Abstract
The gastrin-releasing peptide receptor (BB2r) has shown great promise for tumor targeting due to the increase of the receptor expression in a variety of human cancers including: prostate, breast, small-cell lung and pancreatic cancer. From clinical investigations, prostate cancer has been shown to be among the most hypoxic of the cancers investigated. Many solid tumors contain regions of hypoxia due to poor organization and efficiency of the vasculature. However, hypoxia is typically not present in normal tissue. Nitroimidazoles, a thoroughly investigated class of hypoxia selective drugs, have been shown to be highly retained in hypoxic tissues. The purpose of this study is to determine if the incorporation of hypoxia trapping moieties into the structural paradigm of BB2r-targeted peptides will increase the retention time of the agents in prostate cancer tumors.
The present work involves the design, syntheses, purification and in vitro investigation of hypoxia enhanced 111In-BB2r-targeted radioconjugates. A total of four BB2r-targeted conjugates (1-4) were synthesized and coupled with increasing numbers of 2-nitroimidazoles, a hypoxia trapping moiety. Conjugates were radiolabeled with 111In and purified by HPLC prior to in vitro studies. Receptor saturation assays under both normoxic and hypoxic conditions showed that the BB2r receptor expression on the PC-3 human prostate cancer cell line was not significantly affected by oxygen levels. Competitive binding assays revealed that incorporation of 2-nitroimidazoles had a detrimental effect to BB2r binding when adequate spacer groups, between the hypoxia trapping agent and the pharmacophore, were not employed. All of the 2-nitroimidazole containing BB2r-targeted agents exhibited significantly higher longitudinal retention in PC-3 cells under hypoxic conditions compared to the analogous normoxic studies. Protein association analysis revealed a threefold increase in binding of a 2-nitroimidazole containing BB2r-targeted agent under hypoxic relative to normoxic conditions. The positive nature of these results indicate that further exploration into the potential of hypoxia selective trapping agents for BB2r-targeted agents, as well as other targeted compounds, is warranted.
Introduction
Prostate cancer is among the most hypoxic of cancers found in the clinic.1, 2 This state of hypoxia is a result of the often chaotic and poor vascular organization in the tumor thereby inhibiting the ability of the microvasculature to efficiently deliver oxygen.2 This chaotic vascular architecture often leads to a high degree of oxygenation heterogeneity, with the tumor containing anoxic, hypoxic and normoxic regions, depending on the distance of the tissue from the nearest vascular vessel. Hypoxia has and continues to be a major obstacle in the treatment of numerous cancers, including prostate cancer, due to the increased chemotherapy and radiotherapy resistance of hypoxic tissue.2, 3 While the average fraction of hypoxic tissue in prostate cancer tumors is not well known, it is expected that the fraction of hypoxic cells would be significant.4, 5 Since hypoxia is a property that is not exhibited in most normal human tissues, several classes of bioreductive prodrugs (i.e. nitroimidazoles, aromatic N-oxides and quinones) are under development to exploit the hypoxic nature of tumors for diagnostic and therapeutic applications. One class of bioreductive hypoxia targeting agents that has undergone extensive basic and clinical investigation is nitroimidazoles.6-8 In hypoxic environments, this class of agent is selectively reduced (“triggered”) thereby generating a reactive electrophile that becomes trapped in the hypoxic cell. In particular, 2-nitroimidazole derivatives have been actively investigated for the development of hypoxia targeted agents capable of quantitating the hypoxic burden present in tumors using standard nuclear medicine imaging modalities. 9-13
The BB2 receptor (BB2r), also known as the Gastrin-Releasing Peptide (GRP) receptor, is a subtype of the Bombesin receptor family.14 The BB2r has been thoroughly investigated for the role the receptor plays in neoplastic tissue transformation and growth.15-17 From these studies and subsequent work, it was demonstrated that the BB2r is expressed in significantly higher densities on a number of human tumors and cancer cell lines, including prostate cancer tissues, relative to normal tissues.15, 16, 18, 19 These studies have provided the impetus for the development of BB2r-targeted agents for both diagnostic imaging and therapeutic applications. To date, most of these agents have revolved around synthetic analogs of Bombesin (BBN) - a fourteen amino acid, amphibian peptide that shares a C-terminal binding region sequence homology (Trp-Ala-Val-Gly-His-Leu-Met-NH2, BBN(8-14)NH2) with GRP (the native ligand for the BB2r).20 Analogs utilizing the BBN(8-14)NH2 sequence are able to agonistically bind to the BB2r with high (typically nanomolar) affinity and facilitate endocytosis of the agent into the cell.
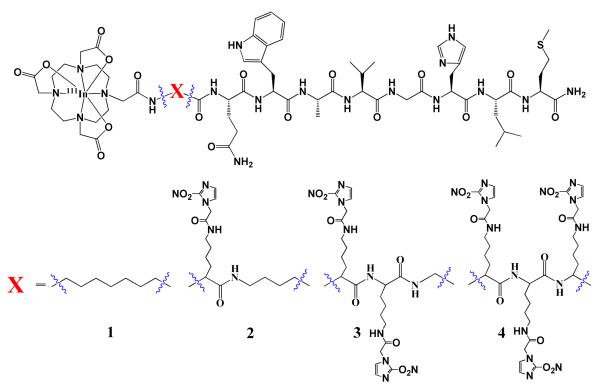
For BB2r-targeted drugs, as well as most small molecule based targeted ligands, the targeted conjugates typically demonstrate maximum tumor accumulation shortly after administration (~ 15-60 min).20-22 Unfortunately, one of the major challenges faced by many targeted peptides is the significant clearance of the drug relatively quickly (~ 1-4 hrs p.i.), after peak accumulation in the tumor, thereby reducing the diagnostic or therapeutic efficacy of the BB2r-targeted agent. In an attempt to increase the effectiveness of BB2r-targeted drugs, we and others have begun to evaluate various means of increasing the retention of the radiolabeled drug in prostate cancer tumors.23 Herein, we report the evaluation of BB2r-targeted agents that include the use of 2-nitroimidazoles as hypoxia trapping moieties to increase the retention of the drug in hypoxic tumor cells. Specifically, we synthesized four BB2r-targeted agents, that include (0 - 3) 2-nitroimidazole moieties, and evaluated the in vitro binding affinity and retention efficacy of the radioconjugates in normoxic and hypoxic PC-3 human prostate cancer cells. The structures of synthesized agents are depicted in Figure 1.
Figure 1.
Hypoxia enhanced 111In-BB2r-targeted conjugates.
Experimental
Unless otherwise noted all solvents were used without further purification. Deionized water was purified by a Millipore (U.S.) Mili-Q-Biocel. Ethylbromoacetate was purchased from Acros Organics (U.S.). Silica and TLC plates were purchased from Sorbent Technologies (U.S.). Acetonitrile, formic acid, N, N-diisopropylethylamine (DIEA), N,N-dimethylformamide (DMF), dichloromethane (DCM), N,N’-dicyclohexylcarbodiimide (DCC) , N-methylpyrrolidone (NMP), thioanisol, L-ascorbic acid, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), bovine serum albumin (BSA) and sodium dodecyl sulfate (SDS) were purchased from Fisher Scientific (U.S.). 2-nitroimidazole was purchased from Amfinecom (U.S.). O-Benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU), Fmoc-protected natural amino acids and the appropriate Rink Amide resins were purchased from Nova Biochem (U.S.). Fmoc-5-AOC and Fmoc-5-Ava were purchased from CreoSalus (U.S). Phosphate buffered saline (PBS) and Mammalian Protein Extraction Reagent (M-PER) were purchased from Thermo Scientific (U.S.). Indium-111 chloride (111InCl3) was purchased from MDS Nordon (Canada). Naturally abundant indium chloride (natInCl3), triisopropyl silane and 3,6-dioxa-1,8-octanedithiol were purchased from Sigma-Aldrich (U.S.). The [125I-Tyr4]-Bombesin was purchased from Perkin Elmer (U.S.). Cation exchange resin was purchased from Bio Rad (USA). Prostate cancer (PC-3) cell lines were obtained from American Type Culture Collection (U.S.) and cultured under vendor recommended conditions. Roswell Park Memorial Institute (RPMI) 1640 media and TrypLE™ Express were purchased from Invitrogen/ GIBCO (U.S.). Hypoxyprobe™-1 Plus Kit was purchased from hypoxyprobe, Inc (U.S). Flow Cytometry Staining Buffer and Foxp3 permeabilization Buffer were purchased from eBioscience (U.S.). The peptides were synthesized on a Liberty microwave peptide synthesizer from CEM (U.S.). Nuclear magnetic resonance spectra were recorded on Varian (U.S.) 500MHz INOVA spectrometer. 1H NMR chemical shifts are expressed as δ values (parts per million) and peaks are described as s for singlet and d for doublet. 13C NMR chemical shifts are expressed as δ values using acetone as an internal reference. HPLC/MS analyses were performed on a Waters (U.S.) e2695 system equipped with a Waters 2489 absorption detector and a Waters Qtof Micro electrospray ionization mass spectrometer. Evaluation and purification of radiolabeled conjugates was performed on a Waters 1515 binary pump equipped with a Waters 2489 absorption detector and a Bioscan (U.S.) Flow Count radiometric detector system. A Phenomenex (U.S.) Jupiter 10μ Proteo 250 × 10 mm semiprep column was used for the purification of bulk amounts of peptides. For the purification of conjugates, natIn-conjugates, and 111In-radioconjugates a Phenomenex Jupiter 10μ Proteo 250 × 4.60 mm analytical column was employed. Solid phase extraction was performed using Empore (U.S.) C18 10 mm high performance extraction disks. The separation of macromolecules and small molecules was performed using Amicon Ultra (Ireland) Ultracel-10K centrifugal filters in protein association analysis. Gamma decay detection of 111In and 125I for the in vitro binding, receptor saturation, efflux studies and protein binding fractionation studies was accomplished using a LTI (U.S.) Multi-Wiper nuclear medicine gamma counter. Flow cytometry studies were performed on BD FACSAria cell sorter (US).
