Abstract
Tumor resistance to chemotherapy is the major obstacle to employ cisplatin, one of the broadly used chemotherapeutic drugs, for effective treatment of various tumors in the clinic. Most acknowledged mechanisms of cancer resistance to cisplatin focus on increased nuclear DNA repair or detoxicity of cisplatin. We previously demonstrated that there was a unique metabolic profile in cisplatinresistant (CP-r) human epidermoid adenocarcinoma KB-CP 20 and hepatoma BEL 7404-CP 20 cancer cells. In this study, we further defined hyperpolarized mitochondrial membrane potentials (Δψm) in CP-r KB-CP 20 and BEL 7404-CP 20 cells compared to the cisplatin-sensitive (CP-s) KB-3-1 and BEL 7404 cells. Based on the mitochondrial dysfunction, mitaplatin was designed with two mitochondrial-targeting moieties [dichloroacetate (DCA) units] to the axial positions of a six-coordinate Pt(IV) center to sensitize cisplatin resistance. It was found that mitaplatin induced more apoptosis in CP-r KB-CP 20 and BEL 7404-CP 20 cells than that of cisplatin, DCA and cisplatin/DCA compared on an equal molar basis. There was more platinum accumulation in mitaplatin-treated CP-r cells due to enhanced transmembrane permeability of lipophilicity, and mitaplatin also showed special targeting to mitochondria. Moreover, in the case of treatment with mitaplatin, the dramatic collapse of Δψm was shown in a dose-dependent manner, which was confirmed by FACS and confocal microscopic measurements. Reduced glucose utilization of CP-r cells was detected with specifically inhibited phosphorylation of pyruvate dehydrogenase (PDH) at Ser-232, Ser-293, and Ser-300 of the E1α subunit when treated with mitaplatin, which was indicated to modulate the abnormal glycolysis of resistant cells. The present study suggested novel mitochondrial mechanism of mitaplatin circumventing cisplatin resistance toward CP-r cells as a carrier across membrane to produce CP-like cytotoxicity and DCA-like mitochondria-dependent apoptosis. Therefore, mitochondria targeting compounds would be more vulnerable and selective to overcome cisplatin resistance due to the unique metabolic properties of CP-r cancer cells.
Keywords: mitaplatin, cisplatin, cancer resistance, mitochondrial dysfunction
INTRODUCTION
cis-Diamminodichloroplatinum II (cisplatin, CP) was the first platinum-based chemotherapeutic drug for clinical treatment of tumors, introduced more than 40 years ago.1 The chemotherapeutic action of cisplatin is to cross-link with DNA, thereby interfering with transcription and/or DNA replication in tumor cells.2,3 Today, cisplatin is widely used to treat cancers, particularly malignant solid tumors such as testicular cancer, ovarian cancer, esophageal cancer, bladder cancer, head and neck cancer, and small-cell lung carcinomas.4,5 However, the development of tumor resistance to chemotherapy leads to the failure of cisplatin treatment and forced higher usage of anti-tumor agents, which also causes formidable side effects experienced by patients.6 There are certain known mechanisms, including reduced drug uptake, enhanced drug inactivation, increased DNA adduct repair, perturbed cell cycle, and the inhibition of apoptosis, of tumor resistance to cisplatin.7 All of these processes have the common effect of limited platinum intracellular accumulation and nuclear DNA damage in cisplatin-resistant tumors. Thus, it is critical to identify novel targets and design potential drugs to overcome tumor resistance to cisplatin.
Mitochondria are recognized as the power plant of cells, providing energy for various cellular activities.8 Mitochondrial disorders are related to many kinds of diseases, such as Alzheimer’s disease,9,10 Parkinson’s disease,11 myopathy,12 osteoarthritis,13 cardiovascular hypoxia,14 and chronic kidney disease.15 However, the importance of mitochondria as targets for cancer chemotherapy has not been adequately explored or clinically exploited. Many years ago, Dr. Otto Warburg suggested that tumor was due to the altered metabolism of cancer cells, converting glucose oxidation to glycolysis, even under aerobic conditions.16 The excessive glycolysis and lower O2 consumption result in the “paradox” of glycolysis under aerobic conditions, and this phenomenon is an adaptation to intermittent hypoxia in premalignant lesions, which contribute to decreased apoptosis of cancer cells.17 Recent studies have indicated that this glycolytic shift makes the mitochondrial membrane less susceptible to permeabilization.18 The resulting “inactivity” of the mitochondria reduces the likelihood of mitochondrially mediated cell death.19 Targeting mitochondria might therefore be an effective way to overcome tumor resistance to chemotherapy.20
Dichloroacetate (DCA) is broadly employed to decrease lactate levels by inhibiting pyruvate dehydrogenase kinase (PDK).21 With good bioavailability, this small molecule drug has been used in humans with inherited diseases involving mitochondrial metabolism for more than 40 years. Recently, DCA has been found to have new action as anticancer agents. It might enhance apoptosis, as it increases the movement of pyruvate into mitochondria, shifts metabolism from glycolysis to glucose oxidation, increases apoptosis, and decreases cellular proliferation by PDK inhibition.22 Thus, DCA causes cancer regression by sensitizing cells to chemotherapy via the mitochondrial apoptotic pathway, with minimal effects on normal cells.
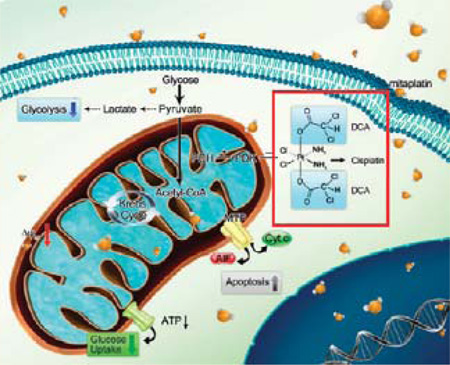
Mitaplatin (cis,cis-diamminedichlorobis(dichloroacetate)-platinum(IV)) is a six-coordinate platinum(IV) complex synthesized from cisplatin containing two DCA molecules in the axial positions. Upon reduction, cisplatin and two DCA molecules are released. Previous studies have shown that mitaplatin is able to induce apoptosis without affecting noncancerous cells.23 In addition, compared to healthy cells, cancer cells are known to have hyperpolarized mitochondrial membrane potentials (Δψm).24 In this study, we focused on the mitochondrial-based difference between cisplatin sensitive and resistant cells. It was found that mitaplatin increased sensitivity of tumor resistant cells by inducing mitochondrial dysfunction. Due to its enhanced lipophilicity, more cytotoxic platinum accumulation in CP-r cells treated with mitaplatin occurred after cisplatin treatment. In addition to inhibiting tumor growth by forming DNA adducts, mitaplatin triggers mitochondrial damage in CP-r cells. There is thus a potential synergistic effect of cisplatin administered with DCA. These properties of mitaplatin provide opportunities for further development of new platinum-based agents with the capability of killing CP-r cells.
MATERIALS AND METHODS
Reagents and Materials
Mitaplatin was synthesized, characterized, and kindly gifted by Prof. Stephen J. Lippard’s group at the Massachusetts Institute of Technology. Cisplatin and DCA were purchased from Sigma-Aldrich Company (St. Louis, MO). Mitaplatin, cisplatin, and DCA were all dissolved in PBS and further diluted in cell culture medium.
