Abstract
In contrast to the wide applications of recombinant bifunctional fusion proteins in clinical usage, the systematic study for the pharmacokinetics (PK) of bifunctional fusion proteins is left blank. In this report, recombinant fusion proteins consisting of transferrin (Tf) and growth hormone (GH) or granulocyte colony-stimulating factor (G-CSF) have been constructed as a model for studying the PK of bifunctional fusion proteins. The results showed that the insertion of different linkers between the two protein domains altered the binding affinities of the fusion proteins to both domain receptors, and that the fusion proteins’ plasma half-lives were greatly affected. A strong correlation between GH receptor binding affinity and plasma half-life of GH-Tf fusion proteins was observed. In addition, we demonstrated that the intracellular processing after receptor binding plays an important role in determining the half-life of fusion proteins. While the binding of the GH domain to the GH receptor will lead to endocytosis and lysosomal degradation in target cells, binding of the Tf domain to the Tf receptor may recycle the fusion protein and prolong its plasma half-life. To further confirm the effects of receptor binding on plasma half-life, G-CSF-Tf bifunctional fusion proteins with the same three linkers as GH-Tf were evaluated. While the 3 fusion proteins showed a similar G-CSF receptor binding affinity, the G-CSF-Tf fusion protein with the higher Tf receptor binding affinity exhibited longer plasma half-life. The linker insertion further demonstrated the involvement of Tf in recycling and prolonging plasma half-life. Based on our results, a model was developed to summarize the factors in determining the PK of the bifunctional fusion proteins. Our findings are useful for predicting the plasma half-lives, as well as for improving the pharmacokinetic profiles of therapeutic bifunctional fusion proteins by applying linker technology.
Keywords: Fusion protein, recombinant, bifunctional, pharmacokinetics, half-life, receptor binding
Introduction
With the emergence of DNA recombinant technology, recombinant bifunctional fusion proteins have become an important class of therapeutics. Several FDA approved drugs such as Enbrel® (TNF-R/Fc-IgG1), Ontak® (IL-2/diphtheria toxin), Orencia® (CTLA-4/Fc-IgG1) and Amevive® (LFA-3/Fc-IgG1) have foreshown the advent of many more fusion protein drugs 1. By fusing with albumin or the Fc portion of IgG, many protein drugs such as insulin, TNF, and Factor IX have exhibited improved pharmacokinetic (PK) and pharmacodynamic (PD) properties 2-5.
In contrast to the rapid development of fusion proteins, the understanding of the determining factors that affect the PK of bifunctional fusion proteins is still very preliminary. There is no established guideline for predicting the plasma half-life of fusion proteins due to the complexity of the bifunctional binding. Different functional and carrier domains possess inherent receptors with different tissue distribution, number of receptors, and nature of binding, and therefore it is difficult to compare the pharmacokinetic parameters of two fusion proteins composed of different protein domains.
In this report, the PK mechanisms of bifunctional fusion proteins were investigated by constructing growth hormone-transferrin (GH-Tf) fusion proteins with various linker peptides inserted between the 2 domains as shown in Scheme 1. These fusion proteins, containing an identical protein drug (GH) and carrier protein (Tf) domains, were used to develop a mechanistic model to elucidate the crucial factors that affect the plasma half-life of bifunctional fusion proteins. Additionally, another fusion protein containing Granulocyte-Colony Stimulating Factor (G-CSF) fused to Tf with the same 3 stable linkers was evaluated to support the findings from the GH-Tf model. The bifunctional Tf fusion proteins resemble other bifunctional fusion proteins such as Fc-fusion proteins or albumin-fusion proteins, which are also composed of a protein drug domain and a carrier protein domain. Therefore, the findings from this report can potentially be applied to many other bifunctional fusion proteins currently under development for therapeutic use.
Scheme 1.
Design of linkers in GH/G-CSF-Tf fusion proteins.*
*(A): Dipeptide linker (Leu-Glu). (B): The cyclopeptide linker is based on the structure of somatostatin modified to contain a thrombin-specific sequence, PRS. Two cysteinyl-residues on somatostatin naturally form a disulfide bond. (C): Helical peptide linker. (D): The disulfide linker is formed by in vitro thrombin treatment of the cyclopeptide linker and is cleavable in vivo 8.
Experimental Section
Cell lines
HEK293 cells, purchased from ATCC (Manassas, VA), were grown as monolayers in Dulbecco’s Modified Eagle’s medium (DMEM) with 10% (vol/vol) fetal bovine serum (FBS) at 37 °C in 5% CO2. Protein-free, chemically defined CD293 medium, obtained from Invitrogen (Carlsbad, CA), was used for protein expression after transfection of HEK293 cells. Colorectal adenocarcinoma Caco-2 cells, purchased from ATCC, were grown in DMEM with 20% (vol/vol) FBS, 2 mM L-glutamine, and 0.1 mM non-essential amino acids. IM-9 cells, purchased from ATCC, were grown in RPMI 1640 medium with 10% (vol/vol) FBS, 10 mM HEPES, and 1 mM sodium pyruvate. Murine myeloblastic NFS60 cells, kindly provided by Dr. James Ihle (St. Jude Children’s Research Hospital, Memphis, TN), were grown in RPMI medium 1640 with 10% (vol/vol) FBS and 0.1 ng/mL recombinant mouse IL-3.
Animals
Male CF-1 mice (25-30 g) from Charles River Laboratories (Kingston, NY) were used for pharmacokinetic studies. The protocol of animal experiments in this study has been approved by the Institutional Animal Care and Use Committee (IACUC) at USC. The animals were handled in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, revised 1985). The animals were fed with a standard laboratory rodent diet (Labdiet, Richmond, IN) and housed on a 12-h light and 12-h dark cycle with room temperature maintained at 22 ± 3 °C and relative humidity at 50 ± 20%.
