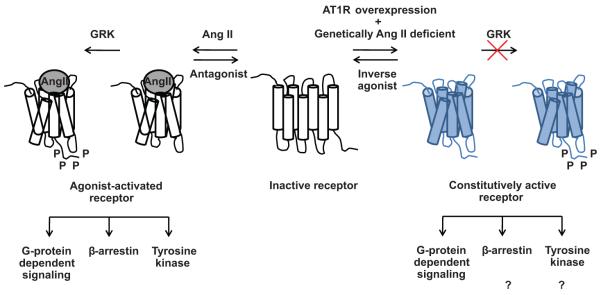
Cell surface receptors and their ligands cooperatively regulate physiological processes. The receptor activity is regulated positively when agonists bind and negatively when antagonists displace the agonists. Complete absence of a hormone should abrogate physiological and pathogenic functions regulated by the cognate receptor. However, awareness of the constitutive activity, the ability of native receptors to become functionally active in absence of hormone, is changing our view of the robustness of ligand-regulated receptor mechanisms.
Paradigms of constitutive activity of G protein-coupled receptors (GPCR) and inverse agonist activity of GPCR-targeted drugs are firmly established. The GPCR, angiotensin II (AngII) type 1 receptor (AT1R) can be spontaneously active1. Ways such as membrane environment, interacting proteins, receptor autoantibodies and single nucleotide polymorphisms (SNPs) that increase expression can increase G-protein signaling in the absence of AngII, using the potential energy of the receptor2. Inverse agonists can suppress the constitutive activity of a receptor, however, classical antagonists cannot perform this action1,2 (Figure 1).
Figure 1.
Mechanism of the constitutively active AT1R signaling is shown in blue. The classical agonist-activated AT1R signaling is shown in black and white. GRK: G protein-coupled receptor kinase, P: Phosphorylation.
Constitutive activity is an inherent property of a GPCR in all, including humans and animal, species1-3. Wild-type AT1R stimulates significant G protein signaling in the absence of AngII, when 1-10 pmol/mg of receptor is expressed in cell lines. The constitutively active pool of wild-type AT1R is less than 5%, which is the reason why it is difficult to detect it with the available functional assays in native tissues expressing the receptor in the fmol/mg-range. In general, effects of constitutive activity of native GPCRs in vivo has been studied in transgenic animals significantly over expressing the receptors. Constitutive activity of many native GPCRs including the AT1R, opioid receptors, D1 dopamine and the 5HT2C and 5HT7 serotonin receptors, the H3 histamine receptor, and the bradykinin B2 receptor have been ascertained this way3. Therefore, the question remains whether constitutive activity observed at such expression levels in the presence of endogenous ligand and GPCR/G-protein stoichiometry is of physiological relevance.
In this issue of Hypertension, Yasuda et al, convincingly demonstrate for the first time that cardiac-specific up-regulation of wild type hAT1R expression leads to spontaneous systolic dysfunction and chamber dilatation, accompanied by severe interstitial fibrosis in mice genetically made angiotensinogen (Agt) deficient4. The Agt-null mice with the endogenous level of AT1R expression did not develop the pathology. Conventionally AngII binding to the hAT1R is thought to initiate signal transduction pathways responsible for the physiological and pathological actions of AngII. Could enhancement of constitutive activity in vivo, due to overexpression of the native hAT1R, lead to cardiac abnormalities when the AngII production is genetically inhibited?
Constitutive activity of the native AT1R (<5%) in cultured cells is low, but introduced mutations such as N111G and N111S significantly enhance constitutive activity of AT1R (25-40%). Transgenic mice with endothelium restricted expression of low level of the AT1R-N111G mutant produced a hypotensive phenotype5. Transgenic mice with inducible cardiomyocyte-specific expression of wild-type or N111G mutant hAT1R from the onset of adolescence, show enhanced myocyte growth and associated cardiac hypertrophy in the adult6. Gene knock-in mice with the N111S mutant hAT1R with a C-terminal deletion (to reduce constitutive internalization) showed low-renin hypertension and progressive fibrosis in kidney and heart7. These studies established that engineered constitutive activating mutations are useful for controlled upregulation of local AT1R activity and mimic various in vivo disease conditions. However, activating mutations of the AT1R gene in humans have not been identified and it remains unclear whether constitutive activity of the native hAT1R has an in vivo pathogenic role.
To elucidate the pathogenic role of AngII-independent AT1R activation in the heart, Yasuda et al. crossed transgenic mice over expressing hAT1R under the control of α-myosin heavy chain (MHC) promoter with the Agt-knockout mice to create AT1Tg-AgtKO hybrid mice, in which the production of AngII is genetically deficient. Over expression of hAT1R in the AT1Tg parental mice was previously shown to induce cardiac remodeling in the presence of endogenous levels of AngII that is prevented by treatment with the AT1R blocker losartan8. The AT1Tg-AgtKO hybrid mice allowed the authors to unequivocally evaluate the AngII-independent constitutive activity in the hearts of mice in vivo, which until now was shown only in cultured cells.
