Synopsis
Successful resuscitation from cardiac arrest requires reestablishment of aerobic metabolism by reperfusion with oxygenated blood of tissues that have been deprived of oxygen for variables periods of time. However, reperfusion concomitantly activates pathogenic mechanisms known as “reperfusion injury.” At the core of reperfusion injury are mitochondria, playing a critical role as effectors and targets of such injury. Mitochondrial injury compromises oxidative phosphorylation and also prompts release of cytochrome c to the cytosol and bloodstream where it correlates with severity of injury. Main drivers of such injury include Ca2+ overload and oxidative stress. Preclinical work shows that limiting myocardial cytosolic Na+ overload at the time of reperfusion attenuates mitochondrial Ca2+ overload and maintains oxidative phosphorylation yielding functional myocardial benefits that include preservation of left ventricular distensibility. Preservation of left ventricular distensibility enables hemodynamically more effective chest compression. Similar myocardial effect have been reported using erythropoietin hypothesized to protect mitochondrial bioenergetic function presumably through activation of pathways similar to those activated during preconditioning. Incorporation of novel and clinical relevant strategies to protect mitochondrial bioenergetic function are expected to attenuate injury at the time of reperfusion and enhance organ viability ultimately improving resuscitation and survival from cardiac arrest.
Keywords: Cardiopulmonary resuscitation, Energy metabolism, Erythropoietin, Ischemia, Mitochondria, Myocardium, Reperfusion injury, Sodium hydrogen antiporter, Ventricular function
More than 90% of individuals who suffer an episode of out-of-hospital sudden cardiac arrest cannot be resuscitated using current CPR techniques despite a large, sustained, coordinated, and costly public health effort that involves the community, emergency medical services, hospitals, and scientific institutions implementing evidence-based guidelines for resuscitation. The number of such victims is staggering totaling >150,000 every year in the United States and many more worldwide.
Main barriers to improving survival after cardiac arrest include: (1) the extremely narrow time window of only a few minutes available after cardiac arrest supervenes for deploying current resuscitation techniques before they become ineffective, (2) the limited hemodynamic capability of current resuscitation techniques to promote the levels of blood flow required to reverse ischemia of critical organs, and (3) the tissue injury – known as reperfusion injury – that stems from uncontrolled reintroduction of oxygen after ischemia. These are critical barriers which drive current resuscitation paradigms forcing efforts to focus on minimizing delays in resuscitation at the scene and on devising approaches to augment the hemodynamic efficacy of the resuscitation efforts. Efforts to overcome the first two barriers coupled with improved post-resuscitation care – including hypothermia in unresponsive victims, percutaneous coronary interventions when suspected coronary etiology, and dedicated post-resuscitation critical care – have resulted in encouraging but modest increases in survival in recent years.
Regarding reperfusion injury, growing basic and translational research supports that reperfusion injury can be attenuated through pharmacological and non-pharmacological interventions resulting in improved resuscitation outcomes. Main contributors to reperfusion injury include Ca2+ overload1,2 and generation of reactive oxygen species3 which exert injury mostly through compromising mitochondrial function. Recent studies aimed at examining the effects of protecting mitochondria from reperfusion injury during cardiac resuscitation indicate that preservation of mitochondrial bioenergetic function in the myocardium helps restoration of cardiac activity and sustained post-resuscitation circulation.
In this article, we first provide a brief overview of mitochondria pertinent to their role in resuscitation followed by a discussion of myocardial abnormalities that occur during cardiac resuscitation and which could be minimized by interventions protecting mitochondrial bioenergetic function. These interventions represent work – mostly from our Resuscitation Institute – using inhibitors of the sodium-hydrogen exchanger isoform-1 (NHE-1) and erythropoietin.
MITOCHONDRIAL ANATOMY, FUNCTION, AND DYSFUNCTION
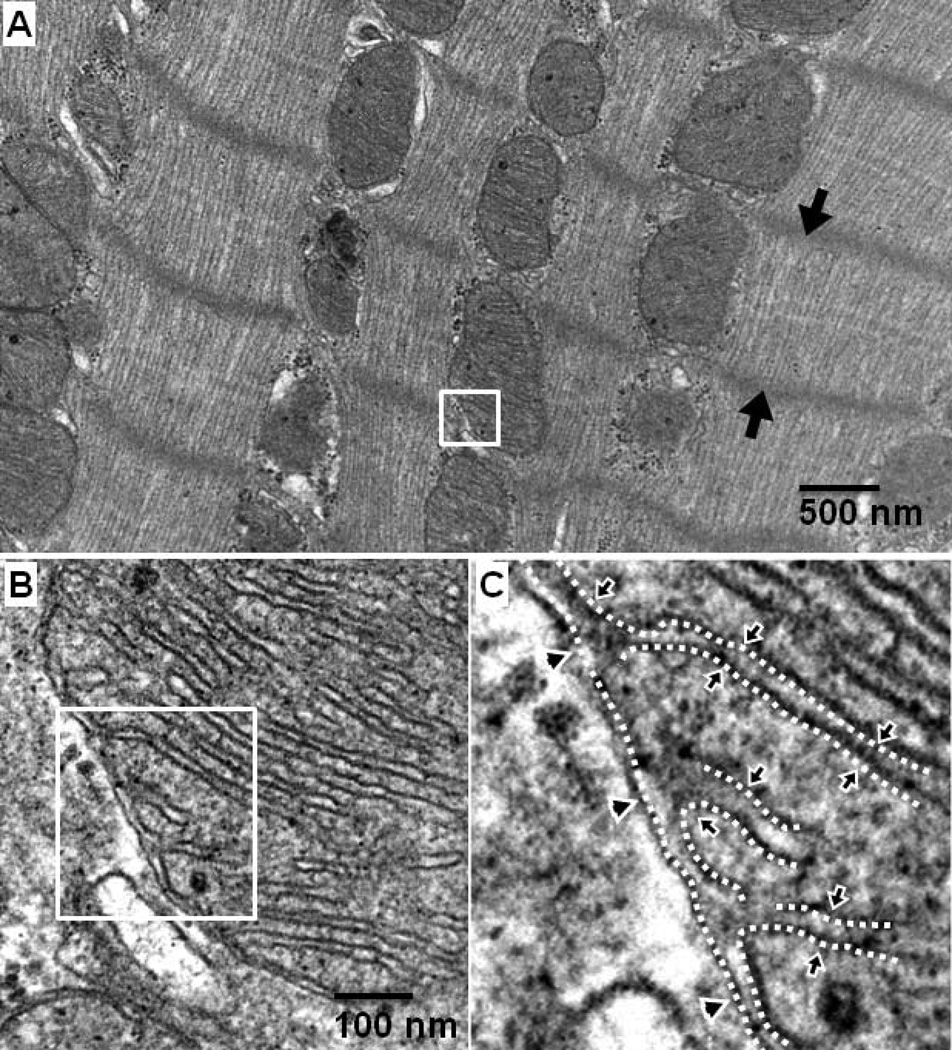
Consistent with their critical role in aerobic energy production, mitochondria are particularly abundant in tissues that have high metabolic activity. In the heart, approximately 35% of the cardiomyocyte volume is comprised of mitochondria which are “strategically” arranged in close proximity to the sarcomeres in a “crystal-like” structure with one mitochondrion per sarcomere4 (Figure 1A). Each mitochondrion has an outer and an inner membrane delimiting key functional components (Figure 1B and 1C). The outer mitochondrial membrane is highly porous and provides the outer boundary of mitochondria. The inner mitochondrial membrane is highly tight and folds inwardly forming convoluted loops known as cristae. These cristae enclose a space known as the intracristae space which communicates with the intermembrane space through bottle-neck like junctions.5,6 The space contained within the inner mitochondrial membrane is the matrix.
Figure 1.
Electron microscope photograph of left ventricular mitochondria isolated from a Sprague-Dawley retired breeder rat at the Resuscitation Institute. A: Intermyofibrillar mitochondria orderly organized within the zone demarcated by two Z lines (black arrow heads); the structure where adjacent sarcomeres connect and thin filaments anchor. B and C: Higher magnification of section in mitochondrion selected in panel A showing the outer mitochondrial membrane (arrow heads) and the inner mitochondrial membrane folding inside the mitochondrial matrix.
ENERGY METABOLISM
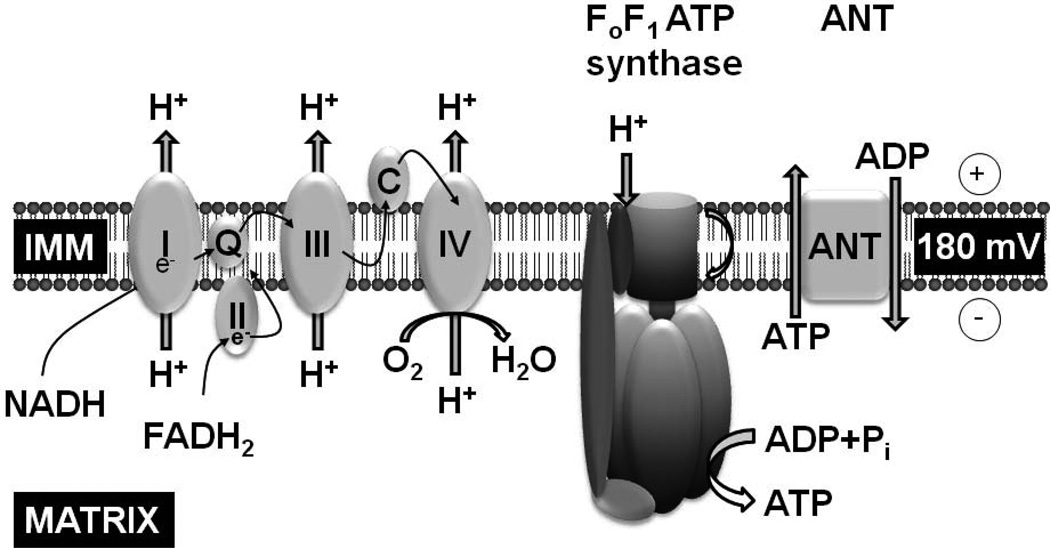
The primary function of mitochondria is the generation of ATP through oxidative phosphorylation. The process starts with reduction of nicotinamide adenine dinucleotide (NAD+) to NADH and flavin adenine dinucleotide (FAD) to FADH2 during oxidation of pyruvate and citric acid cycle substrates. NADH and FADH2 become oxidized again by transferring their electrons down a redox potential through complexes I, II, III, and IV of the electron transport chain (Figure 2). The energy generated is used by complexes I, III, and IV – which are indeed proton pumps embedded in the inner mitochondrial membrane – to translocate H+ from the matrix to outside side the inner mitochondrial membrane against an electrochemical gradient creating the proton motive force required for FoF1 ATPase to synthesize ATP from ADP and inorganic phosphate in the mitochondrial matrix. The newly synthesized ATP is then exchanged for ADP through the inner mitochondrial membrane by the adenine nucleotide translocator (ANT) and is used to phosphorylate creatine into phosphocreatine which is then exported outside the mitochondria to power energy requiring process.
Figure 2.
Schematic rendition of key mitochondrial components involved in ATP synthesis via oxidative phosphorylation. IMM, inner mitochondrial membrane; I, II, III, and IV, respiratory chain complexes; e−, electrons; Q, coenzyme Q; C, cytochrome c; ANT, adenine nucleotide translocator.
Oxygen plays the essential role in energy generation as the final acceptor of electrons from the electron transport chain. Interruption in oxygen delivery (e.g., after onset of cardiac arrest) halts electron flow precluding generation of the proton motive force required for ATP synthesis. Cells can still generate ATP “anaerobically” but at a much lower rate that is not sufficient to meet metabolic demands. ATP is generated anaerobically through glycolysis having lactate as final product which diffuses outside the cell and can be readily measured and used as marker of ischemia and its reversal. ATP can also be regenerated anaerobically from cellular stores of phosphocreatine but this is a non-regenerative mechanism and is rapidly exhausted. Accordingly, after cardiac arrest an intense energy deficit develops in metabolically active organs; i.e., heart and brain, which precludes maintaining their function. Cardiopulmonary resuscitation becomes the means to reoxygenate and restore mitochondrial bioenergetic function. However, as previously stated, reoxygenation is accompanied by reperfusion injury that, in turn, may compromise mitochondrial bioenergetic function. Much of the work discussed in this article provides evidence supporting the concept that resuscitation can be facilitated by protecting mitochondrial bioenergetic function.
CYTOCHROME C RELEASE AS MARKER OF MITOCHONDRIAL INJURY
This brief mitochondrial synopsis, however, would not be complete if the role of mitochondria in modulating cell viability and eventual cell death is not addressed. It is now well-established that mitochondria can signal cell death through the release of various pro-apoptotic proteins, including cytochrome c, apoptosis-inducing factor, Smac/DIABLO, endonuclease G, and a serine protease Omi/HtrA2.7,8 Of these proteins, cytochrome c has been the most widely investigated.