Cell Culture
The human epidermoid adenocarcinoma cell line KB-3-1 and hepatoma cell line BEL 7404 were the parental CP-s cells used in this study. Both of them were originally obtained and authenticated by ATCC, a biological resource center in Manassas, Virginia, and their morphology and propagation assays were performed under standard culture conditions. DNA profiles were analyzed with short tandem repeat (STR) techniques. KB-CP 20 cells were selected from KB-3-1 cells by independent exposure to cisplatin with stepwise increased concentrations up to 20 µg/mL, as were BEL 7404-CP 20 cells.25 BEL 7404-CP 20 and KB-CP 20 cells were cloned and kindly gifted by Michael M. Gottesman’s laboratory at NCI, NIH. Both of them were maintained in medium containing 5 µg/mL cisplatin. The cell lines were all grown as monolayer cultures in atmosphere with 5% CO2 at 37 °C using DMEM (Hyclone, Logan, UT) with 10% fetal bovine serum (ExCell, New Zealand), l-glutamine, penicillin (50 units/mol), and streptomycin (50 µg/mL; Hyclone, Logan, UT).
MTT Assay
To examine the cellular cytoxicity of compounds to different cell lines, 5 × 103 cells were seeded and cultured for 24 h in 96-well plates and incubated with different concentrations of mitaplatin, cisplatin, cisplatin/DCA, and DCA for 72 h. Cells were stained with 100 µL of sterilized MTT dye ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 0.5 mg/mL, Sigma-Aldrich) for 4 h at 37 °C, and 100 µL of DMSO was added. The spectrometric absorbance at 570 nm was measured on a microplate reader (M200, Tecan, Männedorf, Switzerland).
Apoptosis Measurement
Apoptotic cells were evaluated by morphologic observation and flow cytometry analysis as well as DNA fragmentation assays with confocal microscopy. For morphologic evaluation, cells were seeded in 6-well plates and incubated for 24 h under standard conditions. The medium was replaced with fresh medium containing 100 µM mitaplatin, cisplatin, cisplatin/DCA or 200 µM DCA. After 72 h of incubation, cells were observed directly in a bright field or indirectly in fluorescence after being stained with Hoechst 33342 and propidium iodide (PI), as recommended by the manufacturer (Mbchemic, Shanghai, China), for 30 min and washed three times with PBS (MBI3000B, Leica, Stockach, Germany).
To assay apoptosis, cells were treated with mitaplatin, cisplatin, cisplatin/DCA, or DCA alone ([Pt] or [2DCA] was 50, 100, and 150 µM) for 72 h. A positive control was introduced using the Apoptosis Inducers Kit (Beyotime, Shanghai, China). The samples were harvested by centrifugation and washed with PBS. Each sample was stained with 5 µL of Alexa Fluor 488 annexin V and 2 µL of PI according to the protocol of Alexa Fluor 488 annexin V and PI Cell Apoptosis Kit (Invitrogen, Rockville, MD) for 15 min at room temperature. After staining, 400 µL of binding buffer was added to each tube and analyzed within an hour by flow cytometer (Beckman Coulter Quanta SC, Florence, AL).
Transmission Electron Microscopy (TEM) Imaging
For examination of ultrastructure changes of mitochondria, KB-CP 20 and BEL 7404-CP 20 cells were collected for sample preparation after being treated with mitaplatin or cisplatin for 72 h. Briefly, after harvesting with trypsin and washing twice with 0.1 M PBS (pH 7.4), primary fixation was done with 3% glutaraldehyde in the dark for 24 h at 4 °C. The cells were then postfixed in 1% osmium tetroxide (OsO4) for 1 h, dehydrated in a graded ethanol series (50%, 70%, 80%, 90% and 100%), and embedded in a 1:1 mixture of propylene oxide and epoxy resin. Ultrathin sections were cut with diamond knives at 80 nm section thickness using Leica Ultracut UCT microtome (Leica EM UC6, Stockach, Germany). The sections were placed on 300 mesh copper grids and poststained with uranyl acetate and lead citrate. Finally, the sections on grids were observed in an electron microscope (JEOL JEM-1400, Gifu, Japan) at 80 kV.
Mitochondrial Membrane Potential (MMP) Analysis
The mitochondrial membrane potential (Δψm) was measured with 1.5 µM JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) (Invitrogen, Rockville, MD) by flow cytometry. KB-CP 20 and BEL 7404-CP 20 cells were seeded at 2 × 105 cells in 6-well plates and incubated for 24 h. Then mitaplatin, cisplatin, the mixture of cisplatin and DCA, and DCA alone ([Pt] or [2DCA] at 50, 100, and 150 µM) were added, and cells were incubated for 72 h. As a known mitochondrial membrane potential disrupter, 100 µM carbonyl cyanide 3-chlorophenyl hydrazone (CCCP, Alfa Aesar, Haverhill, MA) was used as positive control. After incubation, cells were trypsinized, collected by centrifugation, and then incubated with medium containing JC-1 dye for 20 min at 37 °C. Finally, the cells were washed and resuspended in 1 mL of PBS for fluorescent analysis using a FACScan flow cytometer. J-aggregate fluorescence was recorded by flow cytometry in fluorescence channel 2 (FL2) and monomer fluorescence in fluorescence channel 1 (FL1).
Visualization of MMP Reduction by Confocal Microscopy
After being exposed to different compounds for 72 h, the CP-r KB-CP 20 and BEL 7404-CP 20 cells were labeled with 1.5 µM JC-1 dye for 20 min at 37 °C. Then JC-1 was removed by PBS rinses three times and examined by confocal microscope (Ultraview VOX, Perkin-Elmer, Waltham, MA) with laser excitation at 488 nm.
Measurement of [3H] 2-Deoxyglucose Uptake
The uptake of [3H] 2-deoxyglucose by cell monolayers was measured in glucose-free HBSS containing trace amounts of [3H] 2-deoxyglucose (Perkin-Elmer, Waltham, MA) and 2-deoxyglucose (Sigma, St. Louis, MO) (1:50). After washing, the monolayers were lysed with 300 µL of cell lysis buffer and the protein content was determined by a BCA Protein Assay Kit (Pierce, Rockford, IL). The remaining samples were harvested in 2 mL centrifuge tubes for the measurement of radioactivity by a Wallac 1450 Microbeta TriLux liquid scintillation counter (Turku, Finland).
ELISA Measurement
The activity of pyruvate dehydrogenase (PDH) was examined by the PhosphoPDH In-cell ELISA Kit (MitoScience, Eugene, OR). This is a high-throughput assay for measuring up- or downregulation of PDH subunit E1α, as well as phosphorylation at all three E1α regulatory serines: Ser 232, Ser 293, and Ser 300. The kits use quantitative immunocytochemistry to measure protein levels or posttranslational modifications in cultured cells. Cells were seeded in a 96-well flat bottom cell culture plate, and incubated with drugs for 72 h. Then cells were fixed with 4% paraformaldehyde for 20 min. After washing with PBS three times, 100 µL of permeabilization buffer was added to the wells and incubated for 30 min. The nonspecific sites were blocked with 200 µL of blocking buffer and incubated for 2 h. Highly specific monoclonal antibodies for E1α and all three E1α regulatory serines (Ser 232, Ser 293, and Ser 300) were added and incubated overnight at 4 °C. After the plate was washed, AP/HRP labeled secondary antibody was added and incubated for 1 h. The color was developed in proportion to the amount of E1α and all three phosphorylated E1α regulatory serines.