Synthesis of 2-nitroimidazole acetic acid (2-NIAA)
The synthesis of 2-NIAA was carried out as reported in the literature by Gariépy and co-workers.24 A brief description of the two-step procedure is reported below.
Synthesis of 2-Nitroimidazole Ethyl Acetate (2-NIEA): 2-bromoethyl acetate ( 1.2 mL, 7.18 mmol) was added to a solution of 2-nitroimidazole ( 1 g, 8.84 mmol) and potassium carbonate (2.0 g, 14.5mmol) in dry acetonitrile (15 mL). The mixture was stirred for 24 h at room temperature, and the resulting precipitate was filtered and washed with acetone. Evaporation of the filtrate yielded a yellow oil (1.7 g). The pure product was isolated from the crude oil using flash chromatography (silica gel) eluted with CH2Cl2/MeOH/NH3 (50:7:1) as yellowish oil: 0.9 g (52.9% yield); Analyses for 2-nitroimidazole ethyl acetate: 1H NMR (CDCl3): 7.260 (d, J = 3.7 Hz, 1H, H-imidazole ring), 7.089 (d, J = 1.5 Hz, 1H, H-imidazole ring), 5.10 (s,2H, NCH2COO), 4.29 (q, 2H, OCH2CH3), 1.30 (t, 3H,OCH2CH3); δc (CDCl3): 166.0 (C=O), 128.5 (CH-imidazole ring), 126.5 (CH-imidazole ring), 62.6 (NCH2COO),50.9 (OCH2CH3), 14.0 (OCH2CH3).
Preparation of 2-Nitroimidazole Acetic Acid (2-NIAA): The purified oil of 2-NIEA (0.9 g) was mixed into a solution of NaOH (4 N, 2.5 mL), water (10 mL), and MeOH (10 mL). The resulting solution was stirred at room temperature until no ester derivative was evident by TLC (30 min), at which point a cation-exchange resin (H+, Bio-Rad, 4 g), which had been protonated by washing with H2SO4 (1N; 30 mL; pH 2.5) and drying of the filtrate yielded a yellow paste (1.09 g). Chromatography of crude product on silica gel [mobile phase, CH2Cl2/CH3CN/HCOOH (30:10:1)] yielded a white crystalline solid: 0.25 g. Analyses for 2-nitroimidazole acetic acid: 1H NMR 1H (D2O): δH 7.28 (1H, s, H-imidazole ring), 7.06 (1H, s, H-imidazole ring), 4.93 (2H, s, NCH2COO), 3.80 (1H, broad, COOH); 13C NMR(D2O): 172.20 (C=O), 128.03 (CH-imidazaole ring), 127.27 (CH-imidazole ring), 52.10 (NCH2COOH).
X-ray Crystallographic Analysis. With respect to X-ray crystallography studies, single crystals were obtained by slow evaporation of a methanolic solution of 2-NIAA. Crystal data for 2-NIAA: C5H5N3O4, MW = 171.12 g/mol, monoclinic, space group P21/n, a = 7.3412(8) Å, b = 8.2046(9) Å, c = 11.5678(13) Å, α = γ = 90°, β = 103.796(2)°, V = 676.65(13) Å3, Z = 4, Dcalc = 1.639 Mg/m3, μ = 0.147 mm−1, T = 100(2) K, Data was collected on a Bruker SMART 1K CCD, Refinement of data with I > 2σ(I)(1630 independent reflections, Rint = 0.0217) gave a R1(F) = 0.0349 and a wR2(F2) = 0.0946 for all data with a GOF = 1.055. Crystallographic data for the structural analysis has been deposited with the Cambridge Crystallographic Data Center (CCDC 838909). Copies of this information may be obtained free of charge from the CCDC (http://www.ccdc.cam.ac.uk) or from jcgarrison@unmc.edu
Solid-Phase Peptide Synthesis (SPPS)
Peptide synthesis was performed using an automated solid-phase peptide synthesizer employing traditional Fmoc chemistry and using a Rink Amide resin. Briefly, the resin (100 μmol of the resin substituted peptide anchors) was deprotected using piperidine, resulting in the formation of a primary amine from which the C-terminus of the growing peptide was anchored. The Fmoc protected amino acids (300 μmol) with appropriate orthogonal protection were activated with HBTU and sequentially added to the resin. The resulting peptide was orthogonally deprotected and cleaved from the resin using a cocktail consisting of triisopropyl silane (0.1 ml), water (0.1 ml), 3,6-dioxa-1,8-octanedithiol (0.1 ml) trifluoroacetic acid (4.625 ml) and thioanisole (0.075 ml), respectively. The cleaved peptide was subsequently precipitated and washed using cold (0 °C) methyl-tert-butyl ether (10 ml×3). The crude conjugate was dried by a centrivap concentrator and weighed. The purity of the crude 1, 2*, 3* and 4* conjugates ranged from 58-71 % by RP-HPLC. Isolated yields were 17.5, 11.9, 14.7 and 21.0% for 1, 2*, 3* and 4*, respectively. ES-MS was used to determine the molecular mass of the prepared peptides. All conjugates were peak purified to ≥ 95% purity and quantified by RP-HPLC prior to in vitro investigations.
Coupling of 2-NIAA to Bombesin conjugates
After purification of the conjugates, the 2-nitroimidazole acetic acid was manually coupled to □-amino group of the lysine residue for each peptides 2*, 3* and 4* (15 mg each) using standard amidation chemistry. Briefly, the 2-NIAA (4.95, 9.40, 14.85 mg respectively) was dissolved in M HBTU/DMF (0.45 M, 171 μL) solution followed by addition of DIEA/NMP solution (2.0 M, 96 μL). This solution was allowed to stand for 15 minutes before addition of the conjugate in DMF (200 μL). The reaction mixture was stirred for 3 hours at room temperature and subsequently evaporated to dryness. The residue was re-dissolved in water : acetonitrile (8:2), peak purified by RP-HPLC and characterized by mass spectrometry. Isolated conjugation yields were 34.2, 38.7 and 43.4 % for 2, 3 and 4, respectively.
Labeling with natInCl3
For the convenient characterization of the 111In-Bombesin conjugates, naturally abundant natIn was used to substitute for 111In in the ES-MS and in vitro binding studies. A sample of conjugates (0.75 mg) was dissolved in ammonium acetate buffer (0.5 M, 200 L, pH 5.5) and mixed with a solution of natInCl3 (1.1 mg, 10 mol). The solution was heated for 60 min at 90 °C. After cooling to room temperature, natIn-conjugates were then peak purified by RP-HPLC. Isolated yields were 36.7, 29.6, 33.5 and 41.9 % for natIn-1, natIn-2, natIn-3 and natIn-4, respectively. All natIn-conjugates were ≥ 95% purity before mass spectrometric characterization and in vitro binding studies were performed.