Cytochrome c is a 14 kDa hemoprotein normally present in the intracristae space and the intermembrane space attached to the inner mitochondrial membrane loosely bound to cardiolipin (Figure 2). Cytochrome c plays a key physiological role enabling electron transfer from complex III to complex IV. However, cytochrome c through various pathological mechanisms can be released to the cytosol including ultraviolet irradiation,9 serum deprivation,10 growth factor withdrawal,10–12 and also conditions present during ischemia and reperfusion such as Ca2+ overload,13 hypoxia,14 and generation of reactive oxygen species.15
In our laboratory, we reported using a rat model of VF that cytochrome c is released to the cytosol after resuscitation from cardiac arrest where it activates the “intrinsic” or “mitochondrial” apoptotic pathway through formation of an oligomeric complex known as the apoptosome.16 The apoptosome activates caspase-9 which, in turn, activates downstream executioner caspases 3, 6, and 7.17 Activation of these executioner caspases can lead to apoptotic cell death.18 However, in our rat model activation of the mitochondrial apoptotic pathway did not cause cell death or was responsible for the severe myocardial dysfunction that occurs post-resuscitation, at least within the initial four hours after return of spontaneous circulation. 19,20
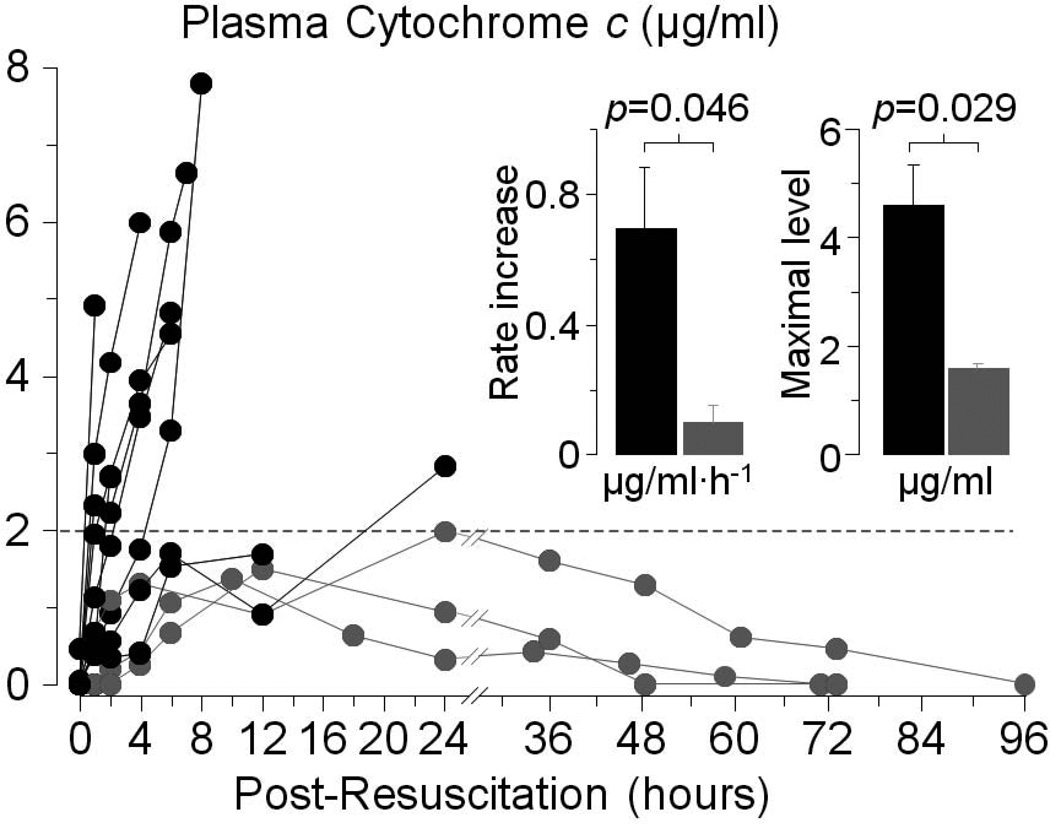
Cytochrome c can also “leak” into the bloodstream under conditions associated with mitochondrial injury such as chemotherapy,21,22 acute myocardial infarction,23 the systemic inflammatory response syndrome,24 and influenza-associated encephalopathy.25,26 Studies in our laboratory further demonstrate that cytochrome c can be also released to the bloodstream after resuscitation from cardiac arrest.19 In these studies, plasma cytochrome c was serially measured using reverse phase high performance liquid chromatography (HPLC) in rats successfully resuscitated from VF. In survivors, plasma cytochrome c gradually increased to levels that did not exceed 2 µg/ml returning to baseline within 48 to 96 hours. In non-survivors, cytochrome c increased at a faster rate and attained levels that substantially exceeded those observed in survivors without reversal before demise from cardiovascular dysfunction (Figure 3). These observation support the idea that plasma cytochrome c could be a marker of mitochondrial injury and be used to assess the effect of interventions designed to protect mitochondria during cardiac resuscitation.
Figure 3.
Serial measurements of plasma cytochrome c by reverse-phase high performance liquid chromatography in rats successfully resuscitated after 8 minutes of untreated ventricular fibrillation. Measurements were made until cytochrome c levels had returned to baseline or the rat had died. Gray symbols represent survivors (n = 3); black symbols represent non-survivors (n = 9) (Adapted from Ayoub et al. Crit Care Med 2008;36:S440).
Two main mechanisms have been proposed to explain cytochrome c release from mitochondria; namely, opening of the mitochondrial permeability transition pore (mPTP) and selective permeabilization of the outer mitochondrial membrane. mPTP opening is typically triggered by abnormalities central to ischemia and reperfusion injury including Ca2+ overload, production of reactive oxygen species, depletion of ATP and ADP, and increases in inorganic phosphate.27 mPTP opening allows molecules up to 1500 Da to enter the mitochondrial matrix along with water and solutes causing mitochondrial swelling, unfolding of the inner mitochondrial membrane cristae, and disrupting the outer mitochondrial membrane ultimately precipitating cytochrome c release to the cytosol.27,28 mPTP opening also causes collapse of the electrochemical gradient across the inner mitochondrial membrane uncoupling oxidative phosphorylation. In addition, release of cytochrome c could also occur through selective permeabilization of the outer mitochondrial membrane associated with oligomerization and formation of channel-like structures by proteins of the B-cell lymphoma-2 (Bcl-2) family.29,30 Release of cytochrome c is further facilitated during ischemia and reperfusion by peroxidation of cardiolipin consequent to mitochondrial Ca2+ overload and production of reactive oxygen species.31–33 Cardiolipin is the principal lipid constituent of the inner mitochondrial membrane and to which a fraction of cytochrome c is bound.
MYOCARDIAL ABNORMALITIES DURING CARDIAC RESUSCITATION
The working heart is a highly metabolic organ that under normal resting conditions extracts nearly 70% of the oxygen supplied by the coronary circulation34,35 representing close to 10% of the total body oxygen consumption. However, the heart has minimal capability for extracting additional oxygen such that increases in metabolic demands can only be met by autoregulatory increases in coronary blood flow through vasodilation of the coronary circuit.36 Consequently, a severe energy imbalance develops when cardiac arrest occurs and coronary blood flow ceases. The severe energy imbalance continues during the ensuing resuscitation effort when current closed-chest resuscitation techniques are used because of the very limited capability for generating systemic and coronary blood flow.37
The magnitude of the energy imbalance is contingent on the metabolic requirements and is particularly severe in the presence of VF when the oxygen requirements are comparable to or exceed those of the beating heart.38,39 A lesser energy deficit is expected during cardiac arrest with a quiescent or minimally active heart (i.e., asystole or pulseless electrical activity precipitated by asphyxia or exsanguination).
Various functional myocardial abnormalities develop during cardiac arrest and resuscitation that are detrimental to cardiac resuscitation. These abnormalities include reductions in left ventricular distensibility during the resuscitation effort followed by reperfusion arrhythmias and post-resuscitation myocardial dysfunction.
LEFT VENTRICULAR DISTENSIBILITY
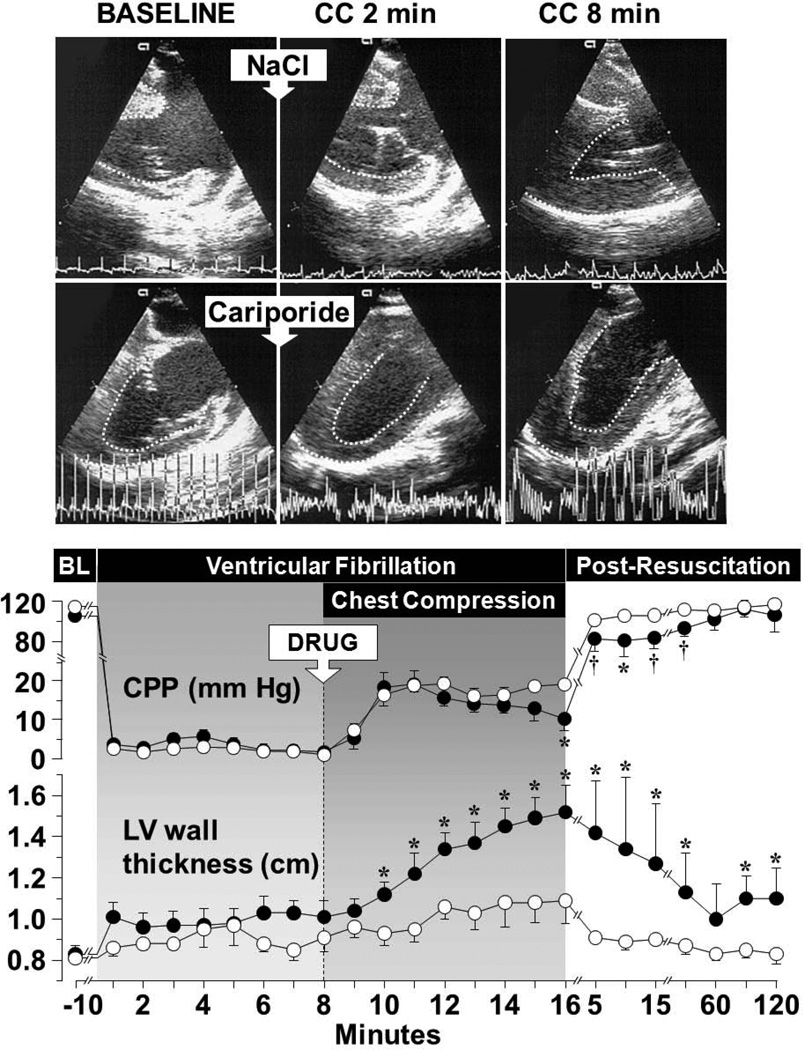
Studies in various animal models of VF and resuscitation have shown progressive thickening of left ventricular wall accompanied by parallel reductions in left ventricular cavity without changes in intracavitary pressures during cardiac resuscitation.41,42 A functionally similar phenomenon known as ischemic contracture was reported in the early seventies during open heart surgery when operations were conducted under normothermic conditions in fibrillating hearts43,44 and more recently after prolonged intervals of untreated VF.45 However, ischemic contracture is associated with profound reductions in myocardial ATP and often leads to a “stony heart” heralding irreversible ischemic injury.46 Reductions in left ventricular distensibility observed during cardiac resuscitation is a different phenomenon: (1) it occurs much earlier than the “stony heart” starting coincident with reperfusion during the resuscitation effort,41,42 (2) it is associated with less ATP depletion,40 (3) it has been attributed to myocardial energy deficit compounded by cytosolic and mitochondrial Ca2+ overload precluding complete relaxation of individual cardiomyocytes, (4) it evolves into diastolic dysfunction upon return of spontaneous circulation,47 (5) it is largely reversible, 48 and (6) it is amenable to therapeutic intervention (Figure 4).
Figure 4.
The upper panel shows left ventricular (LV) wall thickening in a control pig (upper frames) but not in a pig treated with cariporide (lower frames). The images were obtained by transesophageal echocardiography at the end of mechanical diastole (baseline, BL) and at the end of “compression diastole” at 2 and 8 minutes of chest compression (CC). The endocardial border was delineated to facilitate visualization. The lower panel shows progressive decreases in the coronary perfusion pressure (CPP) coincident with progressive thickening of the left ventricular wall in pigs that received 0.9 % NaCl (closed symbols, n = 8) but not in pigs that received cariporide (open symbols, n = 8). NaCl or cariporide (drug, 3 mg/kg) was given immediately before starting chest compression. Mean ± SEM. *p < 0.05, †p < 0.001 vs cariporide by one-way ANOVA (Adapted from Ayoub I et al. Circulation 2003;107:1804).