Isolation of Organelles in CP-r Cells and Qualitative Analysis of Pt Concentration
The CP-r KB-CP 20 and BEL 7404-CP 20 cells were grown at a density of 2 × 107 cells in 150 mm dishes. After adhesion, cells were exposed to 100 µM mitaplatin or cisplatin for 72 h. Afterward, the cells were trypsinized, washed with PBS three times, and centrifuged for 5 min at 600g. Cell nuclei and mitochondria were isolated by a Nuclear Extraction Kit (Solarbio, Shanghai, China) and a Mitochondria Isolation Kit (Pierce, Rockford, IL) for Cultured Cells, respectively. The whole cells or the isolated organelles were lysed and the protein content was quantified by a BCA Protein Assay Kit (Pierce, Rockford, IL). After digestion, the samples were examined by an inductively coupled plasma mass spectroscopy (ICP-MS, Thermo Biosystems, Rockford, IL)). Standards and blanks were prepared in the same way as the samples.
Statistical Analysis
All experiments were performed at least three times and expressed as means ± SD. Data were analyzed for statistical significance using Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Characterization of Mitochondrial Membrane Potentials (MMPs, Δψm) with Sensitive and Resistant Cells
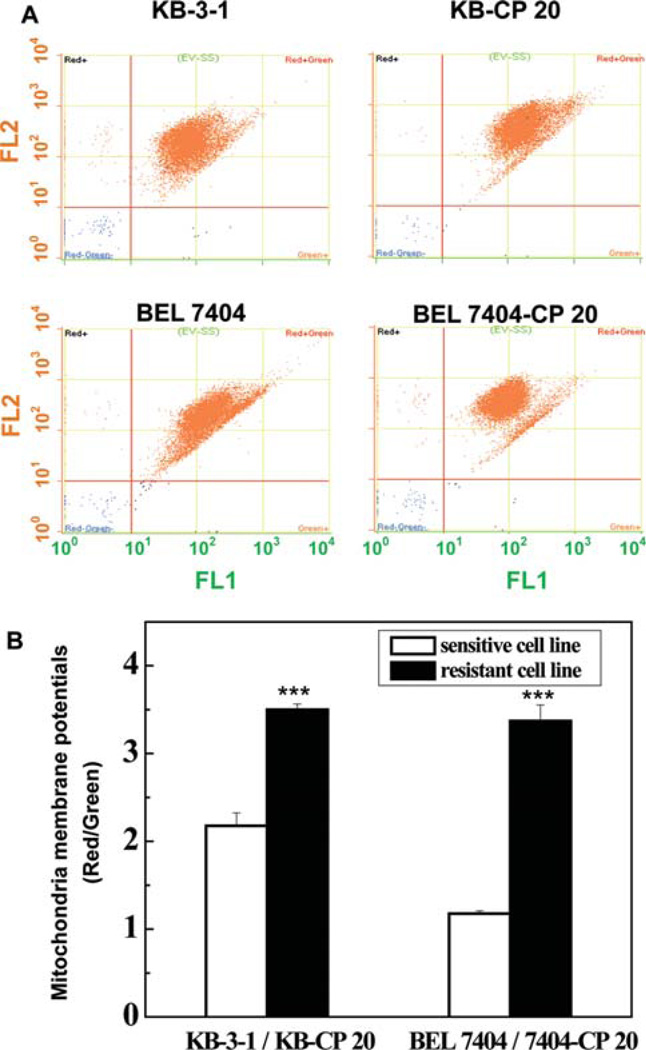
In order to address the distinction between sensitive and resistant cells, the MMPs were measured by JC-1 staining (Figure 1). Changed MMPs were indicated by a fluorescence emission shift from J-monomers with green fluorescence (~529 nm) to J-aggregates with red fluorescence (~590 nm). The higher mitochondrial membrane potentials were defined with the higher ratio of red fluorescent intensity and green fluorescent intensity. Two independently selected CP-resistant (CP-r) cell populations (KB-CP 20 and BEL 7404-CP 20 cells), derived from human KB epidermoid adenocarcinoma cells (KB-3-1) and human BEL 7404 hepatoma cells (BEL 7404), were used. The CP-r cancer cells showed increased Δψm compared to parental CP-sensitive (CP-s) KB-3-1 and BEL 7404 cells, similarly as cancer cells had hyperpolarized Δψm compared to noncancerous (normal) cells.22 Therefore, based on hyperpolarization of resistant cells, designing a drug with mitochondrial targeting was reasonable to sensitive chemotherapy and may selectively destroy tumor resistant cells. Modulating mitochondria-related metabolism may be a promising new approach to reversing cancer resistance to chemotherapeutic agents.
Figure 1.
The mitochondrial membrane potentials (MMPs or Δψm) of CP-sensitive (CP-s) and CP-resistant (CP-r) cells were characterized by JC-1 staining. (A) The different MMPs in CP-r KB-CP 20/BEL 7404-CP 20 cells and CP-s KB-3-1/BEL 7404 cells were measured by flow cytometry. There was hyperpolarized Δψm in CP-r KB-CP 20 and BEL 7404-CP 20 cells compared to CP-s KB-3-1 and BEL 7404 cells. (B) The ratio of red to green fluorescence intensity was used for the MMP measurement in CP-r and CP-s cells. The significant differences of hyperpolarizaion in CP-r cells are shown. Data represent the means ± SD of three independent experiments.
Mitaplatin Was More Effective on Inhibiting CP-r Cancer Cells
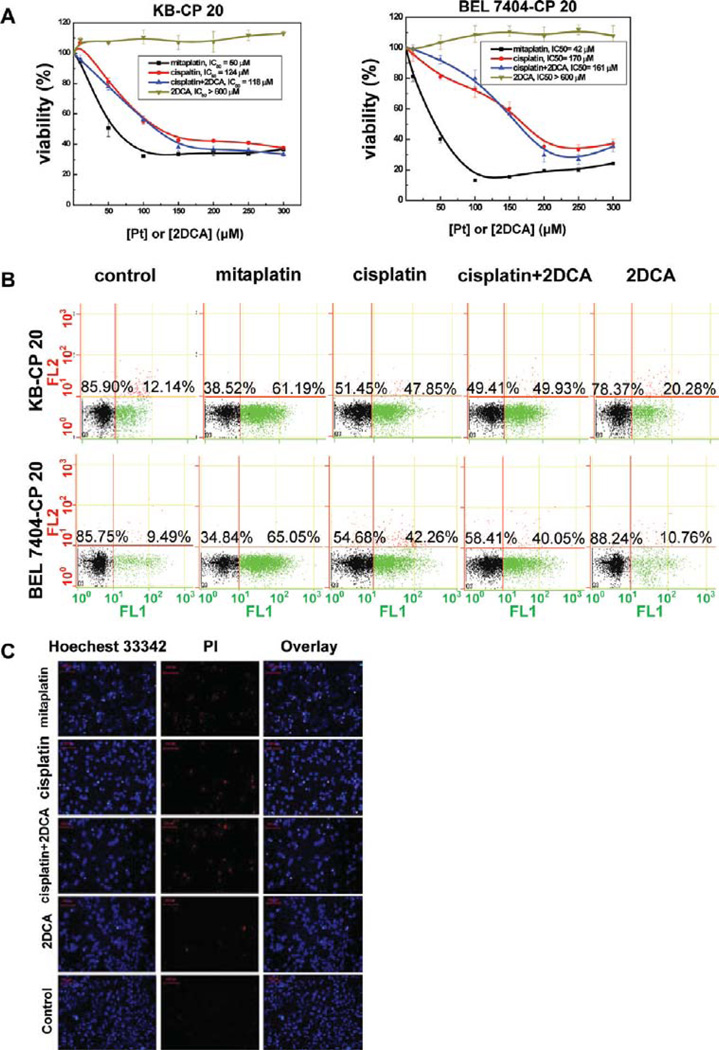
To measure the activity of mitaplatin on chemotherapeutic sensitivity, who has DCA units as mitochondrial targeting motif, the viability of CP-r and CP-s cells were measured by MTT assay. The IC50 value of mitaplatin was much lower than that of cisplatin in CP-r KB-CP 20 and BEL 7404-CP 20 cells measured at 72 h (Figure 2A). As expected, CP-r KB-CP 20 and BEL 7404-CP 20 cells were more susceptible to mitaplatin than cisplatin treatments in a concentration-dependent manner. The IC50 values of mitaplatin were 50 µM and 42 µM for KB-CP 20 and BEL 7404-CP 20, respectively, approximately 2.5- and 4-fold lower than those of cisplatin (Table 1). There was no significant difference between mitaplatin and cisplatin treatment of CP-s KB-3-1 and BEL 7404 cells. However, differences were observed in CP-r KB-CP 20 and BEL 7404-CP 20 cells. These results suggest the enhanced potency of mitaplatin to inhibit CP-r cancer cells.