Radiolabeling with 111InCl3
A 250 μg sample of the conjugate was dissolved in ammonium acetate buffer (0.5 M, 250 L, pH 5.5). 111InCl3 (1 mCi ) was added to the vial containing the conjugate, and the solution was heated for 60 min at 90 °C and allowed to cool to room temperature. The resulting radioconjugates were peak purified using RP-HPLC (≥95%) and concentrated using C18 extraction disk. Elution of the extraction disk with ethanol/sterile saline solution (6:4, 600 L) delivered the radioconjugate in high purity. L-ascorbic acid (~20 mg) was added to all radioconjugates to reduce radiolysis. Radiolabeling yields for 111In-1, 111In-2, 111In-3 and 111In-4 were 32.3, 23.6, 26.9 and 36.1 %, correspondingly.
HPLC Purification and Analysis Methodology
When necessary, bulk sample purification was performed using a semi-preparative Proteo column with a flow rate of 5.0 mL/min. Sample purification for in vitro studies was performed on analytical Proteo column with a flow rate 1.5 mL/min. HPLC solvents consisted of H2O containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). For unlabeled and 111/natIn-conjugates of 1-3, an initial gradient of 85 % A : 15 % B linearly decreased to 75 % A : 25 % B over a 15 minute time period. For unlabeled and 111/natIn-conjugates of 4, an initial gradient of 80 % A : 20 % B linearly decreased to 70 % A : 30 % B over a 15 minute time period. At the end of the run time for all HPLC experiments, the column was flushed with the gradient 5 % A : 95 % B and re-equilibrated to the starting gradient.
In Vitro Competitive Cell-Binding Studies
For in vitro binding studies, the inhibitory concentration (IC50) for all conjugates and natIn-conjugates were determined using the PC-3 human prostate cancer cell line. natIn-conjugates were used as substitutes for the corresponding 111In-radioconjugates. Briefly, the PC-3 cells (~3×104) were suspended in RPMI 1640 media (pH 7.4, 4.8 mg/mL HEPES, and 2 mg/mL BSA) and incubated at 4°C for 40 min in the presence of radiolabeled [125I-Tyr4]-Bombesin and various concentrations of the natIn-conjugate. At the end of the incubation period, the cells were centrifuged, aspirated and washed with media a total of four times. The cell associated activity was measured using a gamma counter and the IC50 values determined by nonlinear regression using the one-binding site model of Graphpad PRISM 5(U.S.).
Flow Cytometric Analysis of Hypoxic Cell Induction
The PC-3 cells were plated at a concentration of 1×106 cells/ well in 6 well plates, and incubated overnight in normoxic (95% air, 5% CO2) and hypoxic (94.9% N2, 0.1% O2, 5% CO2) environments. The cells were washed twice with PBS and incubated with 10 μM pimonidazole in 1 ml fresh medium (control well media did not contain pimonidazole) for 2 hours. Cells were again washed twice with PBS and detached from the well surface using TrypLE for 15 min at 37°C. As per manufacturer’s protocols, 106 cells were treated with Foxp3 permeabilization solution at 4°C for 30 minutes, washed with buffer and resuspended. The PC-3 cells were subsequently incubated with the FITC-labeled Hypoxyprobe-1 monoclonal antibody (mAb1) (1:100 dilution in 0.5% BSA in PBS) in the dark at room temperature for 30 minutes. The stained cells were washed once, fixed in paraformaldehyde and subject to flow cytometric analysis. Cells were analyzed using a FACSAria instrument and FloJo software.
Receptor Saturation Binding Assays
Receptor saturation studies were performed on PC-3 cells under normoxic (95% air, 5% CO2) and hypoxic (94.9% N2, 0.1% O2, 5% CO2) conditions. PC-3 cells (3×104/well) were incubated overnight in 24-well plates at 37°C in RPMI 1640 media (pH 7.4, 4.8 mg/mL HEPES, and 2 mg/mL BSA) in hypoxic conditions. On the day of the experiment, the cells were seeded with fresh culture medium and incubated for 4 hour under normoxic or hypoxic conditions. Upon completion of the incubation, PC-3 cells were incubated with (30,000 cpm) [125I-Tyr4]-Bombesin and a series of [Tyr4]-Bombesin concentrations ranging from 0.469 nM to 120 nM for 1 hour at 4 °C. Non-specific binding was determined using 3 μM of the [Tyr4]-Bombesin in the presence of the radioligand. At the end of the incubation time cells were aspirated and washed thrice with cold media and the remaining radioactivity was measured by solubilizing the cells with 10% SDS. Non-linear regression analysis was then performed using Graphpad PRISM 5 (U.S.) to determine the Bmax and Kd values for each experiment.
Efflux Studies
Efflux studies were performed using PC-3 cells under normoxic (95% air, 5% CO2) and hypoxic (94.9% N2, 0.1% O2, 5% CO2) conditions. PC-3 cells were incubated in six-well plates (0.5 × 106 / well) under hypoxic conditions overnight in RPMI 1640 media (pH 7.4, 4.8 mg/mL HEPES, and 2 mg/mL BSA). On the day of the experiment, the medium was removed, and the cells were washed with cold medium and incubated for 4 hour under normoxic and hypoxic condition. Cells were incubated for an additional 2 hour at 37°C in the presence of 100,000 cpm of each 111In-radioconjugate. Upon completion of the incubation at timepoints 0, 30, 60, 90 and 120 min, cells were washed thrice with media to discard the unbound peptide. Surface bound radioactivity was removed by washing the cells twice with an acid wash (50 mM glycine-HCl/0.1 M NaCl buffer, pH 2.8). The cells were then lysed at 37°C using a 10 % aqueous SDS solution. The radioactivity of the effluxed, surface bound and internalized fractions for each radioconjugate was determined using a gamma counter. Statistical analyses were performed by two-way analysis of variance (ANOVA) using Graphpad PRISM 5 (U.S.).
Cellular Protein Analysis
For the cellular fractionation studies, the procedure for the preparation of normoxic and hypoxic PC-3 was carried out as outlined in the efflux studies above. On the day of the experiment, the medium was removed, and the cells were washed with warm medium and incubated for 4 hours under normoxic and hypoxic conditions. PC-3 cells were incubated for an additional 2 hours at 37°C in the presence of 30,000 cpm of each 111In-radioconjugate. At the end of this time, the cells were washed thrice with warm medium to remove the unbound, extracellular radioconjugates and allowed to incubate for an additional hour at 37°C. Subsequently, PC-3 cells were washed thrice with PBS and lysed using 1ml of M-PER at 37°C. The cellular debris was centrifuged down and the supernatant was then transferred to an Amicon Ultracel 10k filter device with an extra PBS (1ml). The samples were centrifuged at 4000×g for 10 minutes and washed with PBS (1ml×2) per wash. The radioactivity associated with the molecular weight fractions was determined using a gamma counter.
Results
Synthesis of 2-nitroimidazole acetic acid (2-NIAA)
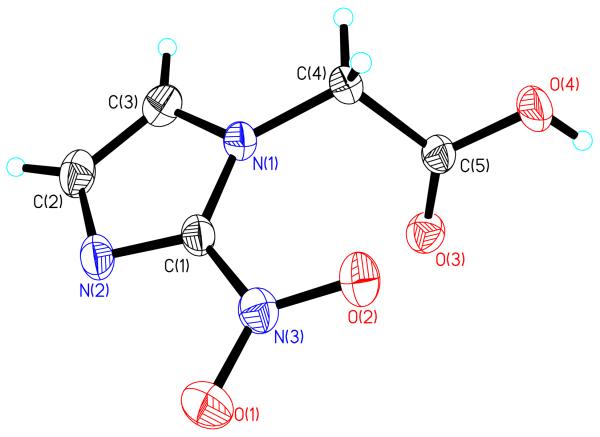
In order to couple the 2-nitroimidazole to the BB2r-targeted peptide conjugates, the acetic acid derivative was synthesized as outlined in literature procedures.24 Initially, the 2-nitroimidazole ethyl acetate was synthesized by formation of the potassium 2-nitroimidazole salt and subsequent reaction with 2-bromoethyl acetate. Conversion of the 2-nitroimidazole ethyl acetate to the acetic acid derivative was achieved using a cation exchange column pre-treated with dilute H2SO4 prior to elution. The chemical constitution of 2-NIAA was confirmed by NMR and single crystal x-ray crystallography. All 1H and 13C NMR data agreed with literature values. The molecular structure of 2-NIAA is depicted in Figure 2.