Reductions in left ventricular distensibility adversely affect the ability of chest compression to generate forward blood flow. As blood returns to the heart during the relaxation phase of chest compression, distensible ventricles are important to properly accommodate the returning blood and establish an adequate preload for the subsequent compression. Progressive reductions in left ventricular distensibility during chest compression contribute to progressive reduction in the hemodynamic efficacy of closed-chest resuscitation. Studies in a porcine model of VF have shown that the severity of this phenomenon is proportional to the duration of untreated VF.41
Work in our laboratory demonstrates that reductions in left ventricular distensibility can be prevented by pharmacologic interventions targeting reperfusion injury leading to hemodynamically more stable closed-chest resuscitation as discuss in the subsequent sections.42,49
In humans, Takino and Okada50 reported on 59 adult patients who suffered non-traumatic out-of-hospital cardiac arrest and underwent open-chest direct manual cardiac compression in the emergency department after failure of closed-chest resuscitation. A “firm” myocardium was noticed during manual cardiac compression in 36 cases affecting predominantly the left ventricle. In the remaining 23 cases the hearts were “soft.” They also noted that some hearts became “firm” during compression.
The presence of a “firm” myocardium was associated with reduced hemodynamic efficacy of cardiac compression as evidenced by a lower end-tidal CO2 tension (PETCO2) – which is a well-documented surrogate measurement of systemic and regional blood flow during cardiac resuscitation.37,51–53 Hearts with “very firm” myocardium never regained spontaneous contractions. Hearts with “less firm” myocardium showed some, albeit insufficient, spontaneous contractions. Hearts with “soft” myocardium regained contractions and were able to generate a peripheral pulse in most instances.
REPERFUSION ARRHYTHMIAS
Premature ventricular complexes and episodes of ventricular tachycardia and VF commonly occur during the early minutes after return of cardiac activity. Post-resuscitation episodes of VF – which require additional electrical shocks – have been reported in up to 79% of patients, with some studies showing and inverse relationship between the number of episodes and survival.54 The underlying cell mechanisms are complex but prominently involve cytosolic Ca2+ overload and afterdepolarizations. There are repolarization abnormalities that include shortening of the action potential duration, decreased action potential amplitude, and development of action potential alternans creating conditions for reentry. Experimentally, these repolarization abnormalities are short-lived (5 to 10 minutes) and coincide with the interval of increased propensity for ventricular arrhythmias and recurrent VF.42 These repolarization abnormalities and reperfusion arrhythmias can be markedly attenuated by NHE-1 inhibition.42
POST-RESUSCITATION MYOCARDIAL DYSFUNCTION
Variable degrees of systolic55–58 and diastolic42,59 dysfunction develop after resuscitation from cardiac arrest. Dysfunction occurs despite full restoration of coronary blood flow and is largely reversible conforming to the definition of myocardial stunning. Systolic dysfunction is characterized by decreases in contractility – documented by load-independent indices derived from varying end-systolic pressure-volume relationship – leading to reductions left ventricular ejection fraction, cardiac index, left ventricular stroke work,56,57 and poor tolerance to afterload increases.60 Diastolic dysfunction is characterized by left ventricular wall thickening with reductions in end-diastolic volume and impaired relaxation.42 Diastolic dysfunction appears to by maximal immediately after restoration of spontaneous circulation, with the magnitude of wall thickness closely correlated with wall thickness during VF,47 suggesting a common pathogenic thread. From a functional perspective, diastolic dysfunction may limit the compensatory ventricular dilatation required to overcome decreases in contractility according to the Frank-Starling mechanism.
Myocardial dysfunction is characteristically responsive to inotropic stimulation and therefore pump function can be improved by administration of agents such as β-agonists (i.e., dobutamine) and phosphodiesterase inhibitors (i.e., milrinone). Dobutamine acts primarily on β1-, β2-, and α1-receptors. Its hemodynamic effects include increases in stroke volume and cardiac output with decreases in systemic and pulmonary vascular resistance. Studies in animal models of cardiac arrest have demonstrated substantial reversal of post-resuscitation systolic and diastolic dysfunction using doses ranging from 5 to 10 µg/kg·min−1.61–63 However, a dose of 5 µg/kg·min−1 was found in domestic pigs (24 ± 0.4 kg) to provide the best balance by restoring post-resuscitation systolic and diastolic function without adverse effects on myocardial oxygen consumption.62 Phosphodiesterase inhibitors such as milrinone also exert inotropic and vasodilator effects and have been shown experimentally to improve post-resuscitation myocardial dysfunction.64 However, the effectiveness of conventional the inotropic agents may be limited by effects on heart rate and the possibility of worsening ischemic injury in settings of critically reduced coronary artery blood flow.
INTERVENTIONS TARGETING MITOCHONDRIAL FUNCTION
Two lines of research at the Resuscitation Institute support the feasibility of targeting mitochondrial bioenergetic function for resuscitation from cardiac arrest. One line relates to work using NHE-1 inhibitors in various animal models of cardiac arrest over a period of approximately 10 years.40,42,49,65–70 The other line relates to more recent work using erythropoietin in a rat model of cardiac arrest71 and in a small clinical study in patients suffering out-of-hospital cardiac arrest.72 Both lines of research support the rationale and feasibility of using either an NHE-1 inhibitor or erythropoietin for preservation of left ventricular myocardial distensibility during cardiac resuscitation.
NHE-1 INHIBITORS
Mechanisms of Injury
The intense myocardial ischemia that develops shortly after the onset of cardiac arrest prompts profound and sustained intracellular acidosis.73–75 Intracellular acidosis activates the sarcolemmal NHE-1 initiating an electro-neutral Na+–H+ exchange that brings Na+ into the cell.76 During the ensuing resuscitation effort, the myocardium is reperfused with blood that typically has a normal pH resulting in the wash-out of protons accumulated in the extracellular space during the preceding interval of ischemia intensifying the sarcolemmal Na+–H+ exchange and the resulting Na+ entry.65,76,77 Na+ accumulates in the cytosol because the Na+-K+ ATPase activity is concomitantly reduced,78 such that progressive and prominent increases in cytosolic Na+ occurs. Na+ may also enter the cell through Na+ channels and the Na+-HCO3− co-transporter. The cytosolic Na+ excess, in turn, drives sarcolemmal Ca2+ influx through reverse mode operation of the sarcolemmal Na+–Ca2+ exchanger leading to cytosolic and mitochondrial Ca2+ overload,79 causing a myriad of detrimental effects.
Ca2+ entering mitochondria can be sequestered in large amounts through a process that involves influx through the Ca2+ uniporter and efflux through the Na+–Ca2+ exchanger.80 However, as mitochondrial Ca2+ rises the mitochondrial Na+–Ca2+ exchanger becomes saturated and mitochondrial Ca2+ overload occurs.80 Mitochondrial Ca2+ overload can compromise the capability of mitochondria to sustain oxidative phosphorylation81 and also promote the release of pro-apoptotic factors including cytochrome c.82
The relevance of this mechanism of injury is highlighted by a large series of preclinical studies demonstrating consistent attenuation of myocardial injury caused by ischemia and reperfusion when sarcolemmal Na+ entry is limited.76,83,84
Effects of NHE-1 Inhibitors on Resuscitation
Research using various rat and pig models of cardiac arrest support a consistent myocardial benefit associated with inhibition of NHE-1 activity during resuscitation from VF, including preservation of left ventricular distensibility, attenuation of reperfusion arrhythmias, and amelioration of post-resuscitation myocardial dysfunction all favoring improved resuscitability and survival.40,42,49,65–70,85–87
Effects on left ventricular distensibility
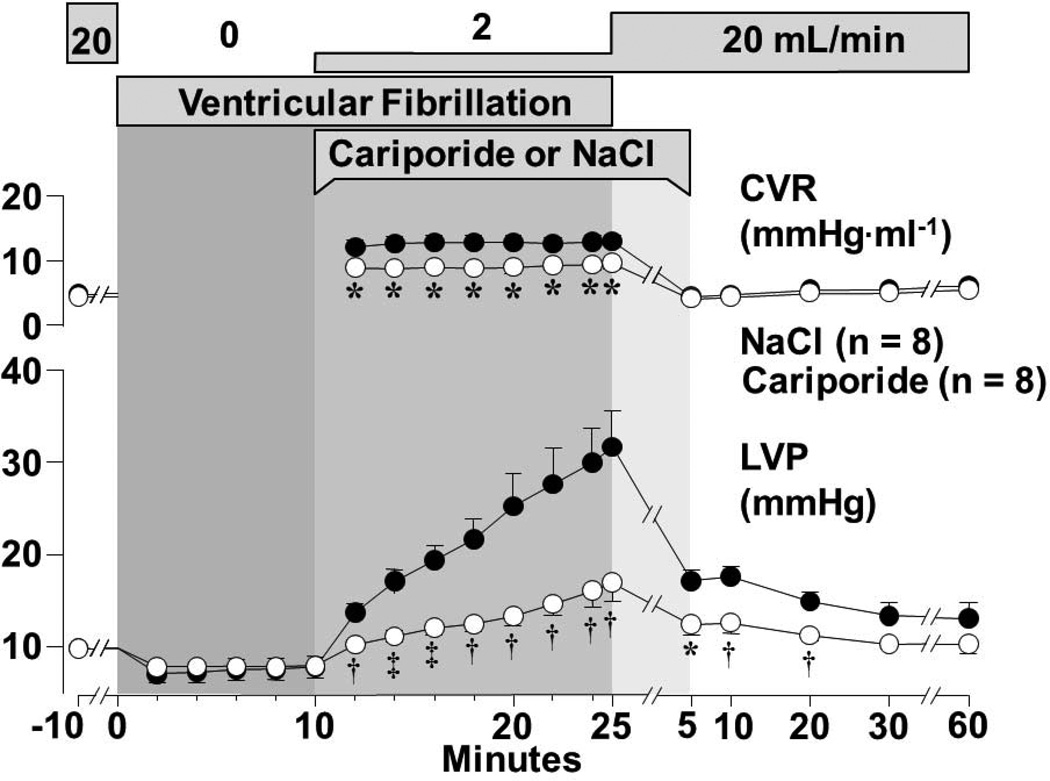
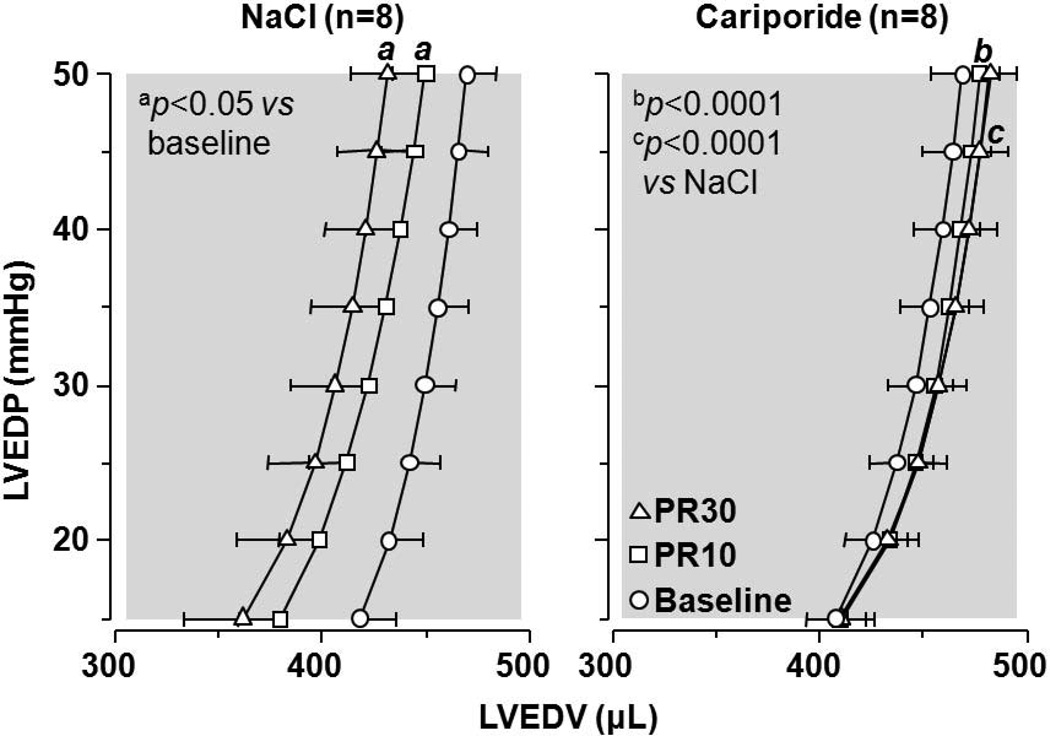
The initial findings suggesting that NHE-1 inhibition could attenuate reductions in left ventricular distensibility during resuscitation and also prevent post-resuscitation diastolic dysfunction were made using an isolated (Langendorff) rat model of VF and simulated resuscitation.65,66 As shown in Figure 5, infusion of the NHE-1 inhibitor cariporide during simulated resuscitation markedly attenuated left ventricular pressure increases, consistent with preservation of left ventricular distensibility. Post-resuscitation, the enddiastolic left ventricle pressure-volume relationship was preserved at baseline levels (Figure 6). Cariporide elicited the effects despite administration starting after 10 minutes of untreated VF supporting the clinical relevance of the findings.
Figure 5.
