadjusted Cost Differences From Baseline |
95% Confidence Interval for EUC-MDDP Difference-in-Difference |
P-Value | |||
|---|---|---|---|---|---|
| EUC | MDDP | Lower | Upper | ||
| Medications | $179.89 | −$57.53 | $64.44 | $410.39 | 0.007 |
| Laboratory | −$51.86 | −$54.54 | −$22.87 | $28.22 | 0.837 |
| Emergency Department | −$8.46 | $7.78 | −$35.55 | $3.06 | 0.099 |
| Outpatient | −$65.60 | $12.56 | −$184.26 | $27.96 | 0.149 |
| Inpatient | $35.15 | −$273.31 | −$78.96 | $695.87 | 0.119 |
| Miscellaneous/Other* | −$171.43 | $49.74 | −$395.22 | −$47.13 | 0.013 |
| Total | −$82.31 | −$315.29 | −$194.92 | $660.88 | 0.286 |
Miscellaneous/Other includes home care, durable medical equipment and additional medical costs not otherwise specified.
Cost results are averaged across the three 6–18 month study periods. There were no significant within-period differences.
As shown in the Table 2 looking at differences-in-differences from baseline, none of the medical cost regressions showed a significant change in costs, whether propensity score adjusted or not and whether log-transformed or not. The MDDP intervention variable was statistically insignificant in all cases (Table 2).
Table 2.
Medical Cost Regression Estimates
| Dependent Variable | Medical Costs | Natural Log of Medical Costs |
||
|---|---|---|---|---|
| Independent Variables |
Estimated Coefficient (P-value) |
Estimated Coefficient (P-value) |
||
| PS* | OLS | PS* | OLS | |
| (Constant) | 395.99 (.755) |
140.91 (.912) |
−.363 (.387) |
−.358 (.391) |
| Age | 26.62 (.089) |
22.82 (.144) |
.015 (.003) |
.015 (.003) |
| Male | 70.12 (.838) |
−30.10 (.930) |
.044 (.700) |
.045 (.689) |
| # of Diabetes Complications |
−52.90 (.670) |
−101.54 (.409) |
.081 (.046) |
.082 (.042) |
| # Economic Stressors | 81.66 (.201) |
58.44 (.356) |
−.008 (.701) |
−.008 (.710) |
| Chronic Disease Score | −18.01 (.688) |
−29.92 (.505) |
−.018 (.236) |
−.018 (.239) |
| Time | −187.08 (.296) |
−189.61 (.291) |
−.082 (.166) |
−.082 (.166) |
| Time Squared | 6.76 (.361) |
6.83 (.358) |
.003 (.276) |
.003 (.277) |
| MDDP Treatment | −32.85 (.903) |
−291.02 (.246) |
.026 (.772) |
.030 (.718) |
| Propensity Score | −1693.64 (.010) |
.027 (.903) |
||
Propensity score estimated using predicted logistic probability of treatment assignment as a correction for observed confounding using baseline predictors (see text).
As shown in Table 3, the MDDP intervention was associated with a significant increase in utility, as measured on the predicted SF-12 utility scale (0.048; P < 0.001; 95% CI 0.028 to 0.068) in the propensity score regression specification. This utility gain translated into an average 0.13 increased QALYs for the MDDP group relative to the control group over the 18 month evaluation period (net of the baseline difference-in-difference values). The depression-free days regressions showed a highly significant improvement in DFDs for the treatment group (Table 3).
Table 3.
Utility and Depression Free Days Regression Estimates
| Dependent Variable | SF-12 Utility |
Depression Free Days |
||
|---|---|---|---|---|
| Independent Variables | Estimated Coefficient (P-value) |
Estimated Coefficient (P-value) |
||
| PS* | OLS | PS* | OLS | |
| (Constant) | .710 (.000) |
.715 (.000) |
−12.62 (.688) |
−22.89 (.469) |
| Age | −.001 (.068) |
−.001 (.089) |
.64 (.102) |
0.48 (.217) |
| Male | .002 (.888) |
.004 (.762) |
−5.89 (.489) |
−9.93 (.245) |
| # of Diabetes Complications | −.019 (.000) |
−.018 (.000) |
−16.92 (.000) |
−18.87 (.000) |
| # Economic Stressors | −.012 (.000) |
−.012 (.000) |
−12.57 (.000) |
−13.51 (.000) |
| Chronic Disease Score | .000 (.902) |
.000 (.788) |
.11 (.921) |
−0.37 (.741) |
| Time | .003 (.603) |
.003 (.597) |
19.25 (.000) |
19.15 (.000) |
| Time Squared | .000 (.700) |
.000 (.696) |
−.03 (.870) |
−0.02 (.883) |
| MDDP Treatment | .048 (.000) |
.053 (.000) |
42.98 (.000) |
32.57 (.000) |
| Propensity Score | .033 (.157) |
−68.26 (.000) |
||
Propensity score regression estimated using predicted logistic probability of treatment assignment as a correction for observed confounding based on baseline predictors (see text).