Figure 2.
An ORTEP representation of the 2-NIAA with thermal ellipsoids drawn at 50% probability. Selected bond lengths (Å) and angles (deg): O(1)-N(3) = 1.2223 (15), O(2)-N(3) = 1.2309 (14), N(3)-C(1) = 1.4387 (17), N(1)-C(1) = 1.3572 (16), N(2)-C(1) = 1.3161 (16), C(2)-C(3) = 1.372 (2), O(3)-C(5)= 1.2012 (16), O(4)-C(5) = 1.3206 (16), C(4)-C(5)= 1.5144 (17), O(1)-N(3)-O(2) = 125.13 (12), O(1)-N(3)-C(1) = 117.15 (11), O(2)-N(3)-C(1) = 117.71 (11), O(3)-C(5)-O(4) = 126.24 (12), O(3)-C(5)-C(4)=124.77 (12), O(4)-C(5)-C(4)=108.98(11).
Conjugate Synthesis and Radiolabeling
The conjugates were synthesized using standard solid-phase peptide synthesis. Specifically, four radioconjugates were synthesized using the DOTA-X-BBN(8-14)NH2 paradigm (Figure 1). The conjugates were purified by RP-HPLC and isolated with yields of 17.5, 11.9, 14.7 and 21.0 % for conjugates 1, 2*, 3* and 4*, correspondingly. RP-HPLC retention times and mass spectrometric identification of the conjugates are listed in Table 1. Subsequently, 2-NIAA was coupled to the □-amino group of lysine residue(s) of the conjugates using HBTU, DIEA and NMP in DMF. Analysis by RP-HPLC revealed that the coupling reactions were completed within 3 hours. The products obtained were consequently purified by RP-HPLC and characterized by ES-MS (Table 1). The yields of conjugates 2, 3 and 4 were 34.2, 38.7 and 43.3 %, respectively.
Table 1.
Mass spectrometric and RP-HPLC characterization of conjugates
| Analog | Molecular Formula |
MS Calculated |
MS Observeda |
RP-HPLC Retention Timeb |
IC50c/nM | |
|---|---|---|---|---|---|---|
| 1 | DOTA-8 AOC–BBN(8-14) NH2 | C67H106N18O17S | 1467.7 | 1468.0 | 14.99 | 10.2 ± 3.5 |
| 2* | DOTA [(D)K - 5-
AVA]–BBN(8- 14) NH2 |
C70H112N20O18S | 1553.8 | 1553.4 | 11.10 | - |
| 3* | DOTA [(D)K - (D)K -G]
–BBN(8- 14) NH2 |
C73H118N22O19S | 1639.8 | 1639.5 | 6.50 | - |
| 4* | DOTA [(D)K - (D)K - (D)K ]
– BBN(8-14) NH2 |
C77H127N23O19S | 1710.9 | 1711.3 | 9.0 | - |
| 2 | DOTA [(D)K (2-NIAA)- 5-
AVA] –BBN(8-14) NH2 |
C75H115N23O21S | 1706.8 | 1707.1 | 12.05 | 10.8 ± 2.8 |
| 3 | DOTA [(D)K (2-NIAA)- (D)K
(2- NIAA)-G] –BBN(8-14) NH2 |
C83H124N28O25S | 1945.9 | 1946.1 | 14.01 | 48.5 ± 27.1 |
| 4 | DOTA [(D)K (2-NIAA)- (D)K (2- NIAA)- (D)K (2-NIAA) ] – BBN(8-14) NH2 |
C92H136N32O28S | 2170.0 | 2170.4 | 14.76 | 1110 ± 205.0 |
| In-1 | In-DOTA-8
AOC–BBN(8-14) NH2 |
C67H103N18O17SIn | 1580.5 | 1580.2 | 15.94 | 23.5 ± 15.9 |
| In-2 | In-DOTA [(D)K (2-NIAA)- 5- AVA] –BBN(8-14) NH2 |
C75H112N23O21SIn | 1819.7 | 1819.5 | 12.67 | 5.70 ± 1.31 |
| In-3 | In-DOTA [(D)K (2-NIAA)-
(D)K (2-NIAA)-G] –BBN(8-14) NH2 |
C83H121N28O25SIn | 2058.9 | 2058.6 | 14.19 | 93.0 ± 37.3 |
| In-4 | In-DOTA [(D)K (2-NIAA)-
(D)K (2-NIAA)- (D)K (2-NIAA) -G] – BBN(8-14) NH2 |
C92H133N32O28SIn | 2283.1 | 2283.2 | 15.04 | 342 ± 99.0 |
For convenient mass spectra analysis 111In was replaced by natIn.
RP-HPLC methods described in Materials and Methods section.
Values represent mean ± SEM. (n=6)
Labeling and purification of the conjugates with natIn and 111In were carried out under nearly identical conditions. In brief, 111/natInCl3 was incubated with the desired conjugate in ammonium acetate buffer (0.1M, pH 5.5) at 90°C for 1 hour. Radiolabeling yields for 111In-1, 111In-2, 111In-3 and 111In-4 were 32.3, 23.6, 26.9 and 36.1 %, correspondingly. Purification of the 111/natIn-conjugates was accomplished by RP-HPLC. Concentration of RP-HPLC eluent was achieved by solid phase extraction on C18 column with typically ≥ 90% recovery. Prior to in vitro analysis, 111In-1-4 were found to have 99.0, 99.3, 97.7 and 95.3% radiochemical purity, correspondingly, as determined by RP-HPLC. Retention times and mass spectrometric characterization of the natIn-conjugates are given in Table 1.
In Vitro Competitive Cell-Binding Studies
The BB2r binding affinity of the unlabeled and natIn Bombesin conjugates were assessed by competitive binding assays performed at 4°C using the BB2r-positive human prostate cancer PC-3 cell line. All conjugates and natIn-conjugates were able to displace [125I-Tyr4]-Bombesin from BB2r binding sites on the PC-3 cell membranes in a dose-dependent manner as shown by the displacement curves (supporting information). For natIn-labeled conjugates, conjugates 1 and 2 demonstrated the highest affinity binding (5.70 ± 1.31, 23.5 ± 15.9 nM) to the BB2r. Conjugates 3 and 4 exhibited substantially poorer binding affinity, relative to 1 and 2, with affinities of 93.0 ± 37.3 nM and 342 ± 99 nM, correspondingly. Some differences in binding affinity were observed between unlabeled and natIn-labeled conjugates, but analysis of the data revealed no overall trend.
Hypoxia Staining and Fluorescence Analysis
Before beginning hypoxia experiments, the conditions used for the generation of hypoxic cells were validated by hypoxia staining. PC-3 cells were placed under hypoxic or normoxic conditions overnight and were subsequently exposed to pimonidazole for 2 hours. Pimonidazole, a 2-nitroimidazole based agent, has been widely used for the detection and quantification of hypoxia.25-27 After exposure to pimonidazole, the cells were permeabilized and incubated with a FITC-conjugated primary antibody against pimonidazole adducts. The fluorescence analysis of the stained PC-3 cells by flow cytometry is depicted in Figure 3. Mean fluorescence of the normoxic PC-3 cells treated with pimonidazole was 56.8. Treatment of hypoxic PC-3 with the hypoxia staining agent yielded a significant increase in antibody binding with a mean fluorescence of 286. The increase in antibody binding for the hypoxic cells corresponds, as expected, with an increase in hypoxia staining due to a drop in the O2 concentration. Based on previous literature reports, the values from this study are consistent with hypoxia being generated in the PC-3 cells and therefore subsequent in vitro studies were carried out employing the hypoxia generating conditions of this experiment.25
Figure 3.

Flow cytometric analysis of pimonidazole binding under control (no pimonidazole), normoxic (95% air, 5% CO2) and hypoxic (94.9% N2, 0.1% O2, 5% CO2) conditions in PC-3 cells.