Isolated perfused rat heart model of VF and simulated resuscitation. Upper horizontal bars represent perfusate flow, VF, and duration of cariporide or NaCl infusion. CVR denotes coronary vascular resistance; LVP, left ventricular end-diastolic pressure during sinus rhythm and “arrest” pressure during VF. Values are mean ± SEM. Closed symbols denote NaCl and open symbols cariporide. Differences in LVP and CVR between treatment groups were significant (p<0.0001 by 2-way ANOVA for treatment effect). *p<0.05, †p<0.01, ‡p<0.001 vs NaCl by 1-way ANOVA (Adapted from Gazmuri et al. Circulation 2001;104:234).
Figure 6.
Left ventricular end-diastolic pressure (LVEDP)–volume (LVEDV) curves. PR denotes post-resuscitation 10 and 30 minutes. Measurements made in hearts from Figure 3 (Adapted from Gazmuri et al. Circulation 2001;104:234).
Subsequent studies in a pig model of VF and closed-chest resuscitation paralleled the findings in the isolated rat heart showing preservation of left ventricular wall thickness and cavity size (Figure 4; data on cavity size not shown).42 Preservation of left ventricular myocardial distensibility enabled the generation of higher coronary perfusion pressures prompting higher resuscitability rates (2/8 vs 8/8; p< 0.05).42
We then reasoned that if left ventricular myocardial distensibility – and therefore preload – could be preserved by NHE-1 inhibition then higher forward blood flows could be generated for a given compression depth; thus, enhancing the relationship between forward blood flow and compression depth.
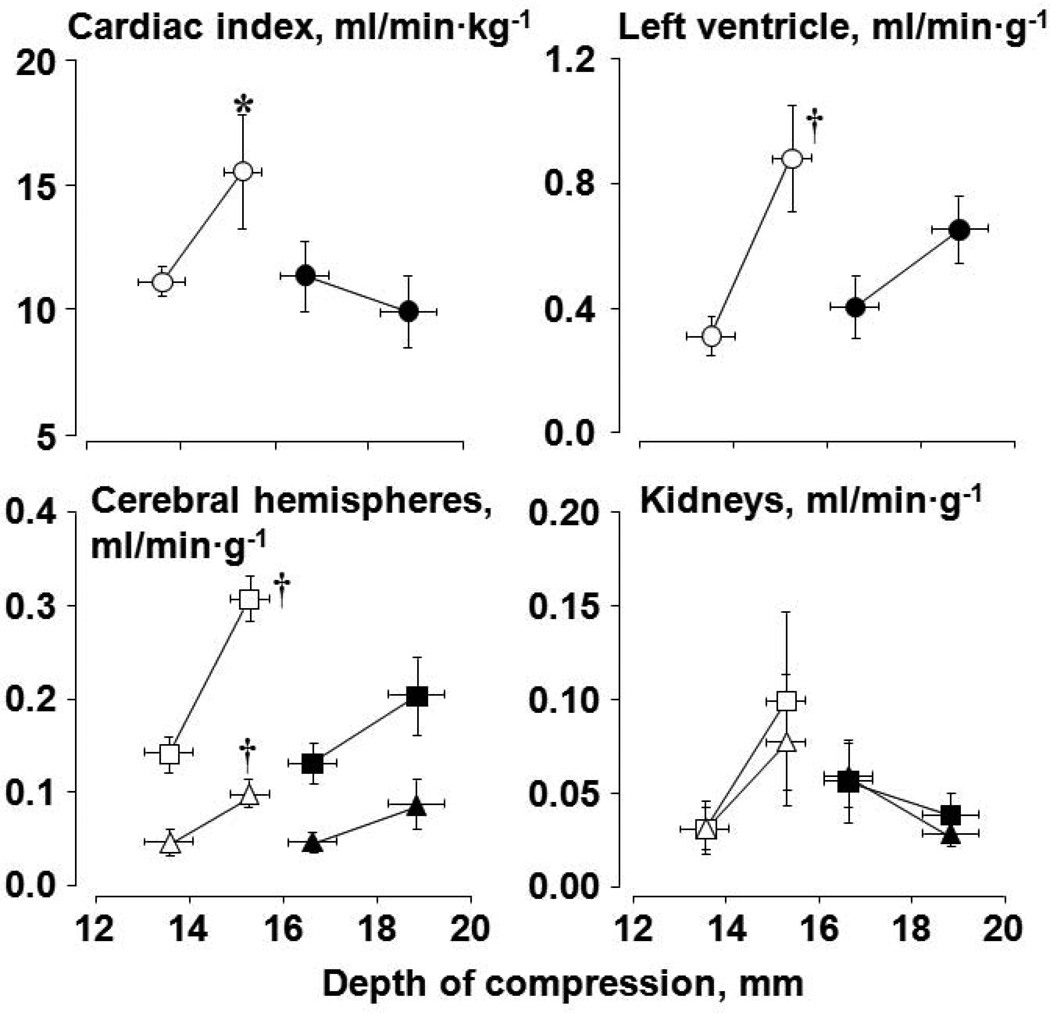
Studies were performed measuring systemic and organ blood flow using fluorescent microspheres in an rat model of VF and closed-chest resuscitation.49 Two series of 14 experiments each were conducted in which rats were subjected to 10 minutes of untreated VF followed by 8 minutes of chest compression before attempting defibrillation. Compression depth was adjusted to maintain an aortic diastolic pressure between 26 and 28 mmHg in the first series and between 36 and 38 mmHg in the second series. Within each series, rats were randomized to receive cariporide (3 mg/kg) or NaCl 0.9% (control) before starting chest compression. In rats that received cariporide, the compression depth required to generate a given systemic and organ blood flow was markedly reduced compared with rats that received the vehicle control (Figure 7). Thus, a higher forward blood flow leading to higher regional organ blood flows could be generated for a given depth of compression in the presence of cariporide.
Figure 7.
Cardiac index and organ blood flow as a function of depth of compression in rats during VF and closed-chest resuscitation. Rats were randomized to receive a bolus of cariporide (open symbols) or vehicle control (closed symbols) at the start of chest compression. The first symbol represents data from series 1 and the second symbol data from series2. For paired organs, triangles denote right and squares left. Values are mean ± SEM; *p<0.05 vs 0.9% NaCl by one-way ANOVA in series 2; †p<0.01 vs series 1 within each treatment group by one-way ANOVA (Adapted from Kolarova et al. Am J Physiol Heart Circ Physiol 2005;288:H2904).
We further reasoned that administration of a vasopressor agent in the presence of an NHE-1 inhibitor could result in a higher systemic and coronary perfusion pressure for the simple reason that pressure is a function of flow and resistance. This was indeed the case as demonstrated in a rat model of VF and closed-chest resuscitation.68 The studies involved two series of 16 experiments each using epinephrine in one series and vasopressin in the other. Within each series rats were randomized to receive cariporide or NaCl control. A significantly higher coronary perfusion pressure was elicited with either vasopressor agent in rats that had received cariporide. A similar effect was observed associated with the administration of epinephrine in a pig model of VF and closed-chest resuscitation.69 These effects on coronary perfusion pressure are important; if translated clinically they could be highly relevant because only small increases in coronary perfusion pressure are required to have a dramatic effect on resuscitability.88
Effects on post-resuscitation arrhythmias and refibrillation
NHE-1 inhibition using cariporide was markedly effective in suppressing ventricular ectopic activity during the early post-resuscitation period.42,66,69,86 This effect was associated with preservation of the action potential duration,42 an effect that could reduce the risk of reentry minimizing the risk of VF during the early post-resuscitation interval.86 This is an important effect, which if translated clinically could help stabilize initially resuscitated victim of out-of-hospital cardiac arrest and avert recurrent episodes of cardiac arrest during initial post-resuscitation period while in route to a hospital.
Effects on post-resuscitation myocardial function
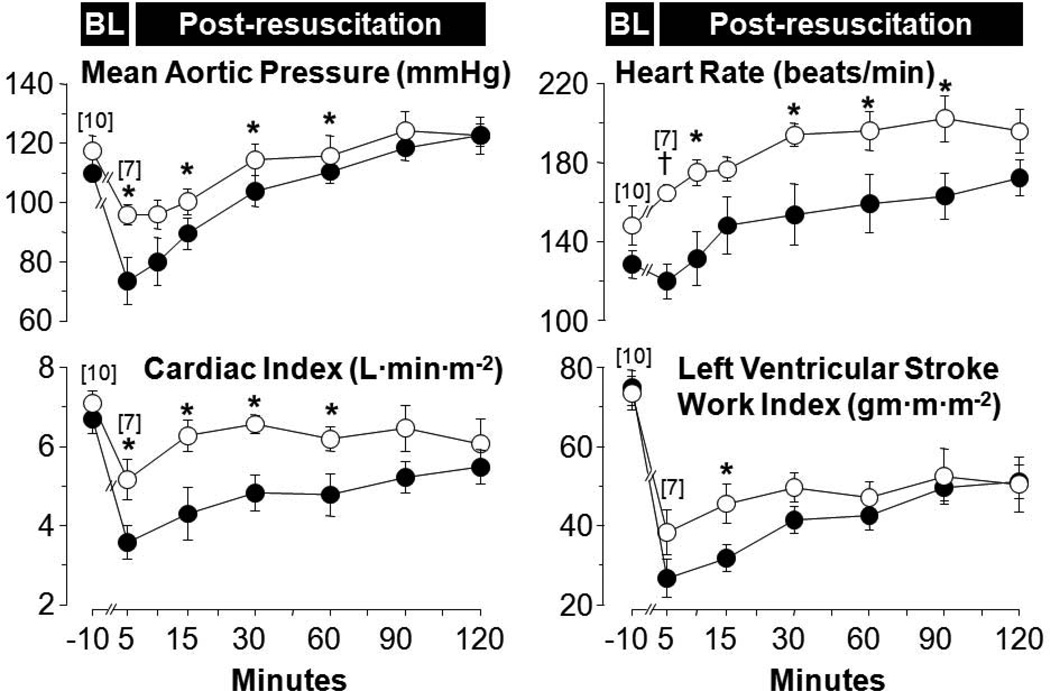
Several series in rat and pig models of VF and resuscitation have shown beneficial effects of NHE-1 inhibitors on post-resuscitation myocardial function as illustrated in Figure 8 using cariporide in a pig model of VF and closed-chest resuscitation.86 A similar post-resuscitation benefit was observed using the NHE-1 inhibitor zoniporide in an open chest pig model of resuscitation by extracorporeal circulation.40 This effect translated in a rat model of VF and closed-chest resuscitation in improved short-term survival. 87
Figure 8.
Baseline and post-resuscitation left ventricular and hemodynamic function in pigs randomized to receive cariporide (open symbols) or 0.9% NaCl (closed symbols). Numbers in brackets indicate sample size. Values are mean±SEM. *p < 0.05; †p < 0.001 vs. 0.9% NaCl analyzed by one-way ANOVA (Adapted from Ayoub I et al. Resuscitation 2009;81:106).
Mechanisms of the Resuscitation Effects
Effects on cytosolic Na+ and mitochondrial Ca2+
A rat model of VF and closed-chest resuscitation was used to examine the effects of NHE-1 inhibition and of Na+ channel blockade (interventions collectively referred to as “Na+-limiting interventions”) on intracellular Na+, mitochondrial Ca2+, cardiac function, and plasma levels of cardiospecific troponin I (cTnI) after resuscitation.70 Limiting sarcolemmal Na+ entry attenuated increases in cytosolic Na+ and mitochondrial Ca2+ overload during chest compression and the post-resuscitation phase. Attenuation of cytosolic Na+ and mitochondrial Ca2+ increases was accompanied by preservation of left ventricular myocardial distensibility during chest compression, less post-resuscitation myocardial dysfunction, and lower levels of cardiospecific troponin I.
Effects on energy metabolism
An open-chest pig model of electrically-induced VF and extracorporeal circulation was developed to study the myocardial energy effects of inhibiting NHE-1 under conditions of controlled coronary perfusion pressure.40 VF was induced by epicardial delivery of an alternating current and left untreated for 8 minutes. Extracorporeal circulation was then started with the flow adjusted to maintain a coronary perfusion pressure at 10 mmHg for 10 minutes before attempting defibrillation and restoration of spontaneous circulation. The target coronary perfusion pressure was chosen to mimic the low coronary perfusion pressure generated by closed-chest resuscitation. Two groups of 8 pigs each were randomized to receive the NHE-1 inhibitor zoniporide (3 mg/kg) or vehicle control as a right atrial bolus immediately before starting extracorporeal circulation. Like in a previous study using the NHE-1 inhibitor cariporide42 (Figure 4), zoniporide also prevented progressive reductions in cavity size and progressive thickening of the left ventricular wall.
The myocardial effects occurred without changes in coronary blood flow or coronary vascular resistance indicating that the favorable myocardial effects of NHE-1 inhibition during resuscitation were not likely the result of increased blood flow and oxygen availability (e.g., by less extrinsic compression of the coronary circuit).