Figure 2.
Mitaplatin selectively overcame chemoresistance and induced concentration-dependent apoptosis in CP-r cells. (A) Cytotoxicity of mitaplatin on KB-CP 20 cells and BEL 7404-CP 20 cells was measured at different concentrations. Data represent the means ± SD of three separate experiments. (B) Quantification of apoptosis induced by mitaplatin, cisplatin, cisplatin/DCA, and DCA using annexin V/PI staining. The FACS analysis with propidium iodide (red) and annexin V (green) showed that most of the resistant cells were apoptotic but not necrotic. Quantification of apoptosis is indicated by the increased % of annexin V-positive cells. (C) Confocal microscopic visualization of KB-CP 20 cells treated with mitaplatin, cisplatin, cisplatin/DCA, and DCA using Hoechest 33342/PI. The condensed chromatin and apoptotic cells (bright white dots) were imaged by fluorescence microscopy.
Table 1.
Comparison of IC50 Values for Mitaplatin, Cisplatin, and DCA to CP-s and CP-r Cells as Determined by the MTT Assaya
| IC50 (µM) | ||||
|---|---|---|---|---|
| KB-3-1 | KB-CP 20 | BEL 7404 | BEL 7404-CP 20 | |
| mitaplatin | 3.47 ± 0.13 | 50.35 ± 4.95 *** | 6.84 ± 0.81 | 41.71 ± 2.30 *** |
| cisplatin | 5.68 ± 1.22 | 123.78 ± 1.86 | 7.20 ± 0.74 | 169.56 ± 4.56 |
| cisplatin + 2DCA | 6.89 ± 0.13 | 117.43 ± 0.85 | 7.13 ± 1.25 | 160.89 ± 0.11 |
| 2DCA | >60 | >600 | >200 | >600 |
Data were the mean ± SD from three independent experiments.
P < 0.001 versus cisplatin treatment group.
Mitaplatin Promoted More Apoptosis in CP-r Cells
To explore the mechanism of mitaplatin, its cytotoxicity on apoptosis was measured with a cellular apoptosis evaluation kit by flow cytometry. Fluor 488 annexin V and propidium iodide (PI) were used to evaluate the percentage of live cells (unstained), early apoptosis cells (green fluorescence for Fluor 488 annexin V), and late apoptosis or necrosis cells (red fluorescence for PI). Mitaplatin treatment led to only 38% viability of CP-r KB-CP 20 cells, compared to 51% with cisplatin and 49% with cisplatin/DCA treatment. There was 78% viability of CP-r KBCP 20 cells treated with DCA only. Similar results were obtained with BEL 7404-CP 20 cells (Figure 2B).
To confirm the activity of mitaplatin on cell viability, CP-r KB-CP 20 (Figure 2C) and BEL 7404-CP 20 (Figure S1 in the Supporting Information) cells were evaluated by staining cells with Hoechst 33342 and PI. Hoechst 33342 is known to penetrate the plasma membrane of live cells and shows blue fluorescence by binding with chromatin. It is usually employed to distinguish live cells and dead cells labeled by PI shown with red fluorescence. Similar to the results obtained by flow cytometry, mitaplatin at 100 µM induced more condensed chromatin (bright white dots) and dead cells compared to cisplatin, cisplatin/DCA, and DCA, as shown in Figure 2C and Figure S1 in the Supporting Information.
Mitaplatin Increased Intracellular Platinum Accumulation in CP-r Cells
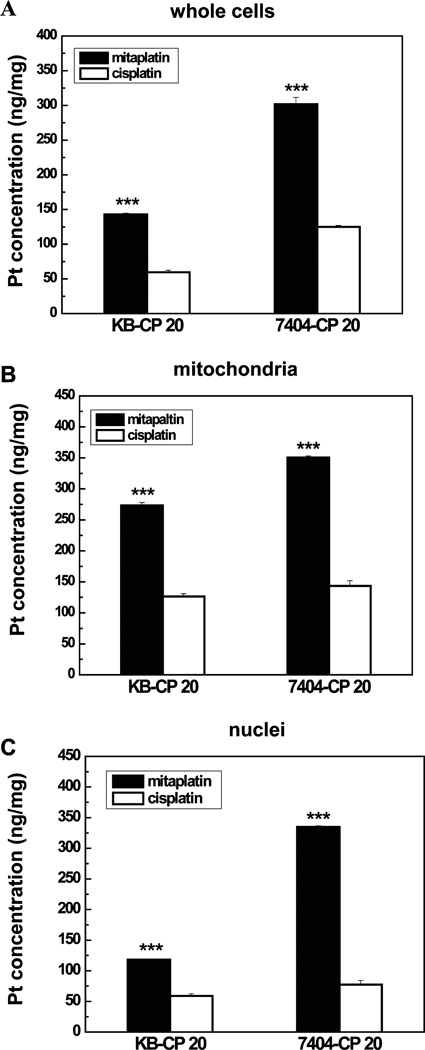
It is generally accepted that tumors become resistant to cisplatin partly because of reduced accumulation of cisplatin in the cytoplasm.26,27 It is possible to sensitize CP-r cells by increasing intracellular platinum accumulation. Recent studies demonstrated platinum(IV) complexes were formed to enhance its lipophilicity and to circumvent cisplatin resistance, since its advanced diffusion facilitated the complexes to cross cell membranes. Additionally, increased kinetic inertness makes the complex more stable and provides the opportunity to reach the intracellular target intact.28–30 To detect the increased mitaplatin accumulation in CP-r cells, intracellular platinum levels were determined by inductively coupled plasma mass spectroscopy (ICP-MS) in the negative ion mode. There was markedly increased platinum (~3-fold) in CP-r whole cell lysates treated with mitaplatin compared to those treated with cisplatin (Figure 3A). This result suggests that apoptosis is more effectively induced and tumor resistance is more efficiently reversed in CP-r cells due to increased drug accumulation.
Figure 3.
Mitaplatin changed the intracellular platinum distribution in CP-r cells. (A) Platinum accumulation was confirmed by ICP-MS. Cells were exposed to 100 µM mitaplatin or cisplatin for 72 h. The concentration of platinum in whole cells was measured in KB-CP 20 and BEL 7404-CP 20 cells. (B) The platinum accumulation in isolated mitochondria was examined by ICP-MS. The mitochondrial platinum amounts were increased ~3-fold after treatment with mitaplatin compared to that of cisplatin (***P < 0.001). (C) The platinum accumulation in isolated cell nuclei was also determined by ICP-MS. Mitaplatin showed its novel dual targets to both nuclei and mitochondria. Data represent the means ± SD of three separate experiments.