Receptor Saturation Binding Assays
In order to determine if and to what extent the receptor expression changes under hypoxic conditions, receptor saturation studies were performed to quantify the receptor expression in PC-3 cells under normoxic and hypoxic conditions. The receptor saturation assays revealed that under normoxic and hypoxic conditions there were 0.35 ± 0.08 million and 0.30 ± 0.06 million binding sites, respectively, per PC-3 cell. The dissociation constant (Kd) values were found to be 1.0 ± 0.05 nM and 0.9± 0.08 nM, correspondingly, for normoxic and hypoxic PC-3 cells. Under the conditions of our experiments, these receptor saturation studies indicate that the BB2r expression at the cell surface does not significantly change with oxygen levels.
Efflux Studies
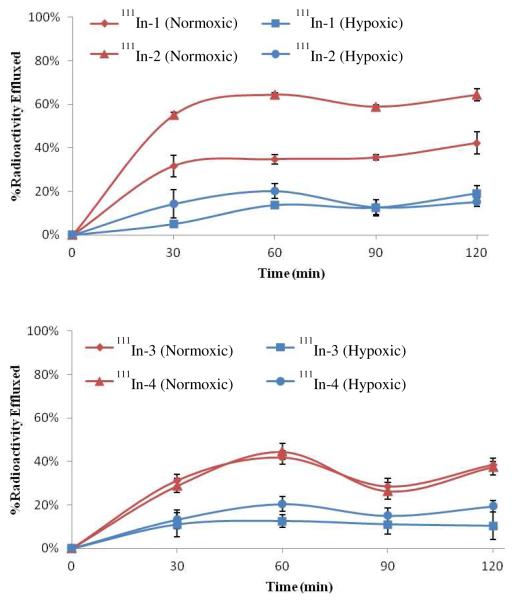
To evaluate the ability of the 2-nitroimidazole hypoxia trapping moieties to enhance the retention of the BB2r-targeted drugs, efflux studies were performed under normoxic and hypoxic environments to determine the rate at which the 111In-labeled radioconjugates 1 - 4 were effluxed from the PC-3 cells. In these studies, 111In-1, which does not have a 2-nitroimidazole incorporated into the structure of the radioconjugate, is the control and will be used to compare the relative effectiveness of the hypoxia trapping conjugates (111In-2-4) in both hypoxic and normoxic conditions.
The radioconjugates were incubated in the presence of the PC-3 cells for 2 hours prior to the start of the efflux studies. During this incubation time period, 111In-1 and 111In-2 demonstrated significant accumulation of the radioactivity with 22.6 and 20.5 %, respectively, of the added radioconjugates internalized under normoxic conditions (supporting information). The same radioconjugates demonstrated a marked decline in overall uptake under hypoxic conditions with 17.8 and 15.0 % internalized, respectively, in the same time span. In comparison, 111In-3 and 111In-4 demonstrated internalization of only 1.4 and 0.8 %, correspondingly, of the added radioactivity under normoxic conditions. Under hypoxic conditions, the 111In-3 and 111In-4 radioconjugates exhibited uptakes of 1.3 and 1.2 %, respectively, during the incubation period. Comparison of the internalization of the radioconjugates revealed that internalization corresponded well with the binding affinities observed in the competitive binding assays. Also, internalization under hypoxic conditions yielded significantly lower uptake for 111In-1 and 111In-2 relative to 111In-3 and 111In-4.
The efflux of the radioconjugates over time viewed as a percentage of the initial internalized activity is depicted in Figure 4. At all timepoints investigated, the radioconjugates demonstrated significantly higher retention of the radioconjugates under hypoxic conditions relative to normoxic. In the normoxic studies, the 111In radioconjugates 1-4 demonstrated substantial efflux with 32, 55, 31 and 29 %, respectively, of the radioactivity material being externalized within the first 30 minutes of the experiment. Under hypoxic conditions radioconjugates 1-4 exhibited significantly lower clearance of the radioactivity with 5, 14, 11 and 13 % of the intracellular radioactivity being externalized from the cell. After the initial 30 minute timepoint, the rate of clearance of the radioactivity substantially declined under both normoxic and hypoxic conditions. With the exception of 111In-2, the radioconjugates demonstrated an increased retention of 20-30 % of the initial internalized activity under hypoxic relative to normoxic conditions. Interestingly, 111In-2 exhibited a 50% increase in retention under hypoxic conditions which is significantly higher than the other radioconjugates.
Figure 4.
Efflux assays depicted as percentage of initial internalized activity for the 111In-radioconjugates in PC-3 cells. Values are mean ± SEM (n=3).
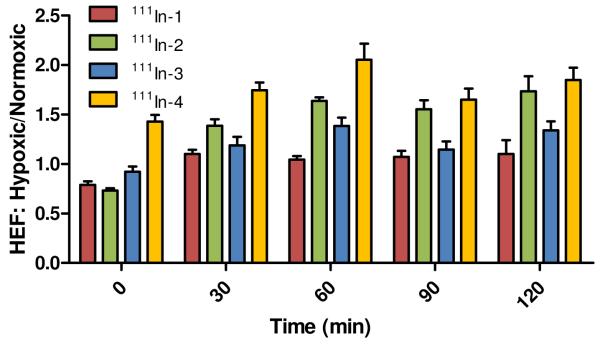
An alternative way to view the effect hypoxia has upon the clearance of the radioconjugates is by directly comparing the internalized activity of the radioconjugate for each timepoint under normoxic and hypoxic conditions. The hypoxia enhancement factor (HEF) is the ratio of the internalized activity of the radioconjugate under hypoxic conditions over the internalized activity observed under a normoxic environment. The HEF ratios for each radioconjugate at each timepoint are depicted in Figure 5. With the exception of 111In-4, all of the radioconjugates at the initial timepoint demonstrated favored retention of the radioconjugates in normoxic conditions. Although, by the 30 minute timepoint, all of the radioconjugates displayed significantly higher retention in hypoxic relative to normoxic cells (i.e. HEF > 1). This observation corresponds with the substantially higher clearance of the radioconjugates under normoxic relative to hypoxic conditions noted during the first 30 minutes of the efflux experiment. From 30 to 120 minutes, the 111In-labeled radioconjugates 1-4 demonstrated an average HEF ratio of 1.08 ± 0.03, 1.58 ± 0.15, 1.27 ± 0.12 and 1.83 ± 0.17, respectively. The radioconjugates 111In-2-4 exhibited significantly (p < 0.001 - 0.05) higher average HEF ratios compared to control (i.e. 111In-1). However, the HEF ratios of 111In-3 were substantially lower than that of either 111In-2 or 111In-4. For 111In-1, the control for this experiment, the radioconjugate exhibited no higher than a 10% increase in retention under hypoxic conditions from timepoints 30 to 120 minutes.
Figure 5.
HEF ratios for the efflux studies of 111In-BB2r-targeted radioconjugates under normoxic (95% air, 5% CO2) and hypoxic conditions (94.9% N2, 0.1% O2, 5% CO2). Values are mean ± SEM (n=3).
Cellular Protein Analysis
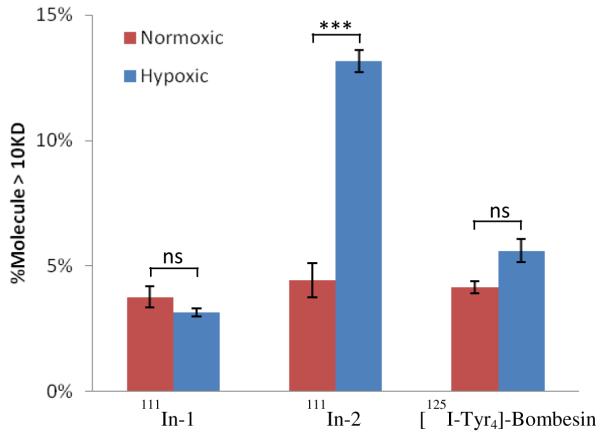
In a hypoxic environment, 2-nitroimdazoles undergo reduction and eventual irreversible conjugation with intracellular nucleophiles. It has been estimated that approximately 20% of such adduct formations are with nucleophilic groups (e.g. thiols) on proteins.28,29 In order to better evaluate the protein binding capabilities of the 2-nitroimidazole containing BB2r-targeted agents, the protein association properties of 111In-2 was evaluated against the controls, 111In-1 and [125I-Tyr4]BBN, under hypoxic and normoxic environments. The radioconjugates 111In-3 and 111In-4 were not evaluated because of the poor internalization of these analogs. After 2 hours of incubation with the radioconjugates under hypoxic or normoxic conditions, the PC-3 cells were lysed using a protein extraction reagent, centrifuged and the supernatant filtered using a 10 kDa centrifugal filter. Analysis of the protein associated radioactivity as a percentage of total intracellular activity is depicted in Figure 6. The radioconjugates 111In-1 and [125I-Tyr4]BBN both demonstrated similar levels, approximately 3.15 to 5.60 %, of protein association during the timespan of the experiment. Statistical analysis of the 111In-1 and [125I-Tyr4]BBN data revealed no significant impact on protein association under hypoxic or normoxic conditions. For the 111In-2 radioconjugate, the protein association under normoxic conditions was statistically the same as those found in controls. However, 111In-2 demonstrated a protein association under hypoxic conditions of approximately 13 % which was three fold higher than that observed under a normoxic environment. These findings strongly imply that the mechanism of protein retention of 111In-2 is associated with the 2-nitroimidazole moiety and is oxygen dependent.