Construction and expression of fusion proteins with various linkers and free GH
GH-Tf fusion proteins with dipeptide, cyclopeptide, and helical peptide linkers (Scheme 1), as well as GH were constructed into the mammalian expression vector pcDNA3.1 (+) (Invitrogen) as previously described 6. Briefly, GH coding sequence was inserted between EcoRV and XhoI cloning sites on pcDNA3.1 (+) vector. Similarly, Tf coding sequence was inserted between XhoI and XbaI cloning sites on pcDNA3.1 (+) vector. The oligonucleotides encoding the desired linkers (except for the dipeptide linker LE) were then inserted between GH and Tf via the XhoI cloning site. The correct sequence was confirmed by DNA sequencing. HEK293 cells were transiently transfected with the plasmids encoding the proteins by using linear polyethylenimine (Polysciences, Inc, Warrington, PA) as previously described 7. Following a 5-hour transfection, serum-free CD293 medium was used for protein expression for an additional 4- day incubation period. The conditioned medium was then concentrated by Tangential Flow Filtration (TFF) (Millipore, Billerica, MA) followed by Amicon centrifugal filter (Millipore) and was stored at − 80 °C until use. The GH-Tf fusion protein with the cyclopeptide linker was treated by thrombin in vitro to create a disulfide linker, denoted as GH-S-S-Tf, as reported in our previous work 8. Similarly, G-CSF-Tf fusion proteins with 3 stable linkers were constructed into the mammalian expression vector pcDNA3.0 (Invitrogen) and expressed from transiently-transfected HEK293 cells as previously described 8-10.
Competitive GH Receptor (GHR) binding assay for GH-Tf fusion proteins
Human GH was expressed from HEK293 cells as described for the GH-Tf fusion proteins. One microgram of GH was radiolabeled with Na-125I (PerkinElmer, Waltham, MA) by the two-phase iodination process to preserve its biological activity as described by Tejedor and Ballesta 11. After iodination, 125I-GH was purified from the reactant by size exclusion chromatography (Sephadex G-25 gel matrix, GE Healthcare, Piscataway, NJ) equilibrated in PBS containing 0.02% Tween 20. IM-9 cells were first washed three times with RPMI 1640 medium with 0.1% bovine serum albumin (BSA) (Sigma, St. Louis, MO). Next, 0.5×106 cells were incubated in 300 μl RPMI 1640 medium containing 0.1% BSA, 2 ng/ml 125I-GH, and serially diluted GH-Tf fusion proteins or free GH recombinantly expressed from HEK293 cells. After 1 hour of incubation at 37 °C, the cells were washed three times with ice-cold PBS, and the radioactivity in the cell pellets was counted using a gamma counter (Packard, Downers Grove, IL).
Competitive G-CSFR binding assay for G-CSF-Tf fusion proteins
Recombinant human G-CSF (Amgen, Thousand Oaks, CA) was radiolabeled with Na-125I (PerkinElmer, Waltham, MA) by the two-phase iodination method used for GH. For the binding assay, NFS-60 cells were first washed three times with RPMI 1640 medium with 0.1% BSA. Then 1×106 cells were incubated in 300 μl RPMI 1640 medium containing 0.1% BSA, 100 ng/ml 125I-G-CSF, and serially diluted G-CSF-Tf fusion proteins or free aglycosylated G-CSF (Neupogen, Amgen, Thousand Oaks, CA). After 3 hours of incubation at 4 °C, the cells were washed with cold PBS three times, and the cell pellets were counted in a gamma counter.
Competitive Tf Receptor (TfR) binding assay for GH-Tf and G-CSF-Tf fusion proteins
Human apo-Tf (Sigma) was dissolved in PBS at 1 mg/ml and incubated with 1 mg/ml ferric ammonium citrate (Sigma) at 37 °C for 2 hours to saturate Tf with Fe3+. The iron-saturated Tf was then dialyzed using Spectra/Por dialysis membrane (14,000 Da MW cut-off, Spectrum Laboratories, Rancho Dominguez, CA) in PBS at pH 7.4 overnight to remove the excess Fe3+. One hundred micrograms of Tf was radiolabeled with Na-125I (PerkinElmer) by Chloramine-T iodination 12. Caco-2 cells were seeded into 12-well plates and grown to confluent. The cells were washed with PBS and then incubated in serum-free DMEM with 0.1% BSA at 37 °C for 30 minutes to remove the endogenous Tf. A mixture of 0.5 μg/ml 125I-Tf with serial-diluted unlabeled fusion protein, or free Tf in DMEM with 0.1% BSA was added to the cells. After 2 hours of incubation at 4 °C, the medium was aspirated, and the cells were washed three times with ice-cold PBS. The cells were then dissolved in 1 M NaOH, and radioactivity in the lysates was counted using a gamma counter (Packard).
In vivo stability of linkers in GH-Tf fusion proteins
The GH-Tf fusion proteins with 3 stable linkers as well as the disulfide linker were administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. Twenty microliters of blood was collected from the saphenous vein as described by Hem et al. 13 at 5 min, 30 min, 1, 3, 6 and 12 hours postdose. The collected blood was mixed with 3 μl heparin (1000 unit/ml, Sigma) to prevent clotting, and was immediately centrifuged at 500 g for 20 minutes to remove the blood cells. The plasma samples were analyzed by non-reducing SDS-PAGE followed by anti-GH Western blot. Fusion proteins are loaded to serve as the controls. Antibody against GH (R & D system, Minneapolis, MN) was used as primary antibody and horseradish peroxidase-conjugated anti-goat IgG antibody (Sigma) was used as secondary antibody. The peroxidase activity was detected by enhanced chemiluminescence using Amersham ECL Plus™ detection reagents (GE Healthcare).