Mitaplatin Altered Intracellular Platinum Distribution
To further explore the mechanism of mitaplatin, mitochondria, and nuclei in KB-CP 20 and BEL 7404-CP 20 were isolated, respectively. It is well-known that cisplatin is a traditional anticancer agent which targets nuclear DNA. Therefore, consistent with the increased platinum accumulation measured in whole cell lysates (Figure 3A), treatment with mitaplatin resulted in the increasing of platinum accumulation in the nuclei as expect (Figure 3C). However, compared to cisplatin, mitaplatin simultaneously exhibited its talent to interact with mitochondria, since there was also increased platinum accumulation in the mitochondria of CP-r cells treated with mitaplatin compared to those treated with cisplatin (Figure 3B). Mitochondrial localization of platinum might be partially due to DCA ligands in mitaplatin compounds, and these results exhibited the ability of mitaplatin for targeting mitochondria. The dual targets (nuclei and mitochondria) may make mitaplatin more distinguishing and unique to sensitize chemotherapy than other antitumor drugs with a single target. To investigate whether the new target contributed to circumvent cisplatin resistance, the effects of mitochondrial dysfunction were further measured.
Mitaplatin Triggered Mitochondrial Dysfunction in CP-r Cells
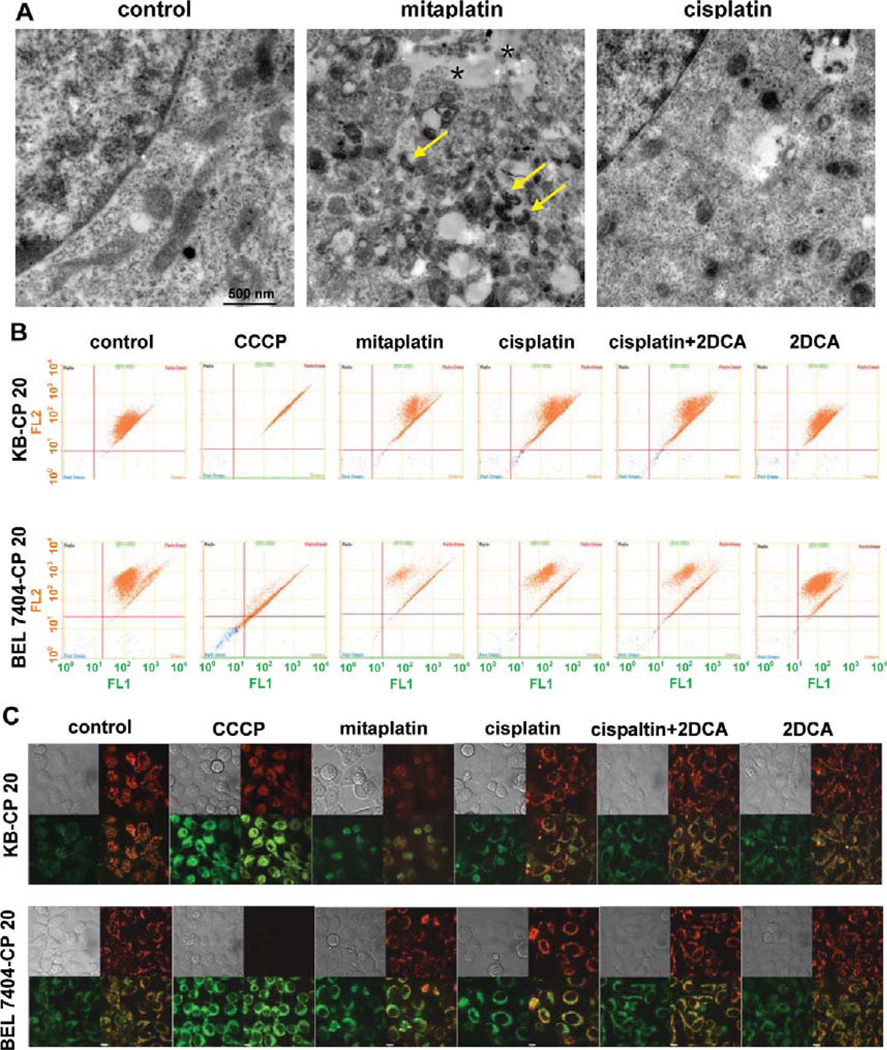
Transmission electron microscopy (TEM) was performed to examine mitochondrial ultrastructures of cells treated with different drugs. The cells as control group without treatment had well-defined integral mitochondrial membranes with regular-appearing cristae. After mitaplatin treatment for 72 h, the mitochondria of KB-CP 20 cells showed irregular ultrastructures with more highly condensed shapes compared to mitochondria with cisplatin treatment (Figure 4A).
Figure 4.
Mitaplatin significantly triggered the dysfunction of mitochondria in CP-r cells. (A) Ultrastructural analysis of mitochondrial changes in KB-CP 20 cells was carried out by transmission electron microscopy. After mitaplatin treatment for 72 h, the mitochondria appeared more irregular. They were much smaller with more highly condensed shapes (arrow) and had zones of intercellular detachment (asterisk), compared to those in cells with cisplatin treatment. (B) Changes of mitochondrial membrane potential in KB-CP 20 and BEL 7404-CP 20 cells were examined by flow cytometry after treatment with 100 µM of various drugs. CCCP was used as a positive control. (C) Changes of mitochondrial membrane potential in KB-CP 20 and BEL 7404-CP 20 cells imaged by confocal microscopy. JC-1 existed either as a green fluorescent monomer at depolarized membrane potentials or as an orange-red fluorescent J-aggregate at hyperpolarized membrane potentials. The shift in membrane charge was observed by disappearance of fluorescent red-orange-stained mitochondria and an increase in fluorescent green-stained mitochondria. The ratio of J-monomer and J-aggregate form was increased after the treatment of mitaplatin for 72 h. The results were consistent with FACS quantitative measurements.
Mitochondrial membrane depolarization was a prelude to apoptosis, and mitochondrial membrane potentials (MMPs) reflected electron transport chain (ETC) activity and mitochondrial function. To explore the unique bioproperties of mitaplatin, activities of mitochondria were measured by quantitative MMPs with JC-1 staining. Mitochondrial depolarization occurring in apoptosis was revealed by a decrease in the red/green fluorescence intensity ratio. Carbonyl cyanide 3-chlorophenyl hydrazone (CCCP), an uncoupled agent that depolarizes mitochondrial membranes, was used as a positive control.
In KB-CP 20 cells, mitaplatin treatment enhanced MMP depolarization when mitaplatin concentration was increased from 50 to 150 µM (Figure S2 in the Supporting Information). Similar results were obtained with BEL 7404-CP 20 cells (Figure 4B and Figure S2 in the Supporting Information). The flow cytometry analysis was consistent with the confocal microscopic measurements allowing direct visualization of mitochondria. The ratio of red to green fluorescence intensity was changed with mitaplatin treatment (Figure 4C). Morphological abnormality and MMP depolarization of mitochondria suggested that mitaplatin induced apoptosis through triggering dysfunction of mitochondria in CP-r cells. In conclusion, a combination of cisplatin with mitochondrial targeting moieties (DCA) would be an attractive therapeutic strategy for effectively attacking CP-r cancer cells.