Figure 6.
Protein association studies for 111In-1, 111In-2 and [125I-Tyr4]-BBN under normoxic (95% air, 5% CO2) and hypoxic (94.9% N2, 0.1% O2, 5% CO2) conditions in PC-3 cells. Values are mean ± SEM (n=3).
Discussion and Conclusion
In the area of cancer diagnostics and therapeutics, targeted, low molecular weight agents, such as those based on small peptides, sugars and steroids, generally offer the advantage of rapid tumor targeting and swift clearance from non-target tissues relative to the analogous large molecular weight counterparts (e.g. antibodies, nanoparticles, etc.).30-33 However, one significant challenge with low molecular weight, targeted agents has been obtaining optimal retention in the target tissue due to significant clearance of the radiotracer from the tumor. Since most of the targeted agents develop maximal accumulation in the target site within a short time (i.e. 15 min to 1 hour) after administration, it would be advantageous to develop techniques to selectively increase retention in the tumor thereby increasing the diagnostic and therapeutic efficacy of the agents.
Tumor hypoxia is the result of the inefficiency of the vascular architecture to adequately deliver nutrients, such as oxygen, to areas within the tumor. Since it has been demonstrated that human cancers, including prostate cancer, have significant levels of hypoxia, the development of hypoxia targeted drugs for both diagnostic and therapeutic applications in cancer has been an active area of research.2, 13, 34-38 In particular, 2-nitroimidazole derivatives, which selectively reduce and become trapped within hypoxic cells, have been among the most thoroughly investigated class of hypoxia selective agents. This study seeks to design BB2r-targeted agents that incorporate the hypoxia selective trapping agent 2-nitroimidazole into the linking group of the radioconjugate. It is estimated that upon reduction and activation of the 2-nitroimidazole approximately 20% of the hypoxia selective agent become irreversibly conjugated to proteins within the cell.29 In order to determine if one or more 2-nitroimidazoles would increase the retention of the radioconjugate in hypoxic cells, 111In-2-4, were synthesized with one, two or three 2-nitroimidazoles, respectively, incorporated into the linking group of the radioconjugate. Radioconjugate 111In-1 is a thoroughly investigated BB2r-targeted peptide that does not contain 2-nitroimidazole moieties and thus was used as the control in our experiments.22
Synthesis of the 111In-labeled radioconjugates 1-4 began with the synthesis of the 2-NIAA according to literature procedures.24 The 2-NIAA was thoroughly characterized by NMR and the structural constitution of the moiety confirmed by x-ray crystallography. Conjugates 1 and 2-4* were synthesized and purified by RP-HPLC in fairly low yields (12-22 %). Coupling of the 2-NIAA to the conjugates 2*-4* was accomplished using standard amidation chemistry. Further purification of 2-4 by RP-HPLC provided the conjugates in modest yields (34 - 44 %). Radiolabeling of conjugates 1-4 proceeded easily and with high purity (≥ 95%) using standard radiolabeling conditions.22
In vitro competitive BB2r binding assays of 111In-1-4 were performed using PC-3 cells with [125I-Tyr4]-Bombesin as the competitive radioligand. For the natural indium labeled conjugates, these studies demonstrated the following trend: 111In-2 > 111In-1 > 111In-3 > 111In-4. Analyzing the structure-activity relationship with respect to binding affinity, it was determined that inclusion of more than one 2-nitroimidazole in the linking group had an unfavorable effect on the binding affinity of the radioconjugate. This strongly indicates that the 2-nitroimidazoles are interfering, through charge or sterics, with the ability of the pharmacophore (i.e. BBN(8-14)NH2) to bind strongly to the BB2r. Since the pKa of 1-substituted-2-nitroimidazoles, such as misonidazole, are generally significantly below physiological pH, the 2-nitroimidazoles are likely not charged.39, 40 Given this, it is probable that the loss in binding affinity in 111In-3 and 111In-4 are primarily a result of steric interference of the 2-nitroimidazole trapping moieties with the BB2r-targeting vector. Based on the 111In-2 radioconjugate, the inclusion of a linker between the 2-nitroimidazole-amino acid residue and the pharmacophore of at least five carbon lengths is needed to achieve more optimal binding. This need for adequate spacers will be considered when designing future 2-nitroimidazole containing radioconjugates.
In order to examine the effect hypoxia has on the uptake and retention of the designed 111In labeled radioconjugates, the conditions for the generation of hypoxic PC-3 cells was first validated. For this purpose pimonidazole, a well-known and commonly used agent for the assessment of cellular hypoxia, was used for the confirmation of hypoxia generation in PC-3 cells.25-27 The targeting mechanism of the pimonidazole utilizes the same 2-nitroimidazole moiety employed in the design of the 111In-2-4 radioconjugates. Exposure of PC-3 cells incubated under the hypoxic conditions of our studies revealed a fivefold increase in pimonidazole binding compared to PC-3 cells incubated under normoxic conditions as determined by flow cytometry. These results are consistent with similar hypoxia analysis utilizing pimonidazole.25 Since pimonidazole utilizes the same 2-nitroimidazole moiety for hypoxia targeting as the 111In labeled radioconjugates in our study, the cellular hypoxia generated utilizing our conditions were determined to be sufficient for the evaluation of the BB2r expression and the efficacy of the radioconjugates in PC-3 cells.
The design of targeted agents that are selectively enhanced toward hypoxic cells requires an understanding of the expression of the target under normoxic as well as hypoxic conditions. Since these agents gain entry into the cell through receptor-mediated endocytosis, if expression of the receptor target is significantly down-regulated under hypoxic conditions, any enhancement in hypoxic retention is likely to be substantially inhibited. Conversely, if under hypoxic conditions the target increases in expression; both the targeting and hypoxia enhancement of the drug would be expected to be improved. With this in mind, receptor saturation studies were undertaken to evaluate the expression of the BB2r on the surface of the PC-3 cells under hypoxic and normoxic conditions. From these studies, it was determined that the BB2r expression was 0.35 ± 0.08 and 0.30 ± 0.06 million receptors per PC-3 cell under normoxic and hypoxic conditions, correspondingly. During this study, oxygen levels, at least throughout the course of our investigation, did not play a significant role in the surface receptor expression of the BB2r. These findings are in accordance with a report from Martínez and colleagues of the BB2r expression in the H209 human lung cancer cell line under normoxic and hypoxic conditions.41 With respect to overall BB2r expression of the PC-3 cells, the values obtained agree well with other reports.42
Efflux studies were performed under normoxic and hypoxic conditions to evaluate the efficacy of the hypoxia trapping moieties to enhance retention in hypoxic cells. The initial 2 hour incubation of the radioconjugates with the PC-3 cells yielded a bimodal uptake pattern. 111In-1, the control for our experiment, and 111In-2 demonstrate significant (22.6 % and 20.5 % of the dose, respectively) uptake, while 111In-3 and 111In-4 exhibited significantly lower internalization (1.4 % and 0.8 %, respectively). Based on the in vitro binding data, the significant decline in internalization with respect to 111In-3 and 111In-4 is likely due to the poor receptor affinity of these radioconjugates relative to 111In-1 and 111In-2. Interestingly, for some of the radioconjugates, oxygen levels seemed to have a significant effect on the initial uptake values of the radioconjugates. This is particularly true for 111In-1 and 111In-2 in which a significant reduction of 25 % was observed under hypoxic conditions for both radioconjugates. The cause for this decline is unknown; however, it was interesting to note that within the first 30 minutes of the efflux study at normoxia, all radioconjugates undergo substantial decline (29 – 55 %) to levels that are significantly below that of the hypoxic levels. Contrarily, for the hypoxic studies, the amount of internalized activity declined relatively little (5 - 15 %) during the same time period. Further investigations are needed to elucidate the exact cause for these contrasting observations. After the initial 30 minute timepoint, there was relatively little decline in internalized activity among the radioconjugates.