Pharmacokinetic studies for GH-Tf, G-CSF-Tf fusion proteins, free GH, and free G-CSF
The GH-Tf or G-CSF-Tf fusion proteins with 3 stable linkers were administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. Free GH was administered at 0.8 mg/kg to maintain the equivalent molar amount as the GH-Tf fusion proteins. Blood was collected at 5 min, 30 min, 1, 3 and 6 hours postdose for GH-Tf or at 5 min, 30 min, 1, 3, 6 and 12 hours postdose for G-CSF-Tf. The plasma samples were analyzed by non-reducing SDS-PAGE followed by quantitative anti-Tf Western blot. Each fusion protein or free GH of known concentration was loaded in serial concentrations to serve as the standards to quantify the plasma concentration of the respective protein. For quantifying the fusion proteins, antibody against human serum Tf (Sigma) was used as primary antibody and horseradish peroxidase-conjugated anti-goat IgG antibody (Sigma) was used as secondary antibody. For quantifying free GH, antibody against human growth hormone (R&D Systems, Minnneapolis, MN) was used as primary antibody and horseradish peroxidase-conjugated anti-goat IgG antibody (Sigma) was used as secondary antibody. The peroxidase activity was detected by enhanced chemiluminescence using the SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Rockford, IL). The Western blot result was captured by the Molecular Imager ChemiDoc XRS system (Bio-Rad, Hercules, CA), and was subsequently analyzed by Quantity One 1-D Analysis Software (Bio-Rad) to determine the amount of fusion protein in the plasma samples.
Free G-CSF was administered intravenously to CF1 mice at a dose of 0.8 mg/kg to maintain the equivalent molar amount as G-CSF-Tf fusion proteins. The blood was collected at 5 min, 15 min, 30 min, 1, 2, and 4 hours postdose. The amount of G-CSF in blood samples was determined via a G-CSF ELISA kit (R&D System).
Pharmacokinetic studies for GH-Tf with excess GH blockage
Human GH, expressed from HEK293 cells, was used to compete with GH-Tf fusion proteins for GHR binding in vivo. Two fold of molar equivalence of GH (1.6 mg/kg) was co-administered with GH-Tf fusion proteins (4 mg/kg) into CF1 mice via i.v. injection. The molar ratio was calculated based on the molecular weights of GH (22 kDa) and GH-Tf (102 kDa), which are about 5 fold different. The blood samples were then taken at 5 min, 30 min, 1, 3 and 6 hours postdose, and the amount of fusion proteins in the plasma was determined by quantitative anti-Tf Western blot as described above.
Pharmacokinetic studies for GH-Tf with excess Tf blockage
Fe3+-saturated Holo-Tf, at a dose of 240 mg/kg, was co-administered with fusion proteins (4 mg/kg) into CF1 mice to compete for TfR binding in vivo. Since the endogenous Tf is highly abundant (about 3 mg/ml as determined in CF1 mice), 240 mg/kg human holo-Tf was co-administered with the GH-Tf fusion proteins to achieve a concentration of 6 mg/ml for total Tf in vivo. As a result, the final level of total Tf is about 2-fold higher than the endogenous level. The blood samples were then taken from CF1 mice at 5 min, 30 min, 1, 3 and 6 hours postdose, and the amount of fusion proteins in plasma was determined by quantitative anti-Tf Western blot as described above.
Data Analysis
Data are presented as mean values ± standard deviation (SD), with n referring to the number of replicates. IC50 values for the competitive receptor binding assays were obtained by sigmoidal curve fitting using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). The IC50 values were used as an indicator of the binding affinity of the fusion proteins for GH, G-CSF, or Tf receptors. Unpaired two-sided Student’s t-test was used to test statistical significance of results. Results with a p-value < 0.05 were considered statistically significantly different. The elimination half-lives of the fusion proteins were calculated by using SAAM II software (University of Washington, Seattle, WA). The weight assigned to data is the inverse of the variance. The variance model used is data-relative, and the fractional standard deviation is set to 0.1 based on previous experiments.
Results
Receptor binding affinities of GH-Tf fusion proteins
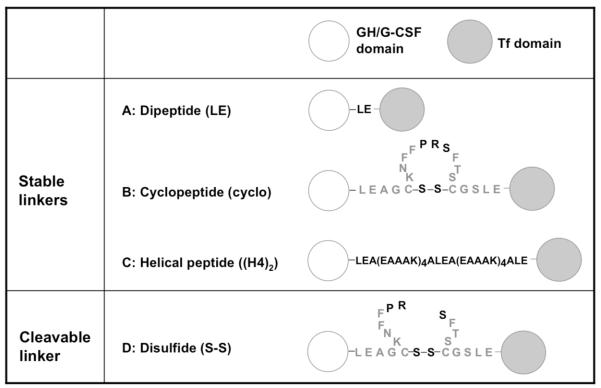
In order to compare the receptor binding affinities after linker insertion, competitive receptor binding assays were performed, in which unlabeled fusion proteins were used to compete with radiolabeled free GH, or free Tf for the respective receptor binding on target cell surface. The IC50 values of fusion proteins for inhibiting the receptor binding of radiolabeled GH or Tf were significantly different from each other (p < 0.05) (Figure 1 and Table 1). The dipeptide-linked GH-LE-Tf, which has the shortest linker, exhibited the weakest binding affinities for both GHR and TfR. On the other hand, fusion proteins with two other linkers (cyclopeptide and helical peptide) exhibited stronger binding capacities to both receptors, with the ranking of IC50 values for GHR binding: GH-LE-Tf (17.7 nM) > GH-cyclo-Tf (8.2 nM) > GH-(H4)2-Tf (7.0 nM), and for TfR binding: GH-LE-Tf (21.2 nM) > GH-(H4)2-Tf (8.7 nM) > GH-cyclo-Tf (4.2 nM). The IC50 values of free GH and free Tf were 5.8 nM and 0.3 nM, respectively. The IC50 values of free GH and Tf proteins are lower than those of all three GH-Tf fusion proteins.
Figure 1.
Competitive receptor binding assays for GH-Tf fusion proteins. (A): competitive GH receptor binding assay on IM-9 cells. (B): competitive Tf receptor binding assay on Caco-2 cells. Values (n = 3) are mean ± SD expressed as percentage of total surface bound tracer in the absence of fusion protein.