Mitaplatin Reduced Glucose Utilization in CP-r Cells
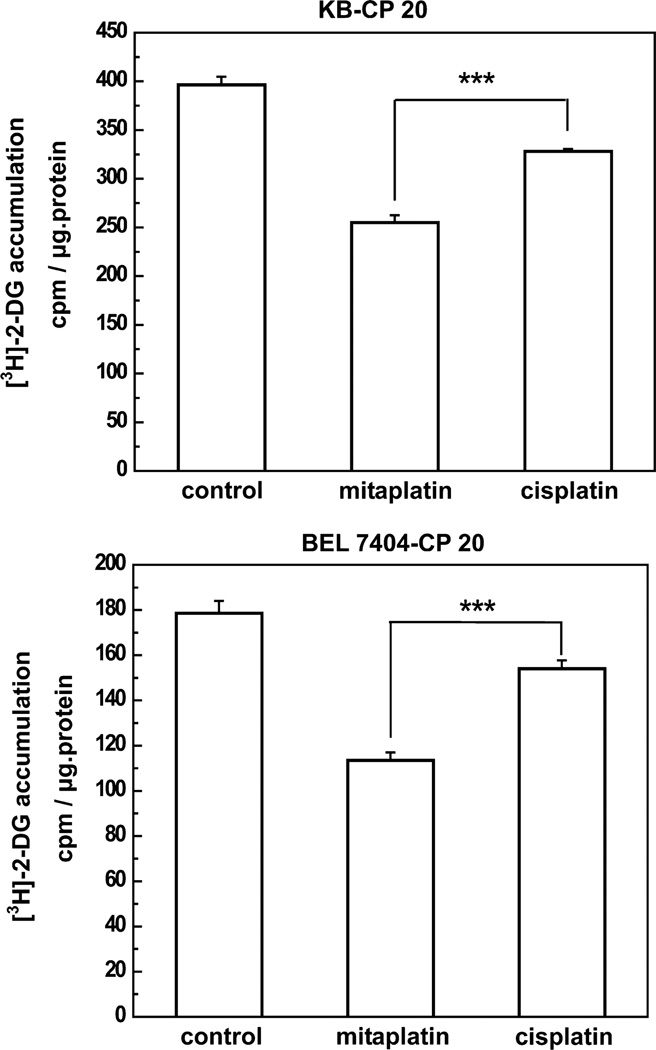
It was demonstrated previously that there was decreased metabolism in CP-r KB-CP 20 and BEL 7404-CP 20 cells at a “less energy consumption state” compared to parental CP-s KB-3-1 and BEL 7404 cells.31 The use of glucose has been recognized as an important feature of tumor development. The utilization of glucose was measured (Figure 5) as the symbol to describe the level of cellular metabolism. There was less [3H] 2-deoxyglucose accumulation in CP-r KB-CP 20 and BEL 7404-CP 20 cells treated with mitaplatin compared to cells treated with cisplatin, which may be partially due to mitochondrial damage-associated apoptosis caused by mitaplatin treatment.32 Glucose oxidation is far more efficient in generating ATP (~36 mol of ATP/mol of glucose) compared with anaerobic glycolysis (~2 mol of ATP/mol of glucose) or aerobic glycolysis (~4 mol of ATP/mol of glucose).33,34 We further determined whether mitaplatin altered the metabolic balance between the ratio of oxidative phosphorylation and aerobic glycolysis.
Figure 5.
Mitaplatin shifted cellular metabolism, leading to reduced glucose uptake. Glucose uptake was decreased in the CP-r cells. When treated with mitaplatin, the accumulation of [3H] 2-deoxyglucose was decreased in KB-CP 20 (a, top) and BEL 7404-CP 20 cells (b, bottom). Each column represents the average of three individual experiments. The t test was performed, and data represent the means ± SD. ***P < 0.001 versus cisplatin-treatment control.
Mitaplatin Decreased the Phosphorylation of Pyruvate Dehydrogenase
Pyruvate dehydrogenase (PDH) is a 9.5 megadalton multienzyme complex localized in the mitochondrial matrix. It has three catalytic components, [pyruvate dehydrogenase (E1), dihydrolipoamide acyltransferase (E2), dihydrolipoyl dehydrogenase (E3)], one binding component (E2/E3 binding protein), and two regulatory components [PDK (PDH kinase) and PDP (PDH phosphatase)].35,36 The pyruvate dehydrogenase complex plays a central role in cellular metabolism.
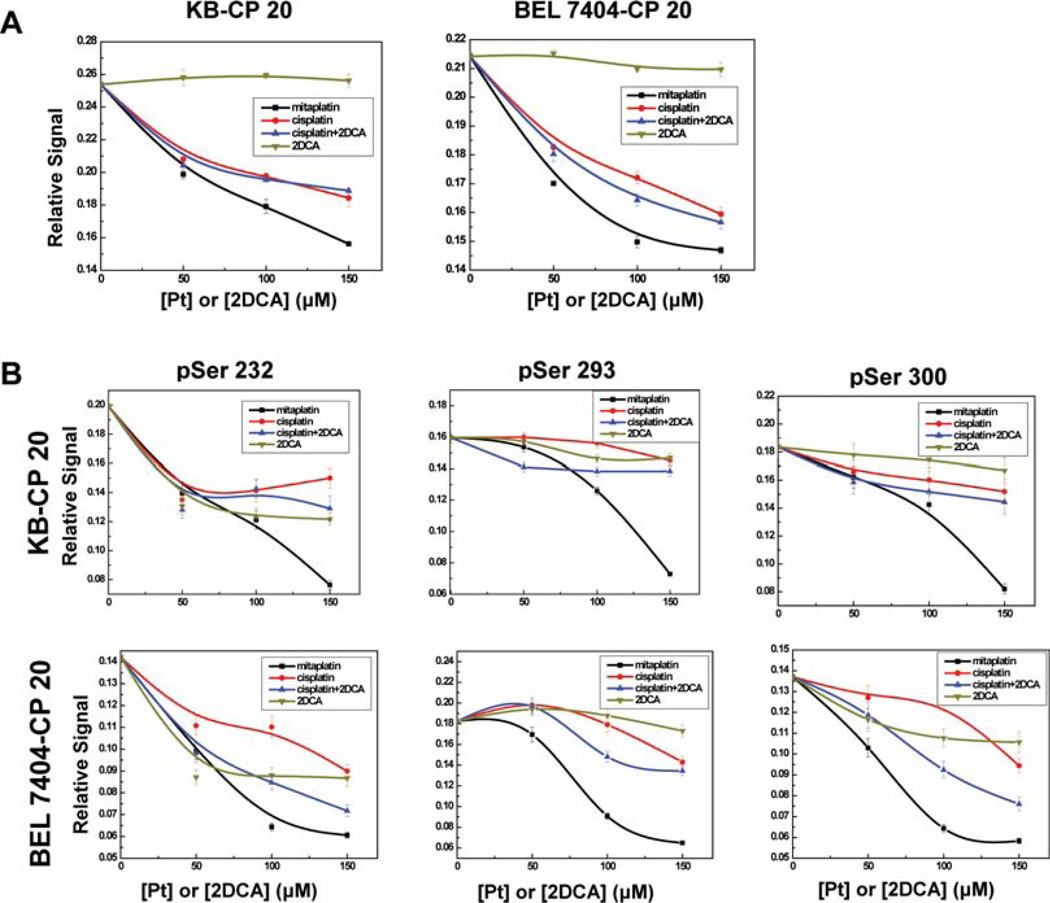
In PDH, the catalytic unit E1 is a heterotetramer of two α and two β subunits. In humans, PDH activity is inhibited by site-specific phosphorylation at three sites on the E1α subunit (Ser 232, Ser 293, and Ser 300). Therefore, the phosphoPDH E1α, as well as the phosphorylation at each individual E1α regulatory serine site, was examined by ELISA (Figure 6). Superior to cisplatin, the phosphorylation of all three regulatory serine sites of E1α was reduced with mitaplatin treatment. Mitaplatin was able to effectively inhibit the PDK activity and reduce phosphorylation at Ser 232, 293, and 300, three regulatory serine residues of E1α.