As stated earlier, normoxic levels of internalized activity initially were greater, excepting 111In-4, in normoxic rather than the hypoxic studies. Although by 30 minutes, the situation had reversed itself with all radioconjugates demonstrating hypoxic/normoxic ratios greater than one. The radioconjugate 111In-4 demonstrated the highest average hypoxia enhancement (1.83 ± 0.17) followed closely by 111In-2 (1.58 ± 0.15). Interestingly, 111In-3 has a fairly low average enhancement of 1.27 ± 0.12 compared to 111In-2. Given that 111In-3 has two 2-nitroimidazole moieties in comparison to one incorporated into 111In-2, the lower hypoxia enhancement is unexpected. The reason for this circumstance is not clear. Remarkably, even the control in our experiment, 111In-1, demonstrated a small (≤ 10 %) increase in retention under hypoxic conditions. Speculatively, the retention in the control under hypoxic conditions may be attributed to slower metabolism kinetics or other hypoxia-impaired cellular functions needed to efflux the radioconjugates. Ultimately, more studies, which are in progress, are needed to obtain a clearer understanding of this process. Nevertheless, all of the experimental radioconjugates (i.e. 111In-2-4) demonstrated significantly higher hypoxia enhancement than observed in the control, strongly suggesting that 2-nitroimidazoles are at least partially responsible for this enhancement.
It is well known that 2-nitroimidazole derivatives based on small molecules bind irreversibly to proteins under hypoxic conditions.25, 26, 29 In order to elucidate whether or not BB2r-targeted agents employing 2-ntiroimidazoles can conjugate to proteins, the cellular protein of the PC-3 cells were isolated after incubation with 111In-1, 111In-2 and [125I-Tyr4]BBN under hypoxic and normoxic conditions. The controls, 111In-1 and [125I-Tyr4]BBN, demonstrated 3.15 to 5.60 % association with the proteins under both normoxic and hypoxic conditions. Statistical analysis of the difference between the hypoxic and normoxic protein association for the controls revealed no statistically significant difference (p > 0.05). Since the controls do not contain reactive moieties that would be expected to irreversibly bind to macromolecules, the protein association for the controls are most likely due to reversible, non-specific binding. Under normoxic conditions the 111In-2 radioconjugate demonstrated similar protein association as controls. However, under hypoxic conditions the 111In-2 radioconjugate demonstrated a threefold increase in protein association (p < 0.001). These results indicate that the increase in protein association of 111In-2 is oxygen dependent and is consistent with the trapping mechanism of the 2-nitroimidazole. While the exact binding mode of the 111In-2 with proteins is not known beyond doubt, the findings from a superfluity of 2-nitroimidazole literature and the results of this study lead us to the conclusion that the increased protein association of the 111In-2 radioconjugate is likely irreversibly bound to the proteins. Assuming the 111In-2 protein association under normoxic conditions is reversible, non-specific binding, the increased protein association of the radioconjugate under hypoxic conditions signifies that ~ 10 % of the internalized cellular activity is likely irreversibly bound to cellular proteins.
In conclusion, we have synthesized and evaluated three BB2r-targeted radioconjugates that have 2-nitroimidazole hypoxia trapping moieties conjugated to the linking group of the peptide for the purpose of enhancing retention in hypoxic cancers. Our studies indicate that placement of the 2-nitroimidazole moieties close to the pharmacophore has a detrimental effect on the ability of the radioconjugate to adequately bind to the BB2r. The BB2r-targeted agents that include 2-nitroimidazole moieties demonstrated improved longitudinal retention in hypoxic relative to normoxic PC-3 cells. Additionally, we found that the inclusion of 2-nitroimidazole moieties in the BB2r-targeted agent design significantly increases protein association, by a likely irreversible conjugation reaction, under hypoxic conditions. Also, evaluation of the PC-3 human prostate cancer cell line reveals that the BB2r expression is not dependent on oxygen levels and suggests that the BB2r would therefore still be a valid target on the hypoxic fraction of BB2r-postive prostate cancer cells. While further work is still needed, this work suggests a potential new avenue to significantly increase the cellular retention of many targeted agents in hypoxic cancers. Our future work will focus on elucidating the mechanisms of retention of the hypoxia enhanced BB2r-targeted agents and evaluating the efficacy of these agents in in vivo cancer models.
Supplementary Material
Acknowledgements
The authors would like to gratefully acknowledge the National Cancer Institute (4 R00 CA137147) and the National Center for Research Resources (5 P20 RR021937) for the funding and support of this research. The author JCG would like to thank Dr. Charles L. Barnes in the Department of Chemistry at the University of Missouri-Columbia for the use of his x-ray diffractometer. Also, JCG would like to acknowledge Dr. Timothy Hoffman and the Harry S. Truman Veterans’ Hospital for the use of laboratory space and equipment during the initial phases of this work. NKW would like to acknowledge Dr. V. M. Kulkarni for encouragement.
Abbreviations
- BB2r
Gastrin-Releasing Peptide Receptor
- BBN
Bombesin
- DIEA
N, N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DCM
dichloromethane
- DCC
N,N’-dicyclohexylcarbodiimide
- NMP
N-methylpyrrolidone
- 111In
111Indium
- natIn
naturalIndium
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- BSA
bovine serum albumin
- SDS
sodium dodecyl sulfate
- HBTU
O-benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate
- PBS
phosphate buffered saline
- 2-NIAA
2-nitroimidazole acetic acid
- 2-NIEA
2-nitroimidazole ethyl acetate
- Bmax
total receptor number
- Kd
dissociation constant
- HEF
hypoxia enhancement factor
Footnotes
Supporting Information
Crystallographic details, selected ES-MS spectra, In Vitro Competitive Cell-Binding displacement curves and Receptor Saturation curves are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Anastasiadis AG, Stisser BC, Ghafar MA, Burchardt M, Buttyan R. Tumor hypoxia and the progression of prostate cancer. Current Urology Reports. 2002;3:222–228. doi: 10.1007/s11934-002-0068-6. [DOI] [PubMed] [Google Scholar]
- (2).Brown JM, William WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews: Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- (3).Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. Journal of the National Cancer Institute. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- (4).Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, Stobbe C, Hanks GE. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age - An Eppendorf pO(2) study. Cancer. 2000;89:2018–2024. doi: 10.1002/1097-0142(20001101)89:9<2018::aid-cncr19>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- (5).Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Pinover WH, Greenberg RE, Stobbe C, Hanks GE. Hypoxia in human prostate carcinoma - An Eppendorf Po-2 study. American Journal of Clinical Oncology - Cancer Clinical Trials. 2001;24:458–461. doi: 10.1097/00000421-200110000-00009. [DOI] [PubMed] [Google Scholar]
- (6).Weinmann M, Welz S, Bamberg M. Hypoxic radiosensitizers and hypoxic cytotoxins in radiation oncology. Current Medicinal Chemistry: Anti-Cancer Agents. 2003;3:364–374. doi: 10.2174/1568011033482350. [DOI] [PubMed] [Google Scholar]
- (7).Nunn A, Linder K, Strauss HW. Nitroimidazoles and Imaging Hypoxia. European Journal of Nuclear Medicine. 1995;22:265–280. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- (8).Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. Journal of Nuclear Medicine. 2008;49(Suppl 2):129S–148S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- (9).Chapman JD, Engelhardt EL, Stobbe CC, Schneider RF, Gerald GE. Measuring hypoxia and predicting tumor radioresistance with nuclear medicine assays. Radiotherapy and Oncology. 1998;46:229–237. doi: 10.1016/s0167-8140(97)00186-2. [DOI] [PubMed] [Google Scholar]