Table 1. Comparison of IC50 values and plasma-half lives of GH and GH-Tf fusion proteins *.
| Protein | IC50, GHR (nM) |
IC50, TfR (nM) |
t1/2 (h) | t1/2 with GH Blockage (h) |
t1/2 with Tf Blockage (h) |
|---|---|---|---|---|---|
| GH | 5.8 | -- | < 0.25 | -- | -- |
| GH-LE-Tf | 17.7 | 21.2 | 4.97 ± 0.34 | 5.95 ± 0.68 | 3.00 ± 0.94 |
| GH-cyclo-Tf | 8.2 | 4.2 | 1.76 ± 0.27 | 8.66 ± 2.98 | 2.14 ± 0.05 |
| GH-(H4)2-Tf | 7.0 | 8.7 | 1.87 ± 0.44 | 6.73 ± 2.05 | 1.61 ± 0.75 |
IC50 values from competitive receptor binding assays were determined by sigmoidal curve fitting using GraphPad Prism 4.0. Half-life values represent mean ± SD from 3 to 4 mice. The half-life of GH is too short to be accurately determined by using the Quantitative Western Blotting method.
The in vivo stability of the linkers
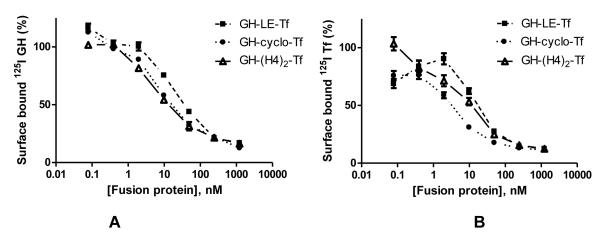
Before determining the PK of the fusion proteins, the in vivo stability of the linkers were first investigated, since the cleavage of the linkers in vivo could lead to the decrease of half-life and confound our interpretation. Therefore, GH-Tf fusion proteins with different linkers were i.v. injected to CF1 mice and the plasma samples were analyzed by anti-GH Western blot to check the integrity of the fusion proteins. As shown in Figure 2, for the GH-Tf with 3 peptide linkers, there is no detectable free GH present in the blood throughout the experiment, indicating that the 3 peptide linkers are stable in vivo. In contrast, free GH was released rapidly from the i.v. injected disulfide-linked fusion protein, GH-S-S-Tf, confirming our previous report on the in vivo rapid cleavage of the linker in the fusion protein 8.
Figure 2.
In vivo stability of the linkers. Anti-GH Western blot analysis for the plasma samples from mice injected with (A): GH-LE-Tf, (B): GH-cyclo-Tf, (C): GH-(H4)2-Tf, (D): GH-S-S-Tf. Data shown are from one representative experiment.
The pharmacokinetics of GH-Tf fusion proteins
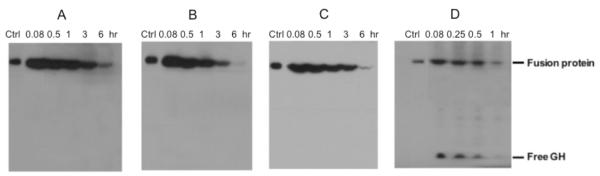
The half-lives of GH-Tf fusion proteins with 3 stable linkers were determined in CF1 mice. Anti-Tf, instead of anti-GH, Western blot was used to quantify the intact fusion protein due to its higher sensitivity. The fusion proteins can be easily distinguished from endogenous Tf due to their higher molecular weight (~102 kDa vs. 80 kDa, respectively). As shown in Figure 3A and Table 1, the half-lives of the fusion proteins were greatly affected by altering the receptor binding affinities with linker insertion. The ranking of the half-lives follows the order of GH-LE-Tf (4.97 hrs) > GH-(H4)2-Tf (1.87 hrs) ≈ GH-cyclo-Tf (1.76 hrs). Notably, the GH-LE-Tf fusion protein has an almost three-fold longer plasma half-life than the others despite having a very similar size and sequence. The plasma half-lives of the 3 GH-Tf fusion proteins were much longer than that of free GH (< 15 minutes).
Figure 3.
PK profiles of GH-Tf fusion proteins with different linkers. (A): The GH-Tf fusion proteins with different linkers were administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. (B): Fusion proteins were co-administered with GH (1.6 mg/kg). (C): Fusion proteins were co-administered with holo-Tf (240 mg/kg). Blood was collected at 5 min, 30 min, 1, 3 and 6 hours postdose and the plasma samples were analyzed by non-reducing SDS-PAGE followed by quantitative anti-Tf Western blot. The half-life was calculated by SAAM II and the values are mean ± SD from 3 to 4 mice.
The pharmacokinetics of GH-Tf fusion proteins with GH competition
In order to elucidate the role of GHR binding in the in vivo clearance of GH-Tf fusion proteins, a pharmacokinetic study of GH-Tf fusion proteins was performed with the competition of a 2 fold excess of free GH. With the blockage of GHR binding, the half-lives of all three GH-Tf fusion proteins were dramatically prolonged, with the ranking of GH-cyclo-Tf (8.66 hrs) > GH-(H4)2-Tf (6.73 hrs) > GH-LE-Tf (5.95 hrs) (Figure 3B and Table 1).
The pharmacokinetics of GH-Tf fusion proteins with Tf competition
In order to elucidate the correlation between TfR binding and plasma half-life, a pharmacokinetic study of GH-Tf fusion proteins was performed with excess Tf competition. As shown in Figure 3C and Table 1, with the co-administration of excess Tf, the half-life of GH-LE-Tf was significantly shortened from 4.97 hours to 3.00 hours (p < 0.05). The half-lives of GH-cyclo-Tf and GH-(H4)2-Tf, however, were not significantly changed with Tf competition.