The average MDDP intervention cost per patient was $515 (95% CI: $469 to $561) (Table 4). Since the cost regressions showed no significant differences in medical treatment costs, we computed the MDDP program cost effectiveness under an assumption of no medical cost savings from the MDDP intervention and including the additional study intervention costs per MDDP patient as captured in the patient study logs. As shown in Table 4, under this assumption the average incremental cost per QALY for the MDDP intervention ([CostMDDP – CostEUC] / [QALYMDDP – QALYEUC]) was $4,053.
Table 4.
Base Case Cost/QALY Estimates*
| Control | MDDP Intervention |
Incremental Cost Effectiveness |
|
|---|---|---|---|
| QALYs Gained | 0.92 | 1.05 | 0.13 |
| MDDP Costs | $0.00 | $515 | $515 |
| Cost/QALY | $4,053 |
All estimates computed relative to baseline.
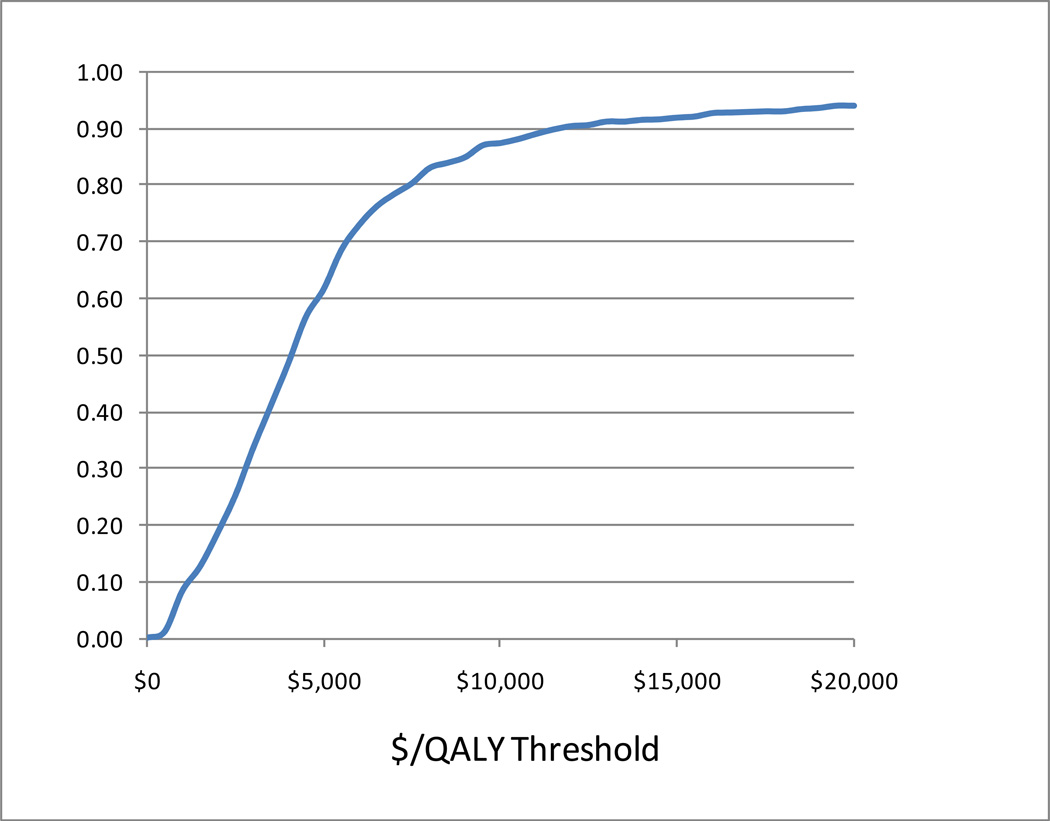
As shown in Figure 1, to capture sampling uncertainty in our cost/QALY estimates using the non-parametric bootstrap method [27], we generated a cost effectiveness acceptability curve from the individual-specific pairs of costs and QALYs for the study subjects, again under the assumption that there were no medical cost savings from the MDDP intervention. This cost effectiveness acceptability curve showed that there was more than a 50% probability that the MDDP intervention was cost effective at a threshold willingness-to-pay of $5,000 per quality adjusted life year and more than a 90% probability that the MDDP intervention was cost effectiveness at a willingness-to-pay threshold of $12,000 per QALY.