Figure 6.
Mitaplatin decreased the phosphorylated PDH level by inhibiting PDK. (A) The relative activity for total E1α demonstrated the inhibition of PDH kinase activity by drugs after treatment for 72 h. Points, mean of three individual experiments; bars, SD. (B) The phosphorylation levels at each E1α pSer 232, 293, and 300 site were examined for further determination.
DISCUSSION
Cisplatin is a drug commonly used to treat many cancers at early clinical stages. However, acquired resistance due to largely unknown mechanisms limits its efficacy. This study demonstrates that the mitochondrial membrane potentials in cisplatin resistant cancer cells are more strongly polarized than in sensitive cells. Recently, the critical role of mitochondria in regulating cell growth metabolism has been investigated to distinguish cancerous cells from nonmalignant cells,22 even to distinguish cancer stem cells from noncancer stem cells.18,37 There are amounts of mitochondrial and metabolism-related properties to be identified, such as masses of mitochondria and mitochondrial DNA, MMPs, oxygen/glucose consumption, ROS and ATP, etc. These distinguishing mitochondrial characteristics aid in understanding and mastering the differences between sensitive and resistant cancer cells. An increasing number of studies have suggested that the function and integrity of mitochondria may impact the viability, proliferation/division, cytotoxic resistance, and hypoxic tolerance. Therefore, the unique properties of mitochondria in this study might become the foundation of designing chemotherapeutic targets for effective cancer treatment. Several mitochondrial targets have been recognized as attractive strategies for overcoming chemotherapeutic resistance. Targets include, for example, (i) mitochondrial permeability transition; (ii) outer membrane permeabilization; (iii) mitochondrial metabolism and metabolic reprogramming.20,38 Concerning the mitochondrial characteristics in cancer cells, drugs were designed to activate cell metabolism machinery as a more effective chemotherapeutic administration. To support this standpoint, mitaplatin was employed to circumvent cancer resistance to cisplatin due to its ability to target the hyperpolarized mitochondria.
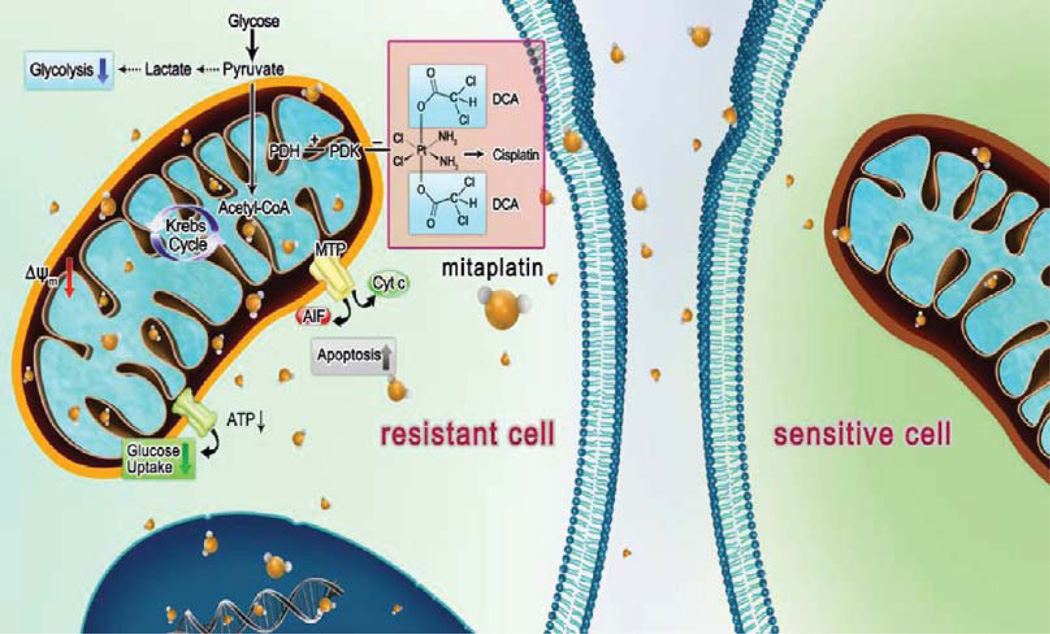
This study highlighted mitaplatin as a dual target molecule that is able to overcome cisplatin chemoresistance in tumor cells. As expected, mitaplatin exhibited the ability to overcome cisplatin resistance in cancer cells. It was hypothesized that mitaplatin induced apoptosis of resistant cells via the pharmacological mechanisms of triggering MMP depolarization (Figure 4B and Figure 4C). Mitaplatin could accumulate inside cells, and the ratio of platinum in mitochondria is 2.2:1 to KBCP 20 cells and 2.5:1 to BEL 7404-CP 20 cells incubated with mitaplatin or cisplatin (Figure 3B); the ratio of platinum in nuclei is 2.5:1 to KB-CP 20 cells and 4.5:1 to BEL 7404-CP 20 cells incubated with mitaplatin or cisplatin (Figure 3C). The advanced mitaplatin intracellular accumulation mainly brought a higher platinum dose in mitochondria and damaged mitochondria, which thereby sensitized the resistant cells and activated a downstream mitochondria-dependent cell death. Furthermore, once the supplying organelles were dysfunctional, the energy metabolism would be changed for sustaining the metabolic needs of tumor cells. As a metabolic modulator, mitaplatin helps to reduce the energy consumption (Figure 5) and transform the metabolic form by downregulated phosphorylation of PDH (Figure 6), leading to shift glycolysis back to oxidative phosphorylation by enchancing the influx of acetyl-CoA into the mitochondria (Figure 7). In conclusion, mitaplatin could be an attractive approach for selective targeting of CP-r cancer cells with mitochondria-based agents.
Figure 7.
Mitaplatin circumvented cancer resistance to cisplatin by targeting mitochondria. In cancer cells, oxidative phosphorylation is inhibited, and cancer cells rely on cytoplasmic glycolysis to produce energy. This metabolic shift induces apoptosis resistance. The enhanced lipophilicity of mitaplatin increases its ability to cross the plasma membrane. After crossing the membrane, mitaplatin targets mitochondria, inhibits the activity of mitochondrial PDK, and leads to activation of PDH, which promotes the influx of acetyl-CoA into mitochondria and increases the Krebs cycle. With triggering hyperpolarized Δψm in resistant cells, mitaplatin also results in reduced glucose utilization. Similar to cisplatin, mitaplatin also enters the nuclei and targets DNA to form 1,2-intrastrand d (GpG) cross-links.