Receptor binding affinities of G-CSF-Tf fusion proteins
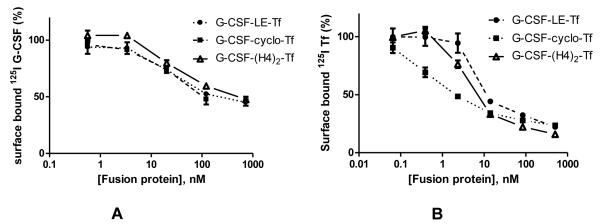
To further evaluate the effects of receptor binding on plasma half-life on bifunctional fusion proteins, another protein drug, G-CSF, was fused with Tf with 3 peptide linkers inserted between the two functional domains. The receptor binding affinities of G-CSF-Tf for G-CSFR, as well as TfR, were determined via competitive receptor binding assays on NFS-60 and Caco-2 cells, respectively. The G-CSFR binding affinity was similar among the 3 fusion proteins, i.e., with IC50 values of 38.0, 39.5, 31.2 nM for G-CSF-LE-Tf, G-CSF-cyclo-Tf and G-CSF-(H4)2-Tf, respectively. However, the TfR binding affinities were significantly different (p < 0.05), with the ranking of IC50 for TfR binding: G-CSF-LE-Tf (7.5 nM) > G-CSF-(H4)2-Tf (4.5 nM) > G-CSF-cyclo-Tf (0.9 nM) (Figure 4 and Table 2). The receptor binding affinities of free Tf and free G-CSF were also measured via competitive binding assays. The IC50 value of free Tf was 0.3 nM, which was lower than those of all 3 G-CSF-Tf fusion proteins. Due to the difficulty to express glycosylated G-CSF from HEK293 cells using the same condition as the fusion proteins, a commercially available G-CSF (Neupogen) was used for the preparation of 125I-G-CSF and for subsequent G-CSF receptor binding assays. The IC50 value of G-CSF (Neupogen) was 113.1 nM, which was slightly higher than those of the G-CSF-Tf fusion proteins. This difference may be due to the fact that Neupogen is a non-glycosylated form of G-CSF. It has been reported that glycosylation could affect the receptor binding affinity of G-CSF 14, 15. Regardless of the difference in G-CSF receptor binding between G-CSF (Neupogen) and G-CSF-Tf fusion proteins, results from receptor competition assays indicate that the 3 forms of fusion protein have a similar affinity to the G-CSF receptor.
Figure 4.
Competitive receptor binding assays for G-CSF-Tf fusion proteins. (A): competitive G-CSF receptor binding assay on NFS-60 cells. (B): competitive Tf receptor binding assay on Caco-2 cells. Values (n = 3) are mean ± SD expressed as percentage of total surface bound tracer in the absence of fusion protein.
Table 2.
Comparison of IC50 values and plasma-half lives of G-CSF and G-CSF-Tf fusion proteins *.
| Protein | IC50, G-CSFR (nM) |
IC50, TfR (nM) | t1/2 (h) |
|---|---|---|---|
| G-CSF** | 113.1 | -- | 1.74 ± 0.14 |
| G-CSF -LE-Tf | 38.0 | 7.5 | 4.15 ± 0.75 |
| G-CSF -cyclo-Tf | 39.5 | 0.9 | 5.69 ± 0.46 |
| G-CSF -(H4)2-Tf | 31.2 | 4.5 | 4.84 ± 1.18 |
IC50 values from competitive receptor binding assays were determined by sigmoidal curve fitting using GraphPad Prism 4.0. Half-life values represent mean ± SD from 3 to 4 mice.
Aglycosylated form (Neupogen)
The pharmacokinetics of G-CSF-Tf fusion proteins
As shown in Figure 5 and Table 2, the plasma half-lives of the G-CSF-Tf fusion proteins follow the ranking of G-CSF-cyclo-Tf (5.69 hrs) > G-CSF-(H4)2-Tf (4.84 hrs) > G-CSF-LE-Tf (4.15 hrs). The half-life of G-CSF-LE-Tf is significantly shorter than that of G-CSF-cyclo-Tf (p < 0.05). Therefore, the half-life of the dipeptide-linked G-CSF-LE-Tf was the shortest among G-CSF-Tf fusion proteins, as opposed to that of GH-LE-Tf, which was the longest among the GH-Tf fusion proteins (Figure 3 and Table 1). Similar to GH-Tf fusion proteins, the half-lives of G-CSF-Tf were much longer than that of free G-CSF (1.74 hrs).
Figure 5.
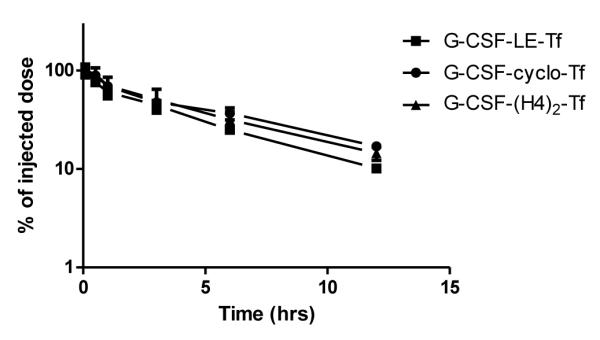
PK profiles of G-CSF-Tf fusion proteins with different linkers. The G-CSF-Tf fusion proteins with different linkers were administered intravenously to CF1 mice via the tail vein at a dose of 4 mg/kg. Blood was collected at 5 min, 30 min, 1, 3, 6 and 12 hours postdose and the plasma samples were analyzed by non-reducing SDS-PAGE followed by quantitative anti-Tf Western blot. The half-life was calculated by SAAM II and the values are mean ± SD from 3 to 4 mice.
Discussion
Bifunctional GH-Tf and G-CSF-Tf fusion proteins with different linkers (as shown in Scheme 1) were prepared. The first linker is a short dipeptide, Leu-Glu (LE), resulting from the cloning site XhoI between the two genes 9. The second linker is a thrombin-sensitive, disulfide cyclopeptide linker 8. This linker was originally used to create a disulfide linker that can be rapidly cleaved in vivo by reduction 8. The third linker is an alpha-helix forming linker LEA(EAAAK)4ALEA(EAAAK)4ALE, which can spatially separate the functional domains 6. A fourth linker was also used to produce a GH-Tf fusion protein containing an in vivo cleavable disulfide linker generated from the in vitro thrombin treatment of the cyclopeptide linker, which is able to release free functional domains from the fusion proteins in vivo 8. GH-Tf and G-CSF-Tf fusion proteins with these four linkers have previously been shown to maintain sufficient biological activity, both in vitro and in vivo 6, 8-10, 16, 17.