- (10).Engelhardt EL, Schneider RF, Seeholzer SH, Stobbe CC, Chapman JD. The synthesis and radiolabeling of 2-nitroimidazole derivatives of cyclam and their preclinical evaluation as positive markers of tumor hypoxia. Journal of Nuclear Medicine. 2002;43:837–850. [PubMed] [Google Scholar]
- (11).Evans SM, Kachur AV, Shiue C-Y, Hustinx R, Jenkins WT, Shive GG, Karp JS, Alavi A, Lord EM, Dolbier WR, Jr., Koch CJ. Noninvasive detection of tumor hypoxia using the 2-nitroimidazole [F-18]EF1. Journal of Nuclear Medicine. 2000;41:327–336. [PubMed] [Google Scholar]
- (12).Yamamoto F, Oka H, Antoku S, Ichiya Y, Masuda K, Maeda M. Synthesis and characterization of lipophilic 1-[F-18]fluoroalkyl-2-nitroimidazoles for imaging hypoxia. Biological & Pharmaceutical Bulletin. 1999;22:590–597. doi: 10.1248/bpb.22.590. [DOI] [PubMed] [Google Scholar]
- (13).Yang DJ, Ilgan S, Higuchi T, Zareneyrizi F, Oh C-S, Liu C-W, Kim EE, Podoloff DA. Noninvasive assessment of tumor hypoxia with Tc-99m labeled metronidazole. Pharmaceutical Research. 1999;16:743–750. doi: 10.1023/a:1018836911013. [DOI] [PubMed] [Google Scholar]
- (14).Jensen RT, Battey JF, Spindel ER, Benya RV. International union of pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacological Reviews. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Research. 1999;59:1152–1159. [PubMed] [Google Scholar]
- (16).Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. American Journal of Pathology. 1999;155:2067–2076. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Fleischmann A, Laderach U, Friess H, Buechler MW, Reubi JC. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Laboratory Investigation. 2000;80:1807–1817. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- (18).Cornelio DB, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Annals of Oncology. 2007;18:1457–1466. doi: 10.1093/annonc/mdm058. [DOI] [PubMed] [Google Scholar]
- (19).Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer J-C, Gugger M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[D-TYR6, β-ALA11, PHE13, NLE14] bombesin(6-14) Clinical Cancer Research. 2002;8:1139–1146. [PubMed] [Google Scholar]
- (20).Ananias HJ, de Jong IJ, Dierckx RA, van de Wiele C, Helfrich W, Elsinga PH. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Current Pharmaceutical Design. 2008;14:3033–3047. doi: 10.2174/138161208786404335. [DOI] [PubMed] [Google Scholar]
- (21).Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: Side-by-side comparison of the CB-TE2A and DOTA chelation systems. Journal of Nuclear Medicine. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- (22).Garrison JC, Rold TL, Sieckman GL, Naz F, Sublett SV, Figueroa SD, Volkert WA, Hoffman TJ. Evaluation of the pharmacokinetic effects of various linking group using the 111In-DOTA-X-BBN(7-14)NH2 structural paradigm in a prostate cancer model. Bioconjugate Chemistry. 2008;19:1803–1812. doi: 10.1021/bc8001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hueting R, Christlieb M, Dilworth JR, Garcia Garayoa E, Gouverneur V, Jones MW, Maes V, Schibli R, Sun X, Tourwe DA. Bis(thiosemicarbazones) as bifunctional chelators for the room temperature 64-copper labeling of peptides. Dalton Trans. 2010;39:3620–32. doi: 10.1039/b925128f. [DOI] [PubMed] [Google Scholar]
- (24).Gariépy J, Rémy S, Zhang XG, Ballinger JR, Bolewska-Pedyczak E, Rauth M, Bisland SK. A simple two-step approach for introducing a protected diaminedithiol chelator during solid-phase assembly of peptides. Bioconjugate Chemistry. 2002;13:679–684. doi: 10.1021/bc0100814. [DOI] [PubMed] [Google Scholar]
- (25).Koch CJ. Importance of antibody concentration in the assessment of cellular hypoxia by flow cytometry: EF5(1) and pimonidazole. Radiation Research. 2008;169:677–688. doi: 10.1667/RR1305.1. [DOI] [PubMed] [Google Scholar]
- (26).Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–45. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- (27).Carnell DM, Smith RE, Daley FM, Saunders MI, Bentzen SM, Hoskin PJ. An immunohistochemical assessment of hypoxia in prostate carcinoma using pimonidazole: Implications for radioresistance. International Journal of Radiation Oncology Biology Physics. 2006;65:91–99. doi: 10.1016/j.ijrobp.2005.11.044. [DOI] [PubMed] [Google Scholar]
- (28).Yaromina A, Zips D, Thames HD, Eicheler W, Krause M, Rosner A, Haase M, Petersen C, Raleigh JA, Quennet V, Walenta S, Mueller-Klieser W, Baumann M. Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: the need for a multivariate approach in biomarker studies. Radiother Oncol. 2006;81:122–9. doi: 10.1016/j.radonc.2006.08.010. [DOI] [PubMed] [Google Scholar]
- (29).Raleigh JA, Koch CJ. Importance of thiols in the reductive binding of 2-nitroimidazoles to macromolecules. Biochemical Pharmacology. 1990;40:2457–2464. doi: 10.1016/0006-2952(90)90086-z. [DOI] [PubMed] [Google Scholar]
- (30).Fischman AJ, Babich JW, Strauss HW. A ticket to ride: peptide radiopharmaceuticals. Journal of Nuclear Medicine. 1993;34:2253–2263. [PubMed] [Google Scholar]
- (31).Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nature Reviews: Drug Discovery. 2003;2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- (32).Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB. Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. European Journal of Endocrinology. 2004;151:15–27. doi: 10.1530/eje.0.1510015. [DOI] [PubMed] [Google Scholar]
- (33).Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nature Reviews: Drug Discovery. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- (34).Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Molecular Medicine Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- (35).Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, Wester HJ, Beck R, McEwan AJB, Wiebe LI, Schwaiger M. Hypoxia-specific tumor imaging with F-18-fluoroazomycin arabinoside. Journal of Nuclear Medicine. 2005;46:106–113. [PubMed] [Google Scholar]
- (36).Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, Hicks RJ. Imaging of tumor hypoxia with [I-124] IAZA in comparison with [F-18] FMISO and [F-18]FAZA - first small animal PET results. Journal of Pharmacy and Pharmaceutical Sciences. 2007;10:203–211. [PubMed] [Google Scholar]
- (37).Eschmann SM, Paulsen F, Bedeshem C, Machulla HJ, Hehr T, Bamberg M, Bares R. Hypoxia-imaging with F-18-misonidazole and PET: Changes of kinetics during radiotherapy of head-and-neck cancer. Radiotherapy and Oncology. 2007;83:406–410. doi: 10.1016/j.radonc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- (38).Hoigebazar L, Jeong JM, Choi SY, Choi JY, Shetty D, Lee YS, Lee DS, Chung J-K, Lee MC, Chung YK. Synthesis and characterization of nitroimidazole derivatives for 68Ga-labeling and testing in tumor xenografted mice. Journal of Medicinal Chemistry. 2010;53:6378–6385. doi: 10.1021/jm100545a. [DOI] [PubMed] [Google Scholar]
- (39).Dennis MF, Stratford MR, Wardman P, Watts ME. Cellular uptake of misonidazole and analogues with acidic or basic functions. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine. 1985;47:629–643. doi: 10.1080/09553008514550871. [DOI] [PubMed] [Google Scholar]
- (40).Gerweck LE. Tumor pH: implications for treatment and novel drug design. Seminars in Radiation Oncology. 1998;8:176–182. doi: 10.1016/s1053-4296(98)80043-x. [DOI] [PubMed] [Google Scholar]
- (41).Martínez A, Zudaire E, Julián M, Moody TW, Cuttitta F. Gastrin-releasing peptide (GRP) induces angiogenesis and the specific GRP blocker 77427 inhibits tumor growth in vitro and in vivo. Oncogene. 2005;24:4106–4113. doi: 10.1038/sj.onc.1208581. [DOI] [PubMed] [Google Scholar]
- (42).Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, Thomas R, Eaton SM, Bogdan NJ, Arunachalam T, Reubi JC, Raju N, Metcalfe EC, Lattuada L, Linder KE, Swenson RE, Tweedle MF, Nunn AD. 177Lu-AMBA: synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. Journal of Nuclear Medicine. 2006;47:1144–1152. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.