In addition, DCA has been approved as to its effectiveness and safety by the FDA, and synthesis of DCA (as mitochondrial moiety) with cisplatin (as traditional antitumor drug) should be a potential strategy for chemotherapeutic drug development. Most recent studies show that treatment with DCA alone can cause the inhibition of PDK or cause tumor regression with relatively high doses (~mM).39–41 However, the high dose inevitably brought extensive adverse reaction to normal tissue, such as neurotoxicity and liver damage,42,43 which may limit its usage and increase health risk. In our study, a synergistic effect of cisplatin with DCA (less than 300 µM, Figure 2A) may be a feasible approach to alleviate this obstacle. Under concentrations used in study, DCA manifested its effect on decreasing the phosPDH E1α level, and there is not cytotoxicity shown in CP-r cells. In conclusion, for future platinum-based drug design, anticancer agents that directly target mitochondria to make CP-r cells more susceptible and/or to bypass resistance mechanisms should be further explored.
ACKNOWLEDGMENTS
The authors thank Dr. Justin J. Wilson for synthesizing the mitaplatin compounds used in this study. The authors also appreciate Drs. Michael M. Gottesman (National Cancer Institute, NIH) and Stephen J. Lippard (MIT) for their critical comments on this project, George Leiman for editorial assistance, and Michael Gottesman for providing the KB and BEL 7404 CP-s and CP-r cell lines used in this work. This work was financially supported by the National Key Basic Research Program of China (MOST 973 Projects 2009CB930200) and the program of National Natural Science Foundation of China (30970784 and 81171455). The work was also partially supported by 2G12RR003048 from RCMI program. The authors are grateful for the support of the Chinese Academy of Sciences (CAS) “Hundred Talents Program” and the CAS Knowledge Innovation Program.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Figures depicting BEL 7404-CP 20 cells imaged by confocal microscopy and depolarization of mitochondrial membrane potentials measured in KB-CP 20 and BEL 7404-CP 20 cells. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Prestayko AW, D’Aoust JC, Issell BF, Crooke ST. Cisplatin (cis-diamminedichloroplatinum II) Cancer Treat. Rev. 1979;6(1):17–39. doi: 10.1016/s0305-7372(79)80057-2. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999;99(9):2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 3.Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007;107(5):1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 4.Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann. Intern. Med. 1984;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 5.Shen D, Pastan I, Gottesman MM. Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res. 1998;58(2):268–275. [PubMed] [Google Scholar]
- 6.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 7.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 8.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16(14):R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J. Alzheimer's Dis. 2006;9(2):101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- 10.Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J. Alzheimer's Dis. 2010;20(Suppl. 2):S591–S607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteves AR, Lu J, Rodova M, Onyango I, Lezi E, Dubinsky R, Lyons KE, Pahwa R, Burns JM, Cardoso SM, Swerdlow RH. Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. J. Neurochem. 2010;113(3):674–682. doi: 10.1111/j.1471-4159.2010.06631.x. [DOI] [PubMed] [Google Scholar]
- 12.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, Hoefler G, Sattler W, Weinstock PH, Breslow JL, Zechner R. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J. Clin. Invest. 1995;96(2):976–986. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7(3):161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 14.Calmettes G, Deschodt-Arsac V, Gouspillou G, Miraux S, Muller B, Franconi JM, Thiaudiere E, Diolez P. Improved energy supply regulation in chronic hypoxic mouse counteracts hypoxia-induced altered cardiac energetics. PLoS ONE. 2010;5(2):e9306. doi: 10.1371/journal.pone.0009306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, Carella M, Schena FP, Grandaliano G, Pertosa G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 17.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 18.Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, Bian XW, Yu SC, Qian GS. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int. J. Cancer. 2011;129(4):820–831. doi: 10.1002/ijc.25944. [DOI] [PubMed] [Google Scholar]
- 19.Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol. Aspects Med. 2010;31(1):60–74. doi: 10.1016/j.mam.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discovery. 2011;9(6):447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 21.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer. 2008;99(7):989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Dhar S, Lippard SJ. Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc. Natl. Acad. Sci. U.S.A. 2009;106(52):22199–22204. doi: 10.1073/pnas.0912276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchier-Hayes L, Munoz-Pinedo C, Connell S, Green DR. Measuring apoptosis at the single cell level. Methods. 2008;44(3):222–228. doi: 10.1016/j.ymeth.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen DW, Akiyama S, Schoenlein P, Pastan I, Gottesman MM. Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br. J. Cancer. 1995;71(4):676–683. doi: 10.1038/bjc.1995.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang XJ, Shen DW, Gottesman MM. A pleiotropic defect reducing drug accumulation in cisplatin-resistant cells. J. Inorg. Biochem. 2004;98(10):1599–1606. doi: 10.1016/j.jinorgbio.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Holford J, Sharp SY, Murrer BA, Abrams M, Kelland LR. In vitro circumvention of cisplatin resistance by the novel sterically hindered platinum complex AMD473. Br. J. Cancer. 1998;77(3):366–373. doi: 10.1038/bjc.1998.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MD, Alderden RA, Zhang M, Beale PJ, Cai Z, Lai B, Stampfl AP, Hambley TW. The fate of platinum(II) and platinum(IV) anti-cancer agents in cancer cells and tumours. J. Struct. Biol. 2006;155(1):38–44. doi: 10.1016/j.jsb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Hall MD, Dillon CT, Zhang M, Beale P, Cai Z, Lai B, Stampfl AP, Hambley TW. The cellular distribution and oxidation state of platinum(II) and platinum(IV) antitumour complexes in cancer cells. J. Biol. Inorg. Chem. 2003;8(7):726–732. doi: 10.1007/s00775-003-0471-6. [DOI] [PubMed] [Google Scholar]
- 30.Modok S, Scott R, Alderden RA, Hall MD, Mellor HR, Bohic S, Roose T, Hambley TW, Callaghan R. Transport kinetics of four- and six-coordinate platinum compounds in the multicell layer tumour model. Br. J. Cancer. 2007;97(2):194–200. doi: 10.1038/sj.bjc.6603854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang XJ, Finkel T, Shen DW, Yin JJ, Aszalos A, Gottesman MM. SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol. Cancer Res. 2008;6(9):1499–1506. doi: 10.1158/1541-7786.MCR-07-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, Lagunoff M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107(23):10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am. J. Physiol. 2003;284(5):E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 35.Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4(14):3224–3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- 36.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 2006;34(Pt2):217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 37.Ye XQ, Wang GH, Huang GJ, Bian XW, Qian GS, Yu SC. Heterogeneity of mitochondrial membrane potential: a novel tool to isolate and identify cancer stem cells from a tumor mass? Stem Cell Rev. 2011;7(1):153–160. doi: 10.1007/s12015-010-9122-9. [DOI] [PubMed] [Google Scholar]
- 38.Don AS, Hogg PJ. Mitochondria as cancer drug targets. Trends Mol. Med. 2004;10(8):372–378. doi: 10.1016/j.molmed.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br. J. Cancer. 2010;102(12):1746–1752. doi: 10.1038/sj.bjc.6605701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heshe D, Hoogestraat S, Brauckmann C, Karst U, Boos J, Lanvers-Kaminsky C. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs. Cancer Chemother. Pharmacol. 2010;67:647–655. doi: 10.1007/s00280-010-1361-6. [DOI] [PubMed] [Google Scholar]
- 41.Han Z, Berendzen K, Zhong L, Surolia I, Chouthai N, Zhao W, Maina N, Srivastava A, Stacpoole PW. A combined therapeutic approach for pyruvate dehydrogenase deficiency using self-complementary adeno-associated virus serotype-specific vectors and dichloroacetate. Mol. Genet. Metab. 2008;93(4):381–387. doi: 10.1016/j.ymgme.2007.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stacpoole PW. The dichloroacetate dilemma: environmental hazard versus therapeutic goldmine--both or neither? Environ. Health Perspect. 2011;119(2):155–158. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, De Vivo DC. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66(3):324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]