The results from the competitive receptor binding assay for GH-Tf showed that the dipeptide linker, which has the shortest length, generated the lowest binding affinities for both GHR and TfR (Figure 1 and Table 1). We reason that this short linker may generate strong steric hindrance or cause interference between functional domains, and therefore decreases the receptor binding capacity. On the other hand, the cyclopeptide linker and helical peptide linker, which have longer lengths (20 and 50 amino acids, respectively) and more rigid structures, generated higher receptor binding affinities (Figure 1 and Table 1). It was reported that the helical peptide linker could improve the biological activity of bifunctional fusion proteins, probably because it can effectively separate domains and provide higher receptor binding affinity 10. By inserting different linkers between the functional domains, we were able to successfully alter the receptor binding affinities of bifunctional fusion proteins.
Next, the in vivo stability of the linkers was evaluated to make sure the plasma half-life of the fusion protein will not be altered by the cleavage of the linkers following in vivo injection. Since the cyclopeptide linker contains a thrombin-sensitive sequence and the helical peptide linker contains many lysine residues, it was a concern that endogenous serine proteases may be able to cleave these linkers in vivo, which would contribute to the disappearance of intact fusion proteins in the plasma. To rule out this possibility, GH-Tf fusion proteins with 3 peptide linkers, as well as an in vivo cleavable disulfide linker, were i.v. injected into CF1 mice and the plasma samples were analyzed by anti-GH Western blot. As shown in Figure 2, for the GH-Tf with 3 peptide linkers, there is no detectable amount of free GH present in the plasma throughout the experiment (5 minutes to 6 hours). In contrast, free GH was released from the i.v. injected GH-S-S-Tf, which, as shown in our previous report 8, results in a significantly shortened plasma half-life of the intact fusion protein due to the cleavability of the linker. This data suggests the 3 peptide linkers are stable in vivo, and there is no preferential protease cleavage of the linkers. Therefore, the differences in plasma half-life are most likely due to the elimination of the intact fusion protein rather than the cleavability of the linker.
The pharmacokinetics of GH-Tf fusion proteins was determined in CF1 mice to evaluate the impact of receptor binding affinities on plasma half-life. GH-LE-Tf exhibited an almost 3-fold longer plasma half-life compared to the others, while GH-cyclo-Tf and GH-(H4)2-Tf had similar half-lives (Figure 3A and Table 1). The half-life of the GH-Tf fusion proteins correlated well with GHR binding affinities, but not with TfR binding affinities (Figure 1 and Table 1). GH-LE-Tf, which had the lowest binding affinity for GHR, exhibited the longest plasma half-life. We reason the binding of fusion protein to GHR will likely lead to endocytosis and lysosomal degradation of the fusion proteins as reported for free GH 18. As a result, GH-LE-Tf exhibited longer half-life due to lower affinity to GHR and consequently less degradation via receptor-mediated endocytosis. In addition, the comparable GHR binding affinities of GH-cyclo-Tf and GH-(H4)2-Tf accorded with their similar half-lives. The PK data indicate that receptor binding affinities could greatly affect the plasma half-life of bifunctional fusion proteins. It also suggests that GHR binding is the primary binding site for determining the plasma half-life of the GH-Tf fusion proteins, as will be further discussed later in this section.
To further confirm the role of GHR binding on in vivo clearance of fusion proteins, the PK of GH-Tf was investigated through the blockage of GHR by excess free GH (Figure 3B and Table 1). In this study, we demonstrated that the blockage of GHR with excess free GH could significantly prolong the plasma half-life of GH-Tf fusion proteins to a similar level (6 to 8 hours, not statistically significantly different from each other). This result has important implications for other bifunctional fusion proteins in addition to GH-Tf. For most protein drugs, the binding to their receptors will lead to the classic pathway of endocytosis and lysosomal degradation 19. This process is the primary factor in determining the plasma half-life, especially for biotechnology pharmaceuticals. Our result indicates that, similar to protein drugs, the receptor binding of a protein drug domain in a bifunctional fusion protein also causes the degradation, and constitutes a major elimination pathway of the fusion proteins.
Similarly, the impact of TfR binding on half-life was investigated through the competition of excess Tf (Figure 3C and Table 1). With the blockage of TfR binding, the half-life of GH-LE-Tf was shortened, from 4.97 to 3.00 h, suggesting that the TfR binding may help recycle the fusion proteins through the classic Tf-TfR recycling pathway 20. This data also indicates that the effect of TfR binding is minor compared to GHR binding, since TfR binding only prolongs half-life of the fusion protein with the weakest GHR binding affinity (i.e. GH-LE-Tf). Again, this result confirmed that GHR binding is the primary binding which overrides TfR binding in determining the plasma half-life. Another interesting finding is that with excess free GH competition, the half-lives of the three GH-Tf fusion proteins correlated very well with their TfR binding affinities. With GHR blockage, GH-cyclo-Tf, which has the strongest TfR binding affinity, exhibited the longest half-life (Figure 1B and Table 1). This result is presumably due to the effect of TfR binding in recycling of the fusion protein.
Besides GH-Tf, G-CSF-Tf fusion proteins with 3 stable linkers were also constructed to broaden the scope of this study, and to further evaluate the importance of the secondary binding to TfR in predicting the plasma half-life. The data showed that the G-CSFR binding affinity was similar among the 3 fusion proteins, while their TfR binding affinity was significantly different (Figure 4 and Table 2). When correlating the binding affinity with plasma half-life, since the fusion proteins have similar G-CSFR binding affinity, the half-life of G-CSF-Tf is determined by the binding to TfR. Our data demonstrate that the stronger TfR binding results in longer plasma half-life (Figure 5 and Table 2). This finding further supports our conclusions from GH-Tf, that the TfR binding leads to the recycling of the fusion protein and prolongs the plasma half-life of the fusion proteins.