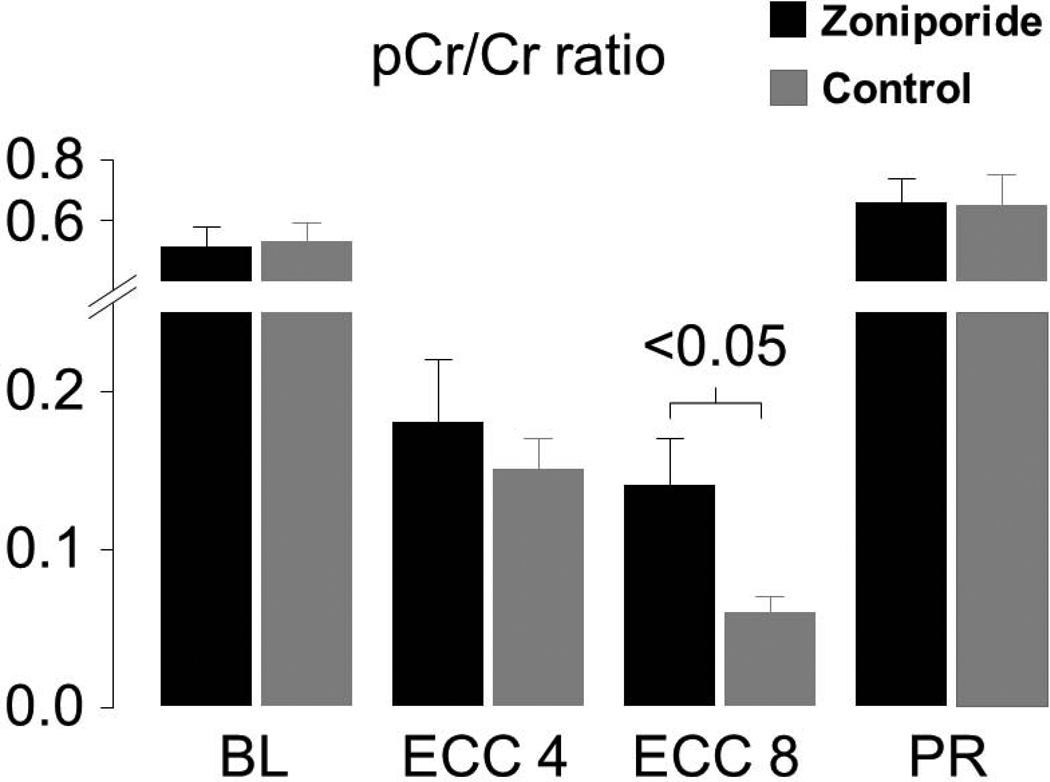
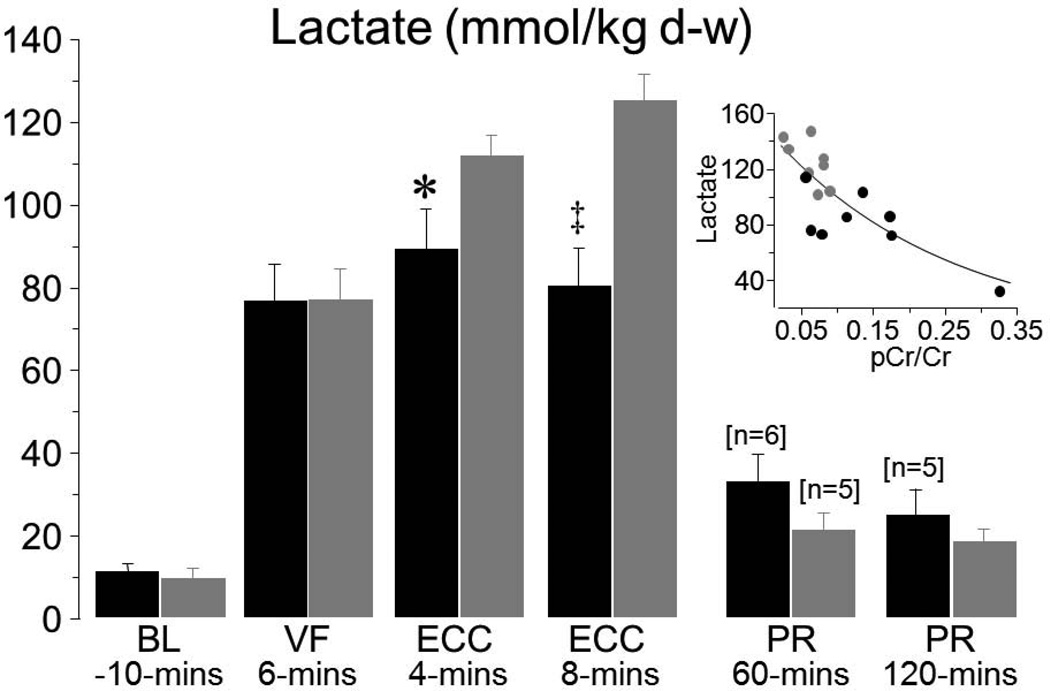
Myocardial tissue measurements indicated that administration of zoniporide prevented progressive loss of oxidative phosphorylation during the interval of simulated resuscitation. Animals that received zoniporide: (1) maintained a higher creatine phosphate to creatine (pCr/Cr) ratio as shown in Figure 9, (2) maintained a higher ATP/ADP ratio, and (3) had lesser increases in adenosine. These measurements are consistent with regeneration of ADP into ATP by mitochondria instead of downstream degradation into adenosine, with the newly formed ATP being used to regenerate creatinine phosphate; all indicative of preserved mitochondrial bioenergetic function. These changes were accompanied by prominent amelioration of myocardial lactate increases, attaining levels which were inversely proportional to the pCr/Cr ratio at 8 minutes of VF and ECC, suggesting a shift away from anaerobic metabolism consequent to preservation of mitochondrial bioenergetic function in pigs treated with zoniporide (Figure 10).
Figure 9.
Myocardial measurements in pigs randomly assigned to receive 3 mg/kg of zoniporide (black bars) or 0.9% NaCl (gray bars) after 8 minutes of untreated VF before starting extracorporeal circulation (ECC). Measurements were obtained at baseline (BL), during VF at ECC 4 and 8 minutes, and at 60 minutes post-resuscitation (PR). Each group had 8 pigs at baseline and ECC and 6 pigs in the zoniporide group and 5 in the NaCl group at PR. Mean ± SEM (Adapted from Ayoub et al. Crit Care Med 2007;35:2329).
Figure 10.
Myocardial lactate measurements in experiments described in Figure 9. Numbers in brackets indicate when sample size decreased from the initial 8 or preceding ones. Insert shows the relationship between lactate and pCr/Cr ratio at ECC 8 minutes. The regression line represents an exponential decay function (R2 = 0.63, p<0.001). Mean ± SEM; *P<0.05, ‡P<0.001 vs NaCl by Student’s t-test (Adapted from Ayoub et al. Crit Care Med 2007;35:2329).
These energy effects are consistent with NHE-1 inhibition protecting mitochondrial bioenergetic function – probably as a result of limiting mitochondrial Ca2+ overload – and supportive of the concept that left ventricular myocardial distensibility during resuscitation is likely to be preserved by activating mitochondrial mechanisms capable of maintaining bioenergetic function.
Erythropoietin
Because development of NHE-1 inhibitors for clinical use has been driven by indications other than resuscitation, and these efforts have been unsuccessful so far, we sought alternative, clinically available, compounds that could elicit similar mitochondrial effect within a time window relevant to resuscitation. One of these compounds is erythropoietin.
Erythropoietin is a 30.4-kDa glycoprotein best known for its action on erythroid progenitor cells and regulation of circulating red cell mass. However, several studies have recently shown that erythropoietin also activates potent cell survival mechanisms during ischemia and reperfusion through genomic and non-genomic signaling mechanisms in a broad array of organs and tissues including the heart,89–98 brain,99–102 spinal cord,103 kidney,104,105 liver,105 and skin.106
The action of erythropoietin begins upon binding to a specific cell membrane receptor known as the erythropoietin receptor (EpoR). This receptor is a member of the type-I superfamily of single-transmembrane cytokine receptors. Erythropoietin binding to EpoR triggers cross-phosphorylation and activation of Janus tyrosine kinases (JAK) 1 and 2. JAK activation, in turn, prompts phosphorylation of tyrosine residues present in the EpoR intracellular domains creating docking sites for the recruitment and activation of multiple signaling proteins that have Src-homology-2 (SH2) domains resulting in well-known anti-apoptotic,92 anti-inflammatory,107,108 and proliferative effects.109,110 The time course of these effects vary contingent on the specific signaling mechanism and the duration of the erythropoietin-EpoR binding.
Without negating the potential benefits of the anti-apoptotic, anti-inflammatory, and proliferative effects of erythropoietin effects, we have been interested in mechanisms responsible for “rapid” activation of cell protective mechanisms. Erythropoietin activates signaling pathways that converge on mitochondria and that favor preservation of bioenergetic function in spite of the adverse conditions present during ischemia and reperfusion. These signaling pathways are likely to involve activation of protein kinase C epsilon (PKCε) and protein kinase B (Akt) with translocation from the cytosol to mitochondria.
PKCε activation is a well-established mechanism of myocardial protection linked to preconditioning and acute protection.111 PKCε is primarily located in the cytosol and it is activated by phosphorylation through various signaling mechanisms, including erythropoietin. Erythropoietin mediates the effect through phosphorylation of phosphoinositide dependent kinase-1 (PDK1) upon activation of phosphatidyl inositide kinase (PI3K), which is an SH2 domain containing signaling protein. Phosphorylated PKCε translocates to the mitochondria where it signals opening of putative mitochondrial ATP-sensitive K+ channels (KATP channels),112,113 activation of the mitochondrial enzymes cytochrome c oxidase114 and aldehyde dehydrogease, 115 and inhibition of the mitochondrial permeability transition pore.116
Akt activation is a powerful survival signal that has been shown to mediate myocardial protection during late preconditioning and after reperfusion.117 Akt is predominantly cytosolic and is activated through phosphorylation by insulin, insulin like growth factor-1, and also erythropoietin. Erythropoietin mediates the phosphorylation of Akt through phosphorylation of PDK1 upon activation of PI3K. Activated Akt can translocate to mitochondria where it has been shown to exert beneficial effects including opening of mitochondrial KATP channels,117 activation of respiratory chain complexes and FoF1 ATPase,118 and inhibition of the mitochondrial permeability transition pore.119
In support of a rapid erythropoietin protective effect, work in our laboratory in a rat model of cardiac arrest71 and work in victims of out-of-hospital cardiac arrest in collaboration with Slovenian investigators72 demonstrated the onset of hemodynamic benefits within minutes after erythropoietin administration. Both studies support the concept that erythropoietin facilitates return of spontaneous circulation by a cell effects that result in the preservation of left ventricular myocardial distensibility enabling preservation of left ventricular preload leading to hemodynamically more effective chest compression. Functionally, the effects are remarkably similar to the effects elicited by administration of NHE-1 inhibitors.42,49
Effects of Erythropoietin during Cardiac Resuscitation
Studies in rats
The effects of erythropoietin for resuscitation were initially studied in a well-standardized rat model of electrically induced ventricular fibrillation (VF) and closed-chest resuscitation using human recombinant erythropoietin (epoetin alpha, Amgen, Thousand Oaks, CA).71 Rats were subjected to a 10-minute interval of untreated VF followed by 8 minutes of closed-chest resuscitation (chest compression and ventilation with 100% oxygen) before attempting defibrillation. Chest compression was adjusted to initially attain and subsequently maintain an aortic diastolic pressure between 26 and 28 mmHg. Three groups of 10 rats each were randomized to receive a right atrial bolus of epoetin alpha (5,000 IU/kg) at baseline 15 minutes before induction of VF (EPOBL-15-min), at 10 minutes of VF before starting chest compression (EPOVF 10-min), or to receive 0.9% NaCl solution (control) instead with the investigators blind to the treatment assignment.