Figure 1.
Cost Effectiveness Acceptability Curve.
COMMENT
To our knowledge this is the first economic evaluation of a randomized controlled trial of collaborative depression care for predominantly low income Hispanic patients with diabetes in public safety net clinics. The findings suggest that a collaborative depression care model, socio-culturally adapted for low-income patients, resulted in significant improvements in quality of life compared to enhanced usual care. The intervention was cost effective, with a conservatively estimated cost per QALY below $5,000 and more than 90% likely to fall below $12,000 per QALY. These cost effectiveness results demonstrated that the MDDP intervention was highly cost effective under conventional cost effectiveness value guidelines reported in the literature [28-32]. The results were well within the highly favorable range compared to widely-accepted medical interventions (www.CEAregistry.org) and well within cost effectiveness value of life thresholds of $100,000-$150,000 per QALY reported in the economic literature for U.S. estimates [28-32].
Improving depression symptoms in patients with diabetes in prior collaborative care studies has been shown to be associated with a high probability of achieving savings in total ambulatory medical costs in comparisons with usual primary care [33]. In that study, the higher costs associated with providing enhanced mental health care were offset by greater savings in medical costs [33]. Black and colleagues [34] found in a large longitudinal study of an aging Hispanic population in the southwestern US that depression markedly increases the risk in patients with diabetes of macro- and microvascular complications, incident physical disability and mortality. Several other studies in diverse populations have also confirmed that comorbid depression increased risk in patients with diabetes of microvascular and macrovascular complications and mortality [13,35].
Our cost regression models did not find that the MDDP intervention was associated with significant medical cost savings, so we did not incorporate medical cost savings into our base case cost effectiveness results. Thus while the MDDP intervention was found to be highly cost effective, further studies are needed to ascertain whether improving outcomes of depression in patients with diabetes would decrease medical costs in this population. Longer term studies would also be needed to establish the impacts of such interventions on changes in patient disability, disease complications and overall survival.
Limitations
Certain study limitations are discussed in the prior publication [5]. The main study limitation relevant to our cost effectiveness results was the statistically significant imbalance of the MDDP and EUC study groups at baseline randomization. We thoroughly investigated all potential causes for this randomization imbalance, and even though it is highly unlikely to have happened by chance, we have no explanation for why so many baseline observable characteristics were significantly different between the MDDP intervention and control groups.
This randomization imbalance necessitated exploration of alternative propensity score adjusted regression estimation methods to control for potential treatment assignment bias. The propensity score that we used for treatment assignment deals with the appropriate source of observable confounding. While our findings, however, were robust to alternative regression specifications based on available study variables, we can’t claim the same degree of robustness for these results as we could have if the baseline randomization had succeeded in balancing the study groups on all observable confounders.
Finally, the estimated MDDP intervention costs did not explicitly include facility, space, or other administrative overhead expenses. However, given that the mean wage for mental health social workers in California in May 2009 was $22.28/hour (http://www.bls.gov/oes/current/oes_ca.htm#21-0000), these intervention cost estimates would conservatively include an approximate 40% margin for such overhead costs.
CONCLUSION
Socioculturally and organizationally adapted collaborative care is highly cost effective in improving quality of life outcomes in a low-income, predominantly Hispanic population in safety net clinics.
ACKNOWLEDGMENTS
We acknowledge the contributions of Dr. Michael Roybal and Dr. Stanley Leong clinic medical directors, their staff and participating patients. We appreciate helpful questions and comments from the anonymous reviewers.