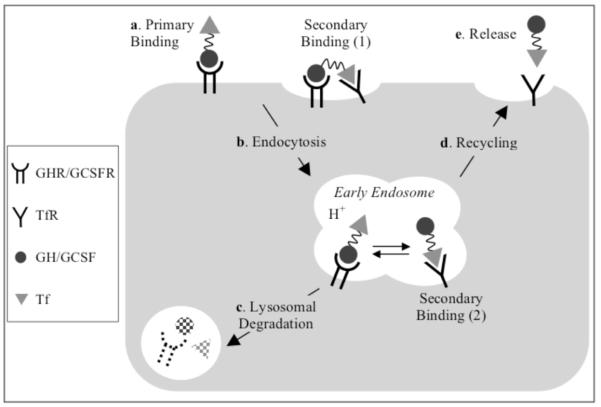
Based on the above results, we proposed a model for the receptor binding and intracellular processing of the GH/G-CSF-Tf fusion proteins (Scheme 2). In the presence of abundant endogenous Tf (about 60 fold higher than the injected fusion protein), the binding of fusion protein to TfR on the cell surface is probably negligible due to the competition from endogenous Tf. Therefore, the fusion proteins likely bind first to GHR/G-CSFR on the plasma membrane of target cells. This binding is considered the primary binding site, which enriches the fusion proteins onto the target cells for the drug action, and may also increase the accessibility of the Tf domain to TfR. TfR binding, which likely occurs after GHR/G-CSFR binding either on the plasma membrane or inside the endosomes, is then referred to as secondary binding. Following the GHR/G-CSFR binding, signal transduction is initiated, and the fusion proteins are endocytosed into the early endosome, where TfR is also present 21. With the acidification of endosome, the fusion proteins may dissociate from GHR/G-CSFR, and bind tightly to TfR via their Tf domain due to the changes in binding affinities at the acidic pH 22. While the binding affinity of GH/G-CSF for GHR/G-CSFR in acidic pH is slightly lower than in neutral pH 23, 24, the affinity of apo-Tf for TfR is increased under the acidic endosomal environment 25, 26. The receptor binding inside the endosomes determines different fates for the fusion proteins: The GHR/G-CSFR binding leads to the lysosomal degradation while the TfR binding promote the recycling of the fusion protein. The relative strength of the binding of the two protein domains inside the endosome will determine the impact of each receptor on the plasma half-life of the fusion proteins.
Scheme 2.
Endocytic pathway and intracellular metabolism of Tf fusion proteins.*
*a. In the presence of abundant endogenous Tf, the fusion proteins first bind to GHR/GCSFR on the target cell membrane via GH/GCSF domain. This binding is considered the primary binding, which enriches the fusion proteins onto the target cells. The GHR/GCSFR binding at the cell surface brings fusion protein close to the plasma membrane surface, which may lead to bivalent binding of the Tf-domain to TfRs, which are present in the clathrin-coated pit regions. This binding, indicated as Secondary Binding (1), is referred to secondary binding since it occurs after the GHR/GCSFR binding. b. The fusion proteins are endocytosed into the early endosome, where TfR is also present. c. Fusion proteins that remain bound to GHR/GCSFR are degraded in the lysosome. With the acidification of endosome, the fusion proteins will retain the binding affinity to TfR via their Tf domain, indicated as Secondary Binding (2). d. The binding to TfR allows the fusion protein to be recycled back to the cell surface. e. The fusion protein is released from TfR into the circulation at cell surface.
The model for GH/G-CSF-Tf fusion proteins may provide an example for many other bifunctional fusion proteins such as Fc-fusion proteins or albumin-fusion proteins, which are also composed of a protein drug domain and a carrier protein domain. The Fc portion of IgG and albumin have a very similar recycling pathway to Tf. By binding to the major histocompatibility complex-related Fc receptor (FcRn) within the endosomes at acidic pH, these proteins are efficiently recycled back to the cell surface 27, 28. Conceivably, these bifunctional Fc- and albumin-fusion proteins may follow similar endocytic pathway and intracellular processing as we demonstrated here for GH/G-CSF-Tf fusion proteins.
Our study provides several implications for the design and development of bifunctional fusion proteins. First, we demonstrated the feasibility of fine-tuning the PK profiles of fusion proteins by linker insertion due to their impact on the receptor binding affinities. Second, our study highlights the importance of determining the receptor binding affinities in predicting the plasma half-life of bifunctional fusion proteins. Third, in contrast to the conventional approach of attempting to maximize the binding affinity of the protein drug such as GH/G-CSF for the highest potency, our study suggests that the binding affinity of the protein drug domain may have an optimal range to achieve less intracellular degradation and a possibly better overall therapeutic effect. When endocytic degradation is considerable, the intracellular trafficking of the fusion protein may be a key target for enhancing plasma half-life and in vivo potency. To our knowledge, this is the first report on a mechanistic study of pharmacokinetic profiles of bifunctional fusion proteins in the context of individual binding, which is vital for the future development of these types of biomolecules as therapeutics. In the development of protein drugs, although the fusion of a carrier protein can diminish the binding affinity to the drug receptor, the overall in vivo bioactivity may be improved due to the prolonged plasma half-life. In the context of G-CSF-Tf fusion proteins, prolonged plasma half-life and improved in vivo biological activity were observed after the fusion of Tf to G-CSF 6, 9, 10. This characteristic has been frequently observed in other fusion proteins (e.g., albumin, Fc-fusion proteins) 3, 5, 29, 30, as well as in protein conjugates (e.g., PEGylation) 31. However, a prolonged plasma half-life may not always correlate with superior in vivo biological activity. For example, GH-Tf displayed comparable in vivo bioactivity as free GH despite prolonged plasma half-life 6, possibly due to the diminished binding affinity, or altered biodistribution 32. Nevertheless, a thorough understanding of the PK characteristics of bifunctional fusion proteins is essential in the development of these biomolecules for therapeutic use.
ACKNOWLEDGMENT
This work was supported partially by NIH grant R01GM063647. WCS is John A. Biles Professor of Pharmaceutical Sciences. We thank Dr. Nurmamet Amet for providing the plasmids for the GH-Tf fusion proteins.