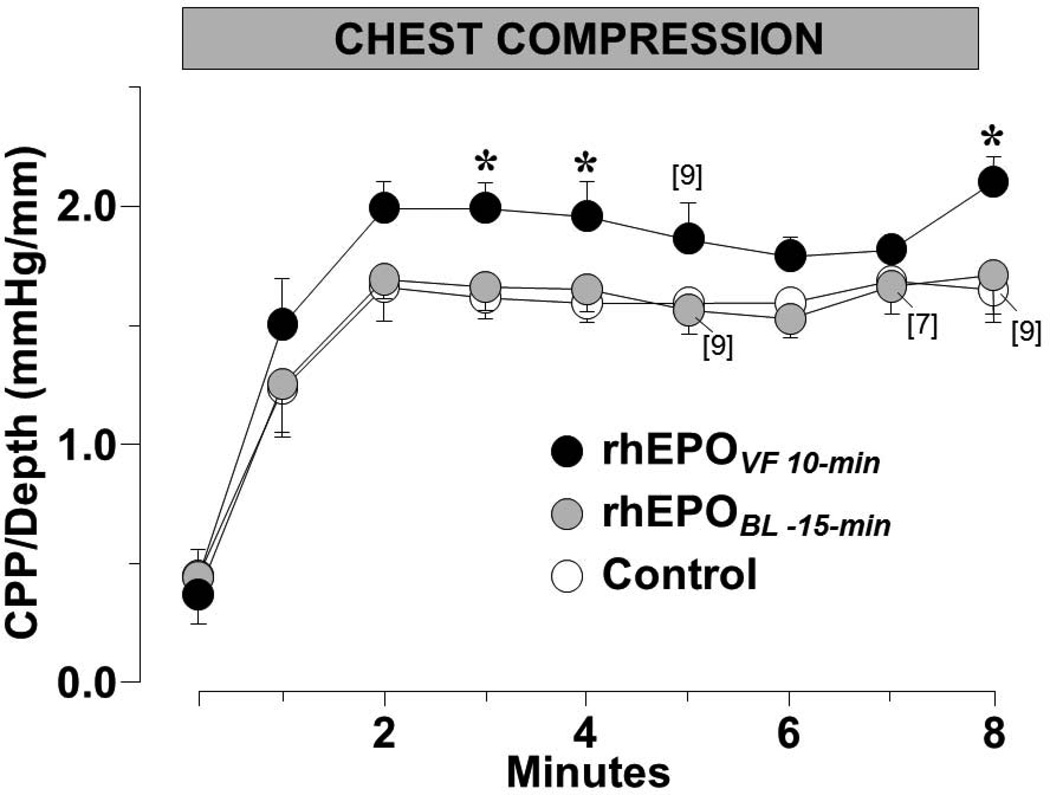
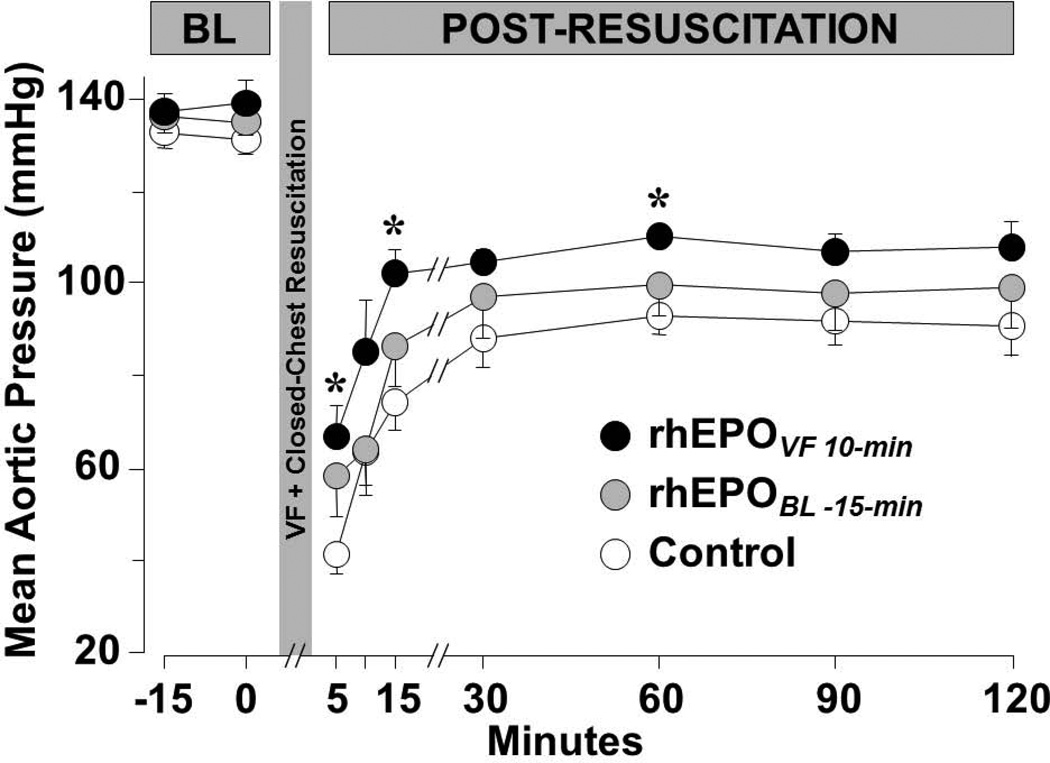
Erythropoietin given coincident with the beginning of chest compression after 10 minutes of untreated VF – but not before inducing VF – promoted hemodynamically more effective chest compression such that the CPP/Depth ratio averaged during the interval of chest compression was 25% higher in the group of rat that received erythropoietin at the beginning of chest compression. In Figure 11, the CPP/depth ratio is depicted throughout chest compression. Post-resuscitation, EPOVF 10-min rats had significantly higher mean aortic pressure (Figure 12) associated with numerically higher cardiac index and higher peripheral vascular resistance. The diminished effectiveness of erythropoietin when given before VF is intriguing and worth of additional investigation.
Figure 11.
Ratio between coronary perfusion pressure and depth of compression (CPP/Depth) during closed-chest resuscitation in rats treated with rhEPO at baseline (rhEPOBL-15-min, n = 10; shaded symbols), during VF before starting chest compression (rhEPOVF 10-min, n = 10; closed symbols), or with 0.9% NaCl (control, n = 10; open symbols). Numbers of rats remaining in VF and therefore receiving chest compression are indicated in brackets. Mean ± SEM. * p < 0.05 vs control by Dunnett’s multicomparison method (Adapted from Singh D et al. Am J Ther 2007;14:361).
Figure 12.
Mean aortic pressure after return of spontaneous circulation in rats treated with rhEPO as described in Figure 11 and the text. BL, baseline. Mean ± SEM. *P<0.05 vs control by Dunnett’s multicomparison method (Adapted from Singh D et al. Am J Ther 2007;14:361).
Similar observations were made in a recent series of experiments in the same rat model of VF and closed-chest resuscitation described above. In this series, hearts were removed after return of spontaneous circulation to examine in left ventricular tissue demonstrating activation of PKCε and Akt in both the cytosolic and the mitochondrial fractions.
Studies in humans
A clinical study was performed in collaboration with Dr. Štefek Grmec, MD, PhD and the Maribor Emergency Medical Services (EMS) system in the city of Maribor and adjacent rural areas encompassing a population of approximately 200,000 inhabitants.72 Resuscitation was attempted using regionally developed protocols that incorporate ILCOR 2005 recommendations. Upon arrival of the rescue squad an endotracheal tube was placed – verifying proper position by capnography. Positive pressure ventilation was provided with a tidal volume of ≈ 6 ml/kg delivered 10 times per minute unsynchronized to compressions. The rescue squad also established an intravenous access through an external vein within approximately 30 seconds. Patients assigned to erythropoietin received 90,000 IU of beta-epoetin (3 vials of NeoRecormon 30,000 IU each, 1.8 ml total, Hoffman La Roche) as a bolus dose within 1 or 2 minutes after starting chest compression followed by a 10-ml bolus of 0.9% NaCl. Beta-epoetin was kept refrigerated (2–8 °C) in the ambulance until immediately before use. In every instance erythropoietin was given before any other drug.
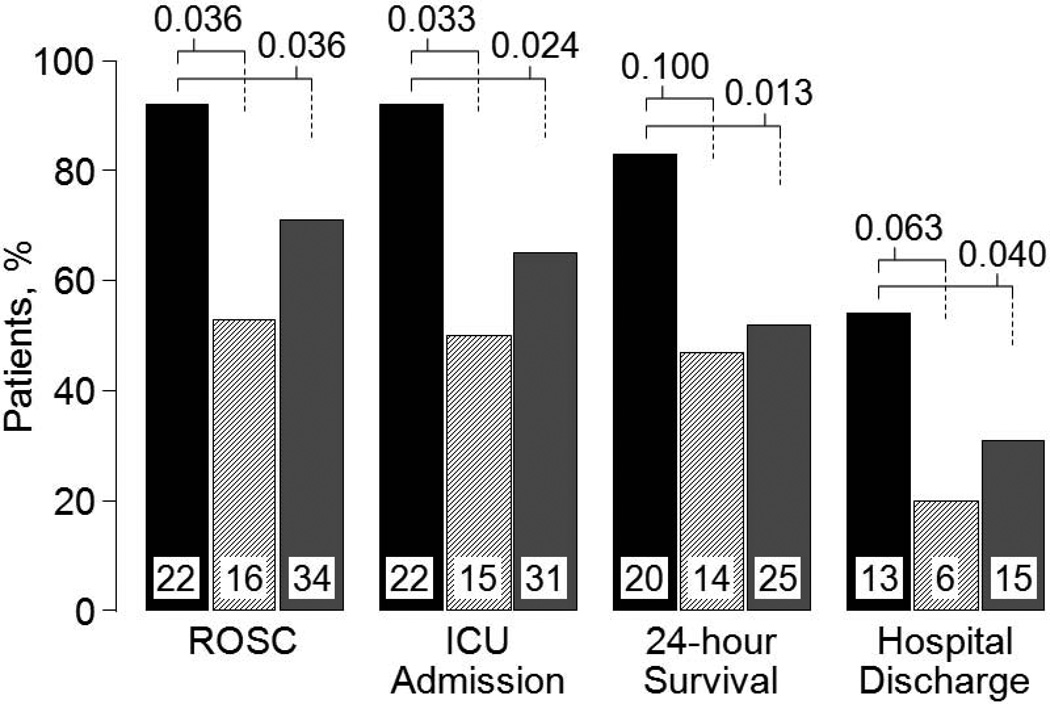
By univariate analysis, administration of erythropoietin was associated with higher rates of admission to the receiving Intensive Care Unit (ICU), return of spontaneous circulation (ROSC), 24-hour survival, and survival to hospital discharge when compared with concurrent controls and associated with higher rates of ICU admission, ROSC, and 24-hour survival when compared with matched controls after adjustment by multiple logistic regression for variables known to influence outcome (i.e., age, male sex, witnessed arrest, time from call to start CPR, pulseless electrical activity, asystole, and bystander CPR). For the comparison with concurrent controls adjustment for these variables reduced the odds ratio, but retained statistical significance for ICU admission and ROSC. For the comparison with matched controls adjustment increased the odds ratio demonstrating statistical significance for all four outcomes (Figure 13).
Figure 13.
Resuscitation and survival outcomes in patients who received erythropoietin (black bars, n = 24) compared with concurrent controls (hatched bars, n = 30) and with matched controls (gray bars, n = 48). ROSC, return of spontaneous circulation; ICU, intensive care unit. Numbers inside bars denote patients for each outcome with the bar representing the percentage of the initial cohort. P-values were calculated by Chi-square test for each outcome adjusted by covariates with known predictive value (i.e., age, male sex, witnessed arrest, time from call to start CPR, pulseless electrical activity, asystole, and bystander CPR) and are shown above bars (Adapted from Grmec S et al. Resuscitation 2009;80:631).
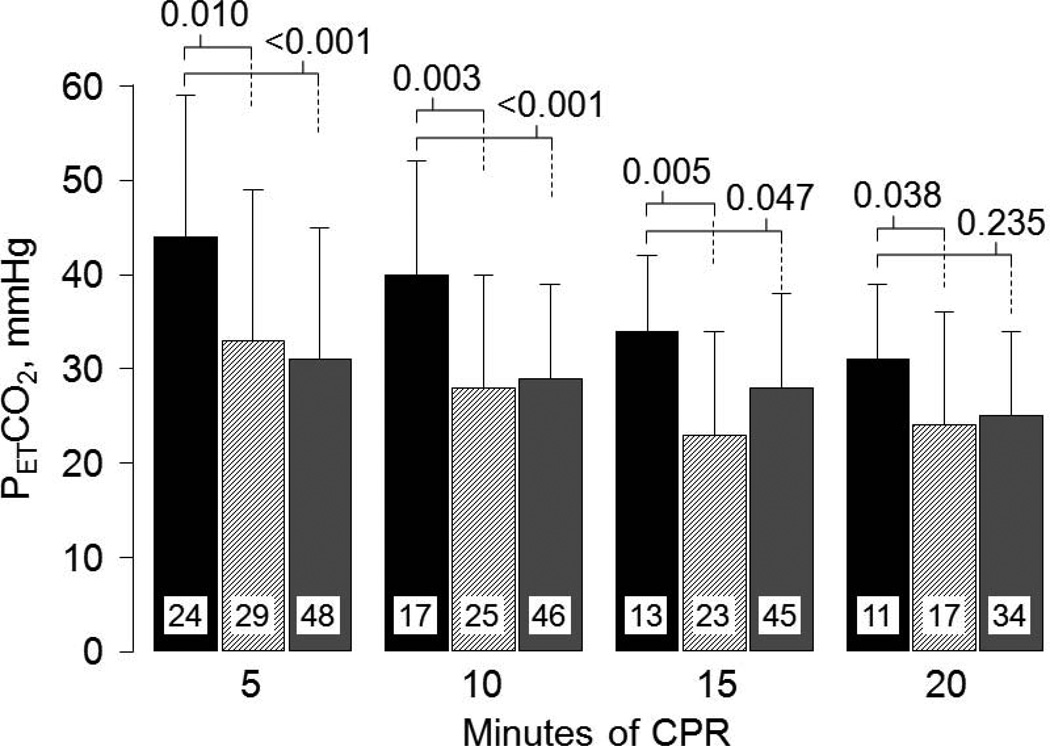
Based on our preceding work in rats, we hypothesized that erythropoietin – by preserving left ventricular myocardial distensibility – would prompt hemodynamically more effective chest compression and this effect would be reflected in a higher PETCO2; which under low flow conditions is proportional to total and regional blood flow.51,52 This was indeed the case. Victims who received erythropoietin had significantly higher PETCO2 during chest compression (Figure 14). Thus, these clinical observations – though based on a small sample size – are consistent with the hypothesis that erythropoietin – by preserving left ventricular myocardial distensibility – leads to hemodynamically more effective chest compression.