Source of financial support: The study is supported by R01 MH068468 from the National Institute of Mental Health (PI, Dr. Ell). Trial Registration NCT00709150, Clinicaltrials.gov/ct/gui.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Center for Disease Control and Prevention, Health disparities experienced by Hispanics: United States. Morb Mortal Wkly Rep. 2004;53:935–937. [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: Department of Health and Human Services (DHHS) ; 2010. [Accessed July 11, 2011]. Table 51. Publication No. 2010-1232. Available at: http://www.cdc.gov/nchs/data/hus/hus09.pdf. [PubMed] [Google Scholar]
- 3.Black SA, Ray LA, Markides KS. The prevalence and health burden of self-reported diabetes in older Mexican Americans: findings from the Hispanic established populations for epidemiologic studies of the elderly. Am J Public Health. 1999;89:546–552. doi: 10.2105/ajph.89.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross R, Olfson M, Gameroff MJ, et al. Depression and glycemic control in Hispanic primary care patients with diabetes. J Gen Intern Med. 2005;20:460–466. doi: 10.1111/j.1525-1497.2005.30003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Ford ES, Strine TW, Mokdad AH. Prevalence of depression among U.S. adults with diabetes: findings from the 2006 behavioral risk factor surveillance system. Diabetes Care. 2008;31:105–107. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- 6.Cabassa LJ, Hansen MC, Palinkas LA, Ell K. Azúcar y Nervios: Explanatory models and treatment experiences of Hispanics with diabetes and depression. Soc Sci & Med. 2008;66:2413–2424. doi: 10.1016/j.socscimed.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Department of Health and Human Services. A report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services; 2001. Mental health: Culture, race, and ethnicity: A supplement to mental health. [PubMed] [Google Scholar]
- 8.Borowsky SJ, Rubenstein LV, Meredith LS, et al. Who is at risk of nondetection of mental health problems in primary care? J Gen Intern Med. 2000;15:381–388. doi: 10.1046/j.1525-1497.2000.12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez HP, Chen J, Rodriguez MA. A national study of problematic care experiences among Latinos with diabetes. J Health Care Poor Underserved. 2010;21:1152–1168. doi: 10.1353/hpu.2010.0923. [DOI] [PubMed] [Google Scholar]
- 10.Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006;163:101–108. doi: 10.1176/appi.ajp.163.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Pinto-Meza A, Fernandez A, Serrano-Blanco A, Haro JM. Adequacy of antidepressant treatment in Spanish primary care: a naturalistic six-month follow-up study. Psychiatr Serv. 2008;59:78–83. doi: 10.1176/ps.2008.59.1.78. [DOI] [PubMed] [Google Scholar]
- 12.Ell K, Katon W, Xie B, et al. Collaborative care management of major depression among low-income, predominantly Hispanics with diabetes: a randomized controlled trial. Diabetes Care. 2010;33:706–713. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with Type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 14.Katon WJ, Unűtzer J, Fan M-Y, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 15.Simon GE, Katon WJ, Lin EHB, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64:65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Vannoy SD, Arean P, Unűtzer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatric Services. 2010;61:160–163. doi: 10.1176/appi.ps.61.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psych. 2007;29:388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Huang FY, Chung H, Kroenke K, et al. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed June, 2009];RBRVS EZ-Fees. 2011 http://www.rbrvs.net/
- 21. [Accessed June, 2009];United States Department of Veterans Affairs. http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx.
- 22.Myer B. Natural and Quasi-Experiments in Economics. J Bus Econ Stat. 1995;13:151–161. [Google Scholar]
- 23.Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates?". Quarterly J Econ. 2004;119:249–275. [Google Scholar]
- 24.Manning WG. The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ. 1998;17:283–295. doi: 10.1016/s0167-6296(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 25.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 27.Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting the results of cost effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 1. 2001:89–100. doi: 10.1586/14737167.1.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Hay JW. Using pharmacoeconomics to value pharmacotherapy. Clin Pharmacol Therapeutics. 2008;84:197–200. doi: 10.1038/clpt.2008.124. [DOI] [PubMed] [Google Scholar]
- 29.Murphy KM, Topel RH, editors. Measuring the Gains from Medical Research: An Economic Approach. Chicago, IL: University of Chicago Press; 2003. [Google Scholar]
- 30.Nordhaus W. NBER Working Paper Series 8818. Cambridge, MA: National Bureau of Economic Research; 2002. The Health of Nations: The Contribution of Improved Health to Living Standards. [Google Scholar]
- 31.Jeffrey D, Sachs, et al. Report of the Commission on Macroeconomics and Health. World Health Organization; 2001. Dec, Macroeconomics and Health: Investing in Health for Economic Development. [Google Scholar]
- 32.Viscusi WK. The Value Of Life: Estimates With Risks By Occupation And Industry. ISSN Discussion Paper Series No. 422 05/2003 [Google Scholar]
- 33.Katon WJ, Russo JE, Von Korff M, et al. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31:1155–1159. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 35.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33:264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]