REFERENCES
- 1.Leader B, Baca Q, Golan D. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 2.Duttaroy A, Kanakaraj P, Osborn B, Schneider H, Pickeral O, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R, Crossan D, Bock J, Kaufman T, Reavey P, Carey-Barber M, Krishnan S, Garcia A, Murphy K, Siskind J, McLean M, Cheng S, Ruben S, Birse C, Blondel O. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251–8. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Osborn B, Olsen H, Nardelli B, Murray J, Zhou J, Garcia A, Moody G, Zaritskaya L, Sung C. Pharmacokinetic and pharmacodynamic studies of a human serum albumin-interferon-alpha fusion protein in cynomolgus monkeys. J Pharmacol Exp Ther. 2002;303:540–8. doi: 10.1124/jpet.102.037002. [DOI] [PubMed] [Google Scholar]
- 4.Müller N, Schneider B, Pfizenmaier K, Wajant H. Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun. 2010;396:793–9. doi: 10.1016/j.bbrc.2010.04.134. [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Low S, Kamphaus G, Dumont J, Amari J, Lu Q, Zarbis-Papastoitsis G, Reidy T, Merricks E, Nichols T, Bitonti A. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115:2057–64. doi: 10.1182/blood-2009-08-239665. [DOI] [PubMed] [Google Scholar]
- 6.Amet N, Wang W, Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. Journal of Controlled Release. 2010;141:177–182. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–50. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Bai Y, Zaro J, Shen WC. Design of an in vivo cleavable disulfide linker in recombinant fusion proteins. Biotechniques. 2010;49:513–8. doi: 10.2144/000113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai Y, Ann D, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102:7292–6. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai Y, Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm Res. 2006;23:2116–21. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 11.Tejedor F, Ballesta JP. Iodination of biological samples without loss of functional activity. Anal Biochem. 1982;127:143–9. doi: 10.1016/0003-2697(82)90156-7. [DOI] [PubMed] [Google Scholar]
- 12.Robinson C, Reit B, Martin T. Proceedings: Effect of iodination by the chloramine T and lactoperoxidase methods upon the biological activity of parathyroid hormone. J Endocrinol. 1974;63:27P–28P. [PubMed] [Google Scholar]
- 13.Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim. 1998;32:364–8. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- 14.Bönig H, Silbermann S, Weller S, Kirschke R, Körholz D, Janssen G, Göbel U, Nürnberger W. Glycosylated vs non-glycosylated granulocyte colony-stimulating factor (G-CSF)-- results of a prospective randomised monocentre study. Bone Marrow Transplant. 2001;28:259–64. doi: 10.1038/sj.bmt.1703136. [DOI] [PubMed] [Google Scholar]
- 15.Höglund M. Glycosylated and non-glycosylated recombinant human granulocyte colony-stimulating factor (rhG-CSF)--what is the difference? Med Oncol. 1998;15:229–33. doi: 10.1007/BF02787205. [DOI] [PubMed] [Google Scholar]
- 16.Amet N, Lee HF, Shen WC. Insertion of the designed helical linker led to increased expression of tf-based fusion proteins. Pharm Res. 2009;26:523–8. doi: 10.1007/s11095-008-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amet N, Chen X, Lee H-F, Zaro JL, Shen WC. Transferrin Receptor-mediated Transcytosis in Intestinal Epithelial Cells for Gastrointestinal Absorption of Protein Drugs. In: Narang AS, Mahato RI, editors. Targeted Delivery of Small and Macromolecular Drugs. Taylor and Francis; London: 2009. [Google Scholar]
- 18.van Kerkhof P, Strous G. The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand. Biochem Soc Trans. 2001;29:488–93. doi: 10.1042/bst0290488. [DOI] [PubMed] [Google Scholar]
- 19.Mager D. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72:1–10. doi: 10.1016/j.bcp.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Grant B, Donaldson J. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller-Eberhard U, Liem H, Grasso J, Giffhorn-Katz S, DeFalco M, Katz N. Increase in surface expression of transferrin receptors on cultured hepatocytes of adult rats in response to iron deficiency. J Biol Chem. 1988;263:14753–6. [PubMed] [Google Scholar]
- 22.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55:1439–66. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Roupas P, Herington A. Intracellular processing of growth hormone receptors by adipocytes in primary culture. Mol Cell Endocrinol. 1988;57:93–9. doi: 10.1016/0303-7207(88)90037-8. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar C, Lowenhaupt K, Horan T, Boone T, Tidor B, Lauffenburger D. Rational cytokine design for increased lifetime and enhanced potency using pH-activated “histidine switching”. Nat Biotechnol. 2002;20:908–13. doi: 10.1038/nbt725. [DOI] [PubMed] [Google Scholar]
- 25.Morgan E. Effect of pH and iron content of transferrin on its binding to reticulocyte receptors. Biochim Biophys Acta. 1983;762:498–502. doi: 10.1016/0167-4889(83)90052-6. [DOI] [PubMed] [Google Scholar]
- 26.Dautry-Varsat A, Ciechanover A, Lodish H. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983;80:2258–62. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung Y, Leabman M, Marvin J, Qiu J, Adams C, Lien S, Starovasnik M, Lowman H. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–71. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- 28.Anderson C, Chaudhury C, Kim J, Bronson C, Wani M, Mohanty S. Perspective-- FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 2006;27:343–8. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Halpern W, Riccobene T, Agostini H, Baker K, Stolow D, Gu M, Hirsch J, Mahoney A, Carrell J, Boyd E, Grzegorzewski K. Albugranin, a recombinant human granulocyte colony stimulating factor (G-CSF) genetically fused to recombinant human albumin induces prolonged myelopoietic effects in mice and monkeys. Pharm Res. 2002;19:1720–9. doi: 10.1023/a:1020917732218. [DOI] [PubMed] [Google Scholar]
- 30.Kostenuik P, Ferrari S, Pierroz D, Bouxsein M, Morony S, Warmington K, Adamu S, Geng Z, Grisanti M, Shalhoub V, Martin S, Biddlecome G, Shimamoto G, Boone T, Shen V, Lacey D. Infrequent delivery of a long-acting PTH-Fc fusion protein has potent anabolic effects on cortical and cancellous bone. J Bone Miner Res. 2007;22:1534–47. doi: 10.1359/jbmr.070616. [DOI] [PubMed] [Google Scholar]
- 31.Fishburn C. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–83. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 32.He XH, Shaw PC, Tam SC. Reducing the immunogenicity and improving the in vivo activity of trichosanthin by site-directed pegylation. Life Sci. 1999;65:355–68. doi: 10.1016/s0024-3205(99)00257-x. [DOI] [PubMed] [Google Scholar]