Figure 14.
End-tidal PCO2 (PETCO2) during cardiopulmonary resuscitation in patients who received erythropoietin (black bars, n = 24) compared with concurrent controls (hatched bars, n = 30) and with matched controls (gray bars, n = 48). Numbers inside bars denote patients remaining in cardiac arrest and receiving CPR. Data are presented as mean values with one standard deviation. P-values were calculated by unpaired t-test or by Mann-Whitney rank sum test for each time period and shown above bars (Adapted from Grmec S et al. Resuscitation 2009;80:631).
A total of five additional studies in cardiac arrest have been reported so far; four in rats71,120–122 and one in humans.123 In three of the animal studies, administration of erythropoietin before chest compression71 or after restoration of spontaneous circulation120,122 exerted beneficial myocardial effects leading to preservation of left ventricular myocardial distensibility during chest compression71 and less post-resuscitation myocardial dysfunction71,120,122 with improved survival.120,122 In the other animal study121 and in the human study123 the focus was on neurological outcome. In the animal study, erythropoietin was given before cardiac arrest and showed no effects on neurological recovery.
The human study was conducted in France by Cariou and colleagues.123 This study enrolled a small group of patients who had suffered out-of-hospital cardiac arrest to examine possible neuroprotective effects of erythropoietin. In this study, five doses of 40,000 IU of EPO-alpha each were given over an interval of 48 hours to 18 patients who remained comatose – with a Glasgow Come Scale of <7 – after return of spontaneous circulation. It is important to emphasize that erythropoietin was given after ROSC and therefore in a setting of potential hemodynamic instability. The first dose of erythropoietin was given at a median time of 62 minutes (42–75, IQR) after ROSC. The effects of erythropoietin were compared with 40 contemporaneous matched controls. There were differences favoring erythropoietin at 28 days in survival (55% vs 48%) and full neurological recovery (55% vs 38%), but the differences were statistically insignificant. The erythropoietin group experienced a high incidence of thrombocytosis (15% vs 5%) and one of these patients in the erythropoietin group suffered an occlusion of a coronary stent.
CONCLUSIONS
The quest for interventions that could prevent or mitigate reperfusion injury has prompted an intense scientific pursuit for decades. This pursuit has broadened our understanding of the underlying pathogenic processes; yet, the development of clinical interventions targeting reperfusion injury has remained an elusive goal. Identification of functional intracellular effectors may lead to the recognition of more robust targets for therapeutic intervention. The growing evidence identifying mitochondria as effectors and targets of reperfusion injury is bringing renewed hope that novel and more effective interventions could be developed for resuscitation from cardiac arrest.
Acknowledgments
The author, Raúl J. Gazmuri MD, PhD, is the holder of a United States Patent entitled “Facilitation of Resuscitation from Cardiac Arrest by Erythropoietin.” The work presented was conducted with grant support from the VA Merit Review system, NHLBI, pharmaceutical companies, and internal institutional funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Contributor Information
Raúl J. Gazmuri, Resuscitation Institute at Rosalind Franklin University of Medicine and Science and Medical Service, Section of Critical Care Medicine, Captain James A. Lovell Federal Health Care Center North Chicago, Illinois 60064.
Jeejabai Radhakrishnan, Research Assistant Professor, Resuscitation Institute at Rosalind Franklin University of Medicine and Science.
BIBLIOGRAPHY
- 1.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 2.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006 April;34(Pt 2):232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 3.Weisfeldt ML, Zweier J, Ambrosio G, Becker LC, Flaherty JT. Evidence that free radicals result in reperfusion injury in heart muscle. Basic Life Sci. 1988;49:911–919. doi: 10.1007/978-1-4684-5568-7_149. [DOI] [PubMed] [Google Scholar]
- 4.Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a "crystal-like" pattern. Am J Physiol Cell Physiol. 2005 March;288(3):C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 5.Frezza C, Cipolat S, Martins de BO, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De SB, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006 July 14;126(1):177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Mannella CA. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim Biophys Acta. 2006 February;1762(2):140–147. doi: 10.1016/j.bbadis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998 August 10;1366(1–2):139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998 August 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 9.Gao W, Pu Y, Luo KQ, Chang DC. Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J Cell Sci. 2001 August;114(Pt 15):2855–2862. doi: 10.1242/jcs.114.15.2855. [DOI] [PubMed] [Google Scholar]
- 10.Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res. 1999 September 3;85(5):403–414. doi: 10.1161/01.res.85.5.403. [DOI] [PubMed] [Google Scholar]
- 11.Martinou I, Desagher S, Eskes R, Antonsson B, Andre E, Fakan S, Martinou JC. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J Cell Biol. 1999 March 8;144(5):883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005 April;46(4):1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 13.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem. 2004 December 17;279(51):53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 14.de Moissac D, Gurevich RM, Zheng H, Singal PK, Kirshenbaum LA. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000 January;32(1):53–63. doi: 10.1006/jmcc.1999.1057. [DOI] [PubMed] [Google Scholar]
- 15.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999 June 8;99(22):2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997 November 14;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 17.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999 April 23;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol. 2007;292:H767–H775. doi: 10.1152/ajpheart.00468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radhakrishnan J, Ayoub IM, Gazmuri RJ. Activation of caspase-3 may not contribute to postresuscitation myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2009 April;296(4):H1164–H1174. doi: 10.1152/ajpheart.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barczyk K, Kreuter M, Pryjma J, Booy EP, Maddika S, Ghavami S, Berdel WE, Roth J, Los M. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int J Cancer. 2005 August 20;116(2):167–173. doi: 10.1002/ijc.21037. [DOI] [PubMed] [Google Scholar]
- 22.Renz A, Berdel WE, Kreuter M, Belka C, Schulze-Osthoff K, Los M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001 September 1;98(5):1542–1548. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- 23.Alleyne T, Joseph J, Sampson V. Cytochrome-c detection: a diagnostic marker for myocardial infarction. Appl Biochem Biotechnol. 2001 February;90(2):97–105. doi: 10.1385/abab:90:2:97. [DOI] [PubMed] [Google Scholar]
- 24.Adachi N, Hirota M, Hamaguchi M, Okamoto K, Watanabe K, Endo F. Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin Chim Acta. 2004 April;342(1–2):127–136. doi: 10.1016/j.cccn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Hosoya M, Nunoi H, Aoyama M, Kawasaki Y, Suzuki H. Cytochrome c and tumor necrosis factor-alpha values in serum and cerebrospinal fluid of patients with influenza-associated encephalopathy. Pediatr Infect Dis J. 2005 May;24(5):467–470. doi: 10.1097/01.inf.0000160995.07461.b8. [DOI] [PubMed] [Google Scholar]
- 26.Hosoya M, Kawasaki Y, Katayose M, Sakuma H, Watanabe M, Igarashi E, Aoyama M, Nunoi H, Suzuki H. Prognostic predictive values of serum cytochrome c, cytokines, and other laboratory measurements in acute encephalopathy with multiple organ failure. Arch Dis Child. 2006 January 27; doi: 10.1136/adc.2005.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 July 15;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 28.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem Soc Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 29.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000 December;7(12):1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 30.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem. 2001 May 25;276(21):18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 31.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001 June;280(6):H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 32.Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol. 2004 July;287(1):H258–H267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- 33.Paradies G, Petrosillo G, Pistolese M, Di VN, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res. 2004 January 9;94(1):53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 34.Binak K, Harmanci N, Sirmaci N, Ataman N, Ogan H. Oxygen extraction rate of the myocardium at rest and on exercise in various conditions. Br Heart J. 1967 May;29(3):422–427. doi: 10.1136/hrt.29.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusa T, Obara S. Myocardial oxygen extraction rate under general anesthesia. Tohoku J Exp Med. 1981 March;133(3):321–324. doi: 10.1620/tjem.133.321. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman JIE. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70:153–159. doi: 10.1161/01.cir.70.2.153. [DOI] [PubMed] [Google Scholar]
- 37.Duggal C, Weil MH, Gazmuri RJ, Tang W, Sun S, O'Connell F, Ali M. Regional blood flow during closed-chest cardiac resuscitation in rats. J Appl Physiol. 1993;74:147–152. doi: 10.1152/jappl.1993.74.1.147. [DOI] [PubMed] [Google Scholar]
- 38.Ditchey RV, Goto Y, Lindenfeld J. Myocardial oxygen requirements during experimental cardiopulmonary resuscitation. Cardiovasc Res. 1992;26:791–797. doi: 10.1093/cvr/26.8.791. [DOI] [PubMed] [Google Scholar]
- 39.Gazmuri RJ, Berkowitz M, Cajigas H. Myocardial effects of ventricular fibrillation in the isolated rat heart. Crit Care Med. 1999 August;27(8):1542–1550. doi: 10.1097/00003246-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Ayoub IM, Kolarova J, Kantola R, Radhakrishnan J, Gazmuri RJ. Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med. 2007;35:2329–2336. doi: 10.1097/01.ccm.0000280569.87413.74. [DOI] [PubMed] [Google Scholar]
- 41.Klouche K, Weil MH, Sun S, Tang W, Povoas HP, Kamohara T, Bisera J. Evolution of the stone heart after prolonged cardiac arrest. Chest. 2002 September;122(3):1006–1011. doi: 10.1378/chest.122.3.1006. [DOI] [PubMed] [Google Scholar]
- 42.Ayoub IM, Kolarova JD, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA, Gazmuri RJ. Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation. 2003;107:1804–1809. doi: 10.1161/01.CIR.0000058704.45646.0D. [DOI] [PubMed] [Google Scholar]
- 43.Cooley DA, Reul GJ, Wukasch DC. Ischemic contracture of the heart: "Stone Heart". Am J Cardiol. 1972;29:575–577. doi: 10.1016/0002-9149(72)90454-7. [DOI] [PubMed] [Google Scholar]
- 44.Katz AM, Tada M. The "Stone Heart": A challenge to the biochemist. Am J Cardiol. 1972;29:578–580. doi: 10.1016/0002-9149(72)90455-9. [DOI] [PubMed] [Google Scholar]
- 45.Sorrell VL, Altbach MI, Kern KB, Squire S, Hilwig RW, Hayes MM, Ewy GA, Berg RA. Images in cardiovascular medicine. Continuous cardiac magnetic resonance imaging during untreated ventricular fibrillation. Circulation. 2005 May 17;111(19):e294. doi: 10.1161/01.CIR.0000165126.38141.1C. [DOI] [PubMed] [Google Scholar]
- 46.Koretsune Y, Marban E. Mechanism of ischemic contracture in ferret hearts: relative roles of [Ca2+]i elevation and ATP depletion. Am J Physiol. 1990;258:H9–H16. doi: 10.1152/ajpheart.1990.258.1.H9. [DOI] [PubMed] [Google Scholar]
- 47.Gazmuri RJ. Effects of repetitive electrical shocks on postresuscitation myocardial function. Crit Care Med. 2000 November;28 11 Suppl:N228–N232. doi: 10.1097/00003246-200011001-00016. [DOI] [PubMed] [Google Scholar]
- 48.Gazmuri RJ, Deshmukh S, Shah PR. Myocardial effects of repeated electrical defibrillations in the isolated fibrillating rat heart. Crit Care Med. 2000;28:2690–2966. doi: 10.1097/00003246-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Kolarova JD, Ayoub IM, Gazmuri RJ. Cariporide enables hemodynamically more effective chest compression by leftward shift of its flow-depth relationship. Am J Physiol Heart Circ Physiol. 2005 February 11;288:H2904–H2911. doi: 10.1152/ajpheart.01181.2004. [DOI] [PubMed] [Google Scholar]
- 50.Takino M, Okada Y. Firm myocardium in cardiopulmonary resuscitation. Resuscitation. 1996;33:101–106. doi: 10.1016/s0300-9572(96)00995-1. [DOI] [PubMed] [Google Scholar]
- 51.Sanders AB, Atlas M, Ewy GA, Kern KB, Bragg S. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985 March;3(2):147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 52.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: A noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 53.Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation. 2005 June;65(3):357–363. doi: 10.1016/j.resuscitation.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 54.van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation. 2003 November;59(2):181–188. doi: 10.1016/s0300-9572(03)00208-9. [DOI] [PubMed] [Google Scholar]