The density of AT1R was increased by > 200-fold in AT1Tg-AgtKO hearts, compared with AgtKO hearts. Constitutive activation of the hAT1R in the AT1Tg-AgtKO hearts was showed by significantly increased distribution of Gαq/11 in the cytosol and phosphorylation of ERKs in AT1Tg-AgtKO hearts compared to AgtKO controls. These molecular changes in AT1Tg-AgtKO mice hearts were associated with progressive chamber dilatation, contractile dysfunction and interstitial fibrosis compared to normal cardiac structure and function in AgtKO mice. Progressive cardiac remodeling in AT1Tg-AgtKO mice was prevented by treatment with the AT1R inverse agonist, candesartan. Cardiac remodeling in offspring of Agt +/− females or Agt −/− females was similar, suggesting that maternal or placental angiotensinogen did not predispose postnatal development of cardiac remodeling in AT1Tg-AgtKO mice. The most logical explanation for the observed G-protein and ERK activation, cardiac remodeling and the AT1R inverse agonist effect in AT1Tg-AgtKO mice is the constitutive receptor activity. Cells, including cardiomyocytes, harbor mechanisms to down regulate activated receptors. Ligand-activated as well as constitutively activated mutant AT1R is phosphorylated by GPCR kinases (GRKs) and recruits β-arrestin, leading to internalization. However, the distribution of GRK2 and β-arrestins in the particulate fraction relative to the cytosolic fraction were comparable between AT1Tg-AgtKO and AgtKO hearts, implying lack of receptor down regulation. Yasuda et al. suggest that stochastic transient activated conformation in wild-type hAT1R may be subtle and not induce detectable receptor internalization4. Thorough experiments are needed for consolidating this mechanism and if proven would be novel.
In classical models of endocrine regulation, abnormal change in the efficacy or level of the hormone is thought to cause pathology. Consequently with regards to pathologies of RAS, the focus of therapeutic strategies has been on controlling circulating and local AngII levels9-10. Up-regulation of AT1R in stressed hearts and vessels in response to various hormones, cytokines, inflammation or metabolic stress would proportionally enhance constitutive activity of the AT1R and accelerate the progression of disease in these tissues which cannot be effectively prevented by strategies targeting AngII supply (e.g., ACE inhibitors) or clearance (e.g., ACE 2), but would require blockade of constitutive activity of the receptor directly through inverse agonists of AT1R. Indeed, AT1R blockers have been superior to ACE inhibitors in newly treated patients9-10. The inverse agonists are even better therapeutics than neutral antagonists in treating diseases caused by genetic variations and constitutively activating mutations of GPCRs. Although a hormone-negative condition in vivo may never arise, the proof-of-principle study by Yasuda et al, details the importance of constitutive activity of a native GPCR in disease pathogenesis5. Activating GPCR mutations underlying diverse diseases have been isolated and transgenic mice expressing these mutant GPCRs have been developed as animal models of human diseases1. The models created will be useful research tools for discovering and evaluating comparative potencies of inverse agonists. The regulatory principle Yasuda et al. have firmly confirmed will have wider relevance across the entire GPCR family.
Acknowledgements
We thank Jacqueline Kemp for useful suggestions.
Sources of Funding: NIH for RO1 grant (HL57470) to Karnik, S. and NRSA award (HL007914) to Unal, H.
Footnotes
Author Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 2.Unal H, Karnik SS. Domain coupling in GPCRs: the engine for induced conformational changes. Trends Pharmacol Sci. 2011 Oct 28; doi: 10.1016/j.tips.2011.09.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda N, Akazawa H, Ito K, Shimizu I, Kudo-Sakamoto Y, Yabumoto C, Yano M, Yamamoto R, Ozasa Y, Minamino T, Naito AT, Oka T, Shiojima I, Tamura K, Umemura S, Nemer M, Komuro I. Agonist-independent constitutive activity of angiotensin II receptor promotes cardiac remodeling in mice. Hypertension. 2012;59 doi: 10.1161/HYPERTENSIONAHA.111.175208. xxx-xxx. [DOI] [PubMed] [Google Scholar]
- 5.Ramchandran R, Takezako T, Saad Y, Stull L, Fink B, Yamada H, Dikalov S, Harrison DG, Moravec C, Karnik SS. Angiotensinergic stimulation of vascular endothelium in mice causes hypotension, bradycardia, and attenuated angiotensin response. Proc Natl Acad Sci USA. 2006;103:19087–19092. doi: 10.1073/pnas.0602715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res. 2009;81:592–600. doi: 10.1093/cvr/cvn230. [DOI] [PubMed] [Google Scholar]
- 7.Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–1925. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA. 2000;97:931–936. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber MA. Interrupting the renin-angiotensin system: the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of hypertension. Am J Hypertens. 1999;12:189–194. doi: 10.1016/s0895-7061(99)00105-3. [DOI] [PubMed] [Google Scholar]
- 10.Vegter S, Nguyen NH, Visser ST, de Jong-van den Berg LT, Postma MJ, Boersma C. Compliance, persistence, and switching patterns for ACE inhibitors and ARBs. Am J Manag Care. 2011;17:609–616. [PubMed] [Google Scholar]