- 55.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med. 1996 June;24(6):992–1000. doi: 10.1097/00003246-199606000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 57.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002 December 18;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Bailen M, Aguayo dH, Ruiz-Navarro S, Diaz-Castellanos MA, Rucabado-Aguilar L, Gomez-Jimenez FJ, Martinez-Escobar S, Moreno RM, Fierro-Roson J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005 August;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Xu T, Tang W, Ristagno G, Wang H, Sun S, Weil MH. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2008 January;36(1):188–192. doi: 10.1097/01.CCM.0000295595.72955.7C. [DOI] [PubMed] [Google Scholar]
- 60.Hilwig RW, Berg RA, Kern KB, Ewy GA. Endothelin-1 vasoconstriction during swine cardiopulmonary resuscitation improves coronary perfusion pressures but worsens postresuscitation outcome. Circulation. 2000 May 2;101(17):2097–2102. doi: 10.1161/01.cir.101.17.2097. [DOI] [PubMed] [Google Scholar]
- 61.Kern KB, Hilwig RW, Berg RA, Rhee KH, Sanders AB, Otto CW, Ewy GA. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997 June 17;95(12):2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 62.Vasquez A, Kern KB, Hilwig RW, Heidenreich J, Berg RA, Ewy GA. Optimal dosing of dobutamine for treating post-resuscitation left ventricular dysfunction. Resuscitation. 2004 May;61(2):199–207. doi: 10.1016/j.resuscitation.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Studer W, Wu X, Siegemund M, Marsch S, Seeberger M, Filipovic M. Influence of dobutamine on the variables of systemic haemodynamics, metabolism, and intestinal perfusion after cardiopulmonary resuscitation in the rat. Resuscitation. 2005 February;64(2):227–232. doi: 10.1016/j.resuscitation.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Niemann JT, Garner D, Khaleeli E, Lewis RJ. Milrinone facilitates resuscitation from cardiac arrest and attenuates postresuscitation myocardial dysfunction. Circulation. 2003 December 16;108(24):3031–3035. doi: 10.1161/01.CIR.0000101925.37174.85. [DOI] [PubMed] [Google Scholar]
- 65.Gazmuri RJ, Hoffner E, Kalcheim J, Ho H, Patel M, Ayoub IM, Epstein M, Kingston S, Han Y. Myocardial protection during ventricular fibrillation by reduction of proton-driven sarcolemmal sodium influx. J Lab Clin Med. 2001 January;137(1):43–55. doi: 10.1067/mlc.2001.111693. [DOI] [PubMed] [Google Scholar]
- 66.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation. 2001;104:234–239. doi: 10.1161/01.cir.104.2.234. [DOI] [PubMed] [Google Scholar]
- 67.Gazmuri RJ, Ayoub IM, Kolarova JD, Karmazyn M. Myocardial protection during ventricular fibrillation by inhibition of the sodium-hydrogen exchanger isoform-1. Crit Care Med. 2002 April;30 4 Suppl:S166–S171. doi: 10.1097/00003246-200204001-00010. [DOI] [PubMed] [Google Scholar]
- 68.Kolarova J, Yi Z, Ayoub IM, Gazmuri RJ. Cariporide potentiates the effects of epinephrine and vasopressin by nonvascular mechanisms during closed-chest resuscitation. Chest. 2005 April;127(4):1327–1334. doi: 10.1378/chest.127.4.1327. [DOI] [PubMed] [Google Scholar]
- 69.Ayoub IM, Kolarova J, Kantola RL, Sanders R, Gazmuri RJ. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Crit Care Med. 2005 November;33(11):2599–2605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol. 2007 April 12;103:55–65. doi: 10.1152/japplphysiol.01167.2006. [DOI] [PubMed] [Google Scholar]
- 71.Singh D, Kolarova JD, Wang S, Ayoub IM, Gazmuri RJ. Myocardial protection by erythropoietin during resuscitation from ventricular fibrillation. Am J Ther. 2007;14:361–368. doi: 10.1097/01.pap.0000249957.35673.f0. [DOI] [PubMed] [Google Scholar]
- 72.Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80:631–637. doi: 10.1016/j.resuscitation.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 73.von Planta M, Weil MH, Gazmuri RJ, Bisera J, Rackow EC. Myocardial acidosis associated with CO2 production during cardiac arrest and resuscitation. Circulation. 1989;80:684–692. doi: 10.1161/01.cir.80.3.684. [DOI] [PubMed] [Google Scholar]
- 74.Kette F, Weil MH, Gazmuri RJ, Bisera J, Rackow EC. Intramyocardial hypercarbic acidosis during cardiac arrest and resuscitation. Crit Care Med. 1993 June;21(6):901–906. doi: 10.1097/00003246-199306000-00020. [DOI] [PubMed] [Google Scholar]
- 75.Noc M, Weil MH, Gazmuri RJ, Sun S, Bisera J, Tang W. Ventricular fibrillation voltage as a monitor of the effectiveness of cardiopulmonary resuscitation. J Lab Clin Med. 1994;124:421–426. [PubMed] [Google Scholar]
- 76.Karmazyn M, Sawyer M, Fliegel L. The na(+)/h(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005 August;5(4):323–335. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 77.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999 June 25;84(12):1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 78.Avkiran M, Ibuki C, Shimada Y, Haddock PS. Effects of acidic reperfusion on arrhythmias and Na(+)-K(+)-ATPase activity in regionally ischemic rat hearts. Am J Physiol. 1996 March;270(3 Pt-2):H957–H964. doi: 10.1152/ajpheart.1996.270.3.H957. [DOI] [PubMed] [Google Scholar]
- 79.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol. 2001 December;281(6):H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 80.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000 November;28(5–6):285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+ /H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/ reperfused rat hearts. J Cardiovasc Pharmacol. 2002 April;39(4):569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003 April 24;541(1–3):1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 83.Nasser FN, Walls JT, Edwards WD, Harrison CE., Jr Lidocaine-induced reduction in size of experimental myocardial infarction. Am J Cardiol. 1980 December 1;46(6):967–975. doi: 10.1016/0002-9149(80)90353-7. [DOI] [PubMed] [Google Scholar]
- 84.Hinokiyama K, Hatori N, Ochi M, Maehara T, Tanaka S. Myocardial protective effect of lidocaine during experimental off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2003 February;9(1):36–42. [PubMed] [Google Scholar]
- 85.Ayoub IM, Radhakrishnan J, Gazmuri RJ. Targeting mitochondria for resuscitation from cardiac arrest. Crit Care Med. 2008;36:S440–S446. doi: 10.1097/ccm.0b013e31818a89f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayoub IM, Kolarova J, Gazmuri RJ. Cariporide given during resuscitation promotes return of electrically stable and mechanically competent cardiac activity. Resuscitation. 2009 October 21;81:106–110. doi: 10.1016/j.resuscitation.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radhakrishnan J, Kolarova JD, Ayoub IM, Gazmuri RlJ. AVE4454B--a novel sodium-hydrogen exchanger isoform-1 inhibitor--compared less effective than cariporide for resuscitation from cardiac arrest. Translational Research. 2011 February;157(2):71–80. doi: 10.1016/j.trsl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 89.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003 July 8;108(1):79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 90.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003 April 15;100(8):4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003 September 30;100(20):11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003 October;112(7):999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003 September 5;308(4):990–994. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- 94.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004 May 4;109(17):2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 95.Lipsic E, van der MP, Henning RH, Suurmeijer AJ, Boddeus KM, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Timing of erythropoietin treatment for cardioprotection in ischemia/reperfusion. J Cardiovasc Pharmacol. 2004 October;44(4):473–479. doi: 10.1097/01.fjc.0000140209.04675.c3. [DOI] [PubMed] [Google Scholar]
- 96.Parsa CJ, Kim J, Riel RU, Pascal LS, Thompson RB, Petrofski JA, Matsumoto A, Stamler JS, Koch WJ. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem. 2004 May 14;279(20):20655–20662. doi: 10.1074/jbc.M314099200. [DOI] [PubMed] [Google Scholar]
- 97.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004 June;18(9):1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 98.Namiuchi S, Kagaya Y, Ohta J, Shiba N, Sugi M, Oikawa M, Kunii H, Yamao H, Komatsu N, Yui M, Tada H, Sakuma M, Watanabe J, Ichihara T, Shirato K. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005 May 3;45(9):1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 99.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000 September 12;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythro-poietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001 March 27;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002 December 1;22(23):10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004 July;11:S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 103.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002 February 19;99(4):2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vesey DA, Cheung C, Pat B, Endre Z, Gobe G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004 February;19(2):348–355. doi: 10.1093/ndt/gfg547. [DOI] [PubMed] [Google Scholar]
- 105.Abdelrahman M, Sharples EJ, McDonald MC, Collin M, Patel NS, Yaqoob MM, Thiemermann C. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004 July;22(1):63–69. doi: 10.1097/01.shk.00001276869.21260.9d. [DOI] [PubMed] [Google Scholar]
- 106.Buemi M, Vaccaro M, Sturiale A, Galeano MR, Sansotta C, Cavallari V, Floccari F, D'Amico D, Torre V, Calapai G, Frisina N, Guarneri F, Vermiglio G. Recombinant human erythropoietin influences revascularization and healing in a rat model of random ischaemic flaps. Acta Derm Venereol. 2002;82(6):411–417. doi: 10.1080/000155502762064520. [DOI] [PubMed] [Google Scholar]
- 107.Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005 February 15;65(3):719–727. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 108.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006 September 1;71(4):684–694. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 109.van der MP, Lipsic E, Henning RH, Boddeus K, van d V, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol. 2005 July 5;46(1):125–133. doi: 10.1016/j.jacc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 110.Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, Tsukamoto O, Okada K, Koyama H, Komamura K, Takashima S, Shinozaki Y, Mori H, Shiraga M, Kitakaze M, Hori M. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol. 2006 July 4;48(1):176–184. doi: 10.1016/j.jacc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998 November;275(5 Pt-2):H1567–H1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- 112.Wald M, Gutnisky A, Borda E, Sterin-Borda L. Erythropoietin modified the cardiac action of ouabain in chronically anaemic-uraemic rats. Nephron. 1995;71(2):190–196. doi: 10.1159/000188711. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, Zhang HY, Zhu X, Shao Z, Yao Z. Preconditioning blocks cardiocyte apoptosis: role of K(ATP) channels and PKC-epsilon. Am J Physiol. 2002 April;282(4):H1380–H1386. doi: 10.1152/ajpheart.00348.2001. [DOI] [PubMed] [Google Scholar]
- 114.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, Caldwell RW, Johnson JA. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. Am J Physiol Heart Circ Physiol. 2007 October;293(4):H2219–H2230. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 115.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008 September 12;321(5895):1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003 May 2;92(8):873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006 June;290(6):H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 118.Shaik ZP, Fifer EK, Nowak G. Akt activation improves oxidative phosphorylation in renal proximal tubular cells following nephrotoxicant injury. Am J Physiol Renal Physiol. 2008 February;294(2):F423–F432. doi: 10.1152/ajprenal.00463.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kobayashi H, Miura T, Ishida H, Miki T, Tanno M, Yano T, Sato T, Hotta H, Shimamoto K. Limitation of infarct size by erythropoietin is associated with translocation of Akt to the mitochondria after reperfusion. Clin Exp Pharmacol Physiol. 2008 July;35(7):812–819. doi: 10.1111/j.1440-1681.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 120.Huang CH, Hsu CY, Chen HW, Tsai MS, Cheng HJ, Chang CH, Lee YT, Chen WJ. Erythropoietin improves postresuscitation myocardial dysfunction and survival in the asphyxia-induced cardiac arrest model. Shock. 2007 July;28(1):53–58. doi: 10.1097/shk.0b013e31802f0218. [DOI] [PubMed] [Google Scholar]
- 121.Popp E, Vogel P, Teschendorf P, Bottiger BW. Effects of the application of erythropoietin on cerebral recovery after cardiac arrest in rats. Resuscitation. 2007 August;74(2):344–351. doi: 10.1016/j.resuscitation.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 122.Huang CH, Hsu CY, Tsai MS, Wang TD, Chang WT, Chen WJ. Cardioprotective effects of erythropoietin on postresuscitation myocardial dysfunction in appropriate therapeutic windows. Crit Care Med. 2008;36:S467–S473. doi: 10.1097/ccm.0b013e31818a8cec. [DOI] [PubMed] [Google Scholar]
- 123.Cariou A, Claessens YE, Pene F, Marx JS, Spaulding C, Hababou C, Casadevall N, Mira JP, Carli P, Hermine O. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008 March;76(3):397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Incagnoli P, Ramond A, Joyeux-Faure M, Pepin JL, Levy P, Ribuot C. Erythropoietin improved initial resuscitation and increased survival after cardiac arrest in rats. Resuscitation. 2009 June;80(6):696–700. doi: 10.1016/j.resuscitation.2009.03.024. [DOI] [PubMed] [Google Scholar]