Abstract
Background
Molecules taken up by olfactory and trigeminal nerve neurons directly access the brain by the nose-to-brain pathway. In situ-forming mucoadhesive gels would increase the residence time of intranasal material, favoring the nose-to-brain delivery. In this first approach, brain radioactivity after intranasal administration of 32P-small interference RNA (siRNA) complexed with poly(amidoamine) G7 dendrimers (siRNA dendriplexes) within in situ-forming mucoadhesive gels, was determined.
Materials
32P-siRNA dendriplexes were incorporated into in situ-forming mucoadhesive gels prepared by blending thermosensitive poloxamer (23% w/w) with mucoadhesive chitosan (1% w/w, PxChi) or carbopol (0.25% w/w, PxBCP). Rheological properties, radiolabel release profile, and local toxicity in rat nasal mucosa were determined. The best-suited formulation was intranasally administered to rats, and blood absorption and brain distribution of radioactivity were measured.
Results
The gelation temperature of both formulations was 23°C. The PxChi liquid showed non-Newtonian pseudoplastic behavior of high consistency and difficult manipulation, and the gel retained 100% of radiolabel after 150 minutes. The PxCBP liquid showed a Newtonian behavior of low viscosity and easy manipulation, while in the gel phase showed apparent viscosity similar to that of the mucus but higher than that of aqueous solution. The gel released 35% of radiolabel and the released material showed silencing activity in vitro. Three intranasal doses of dendriplexes in PxCBP gel did not damage the rat nasal mucosa. A combination of 32P-siRNA complexation with dendrimers, incorporation of the dendriplexes into PxCBP gel, and administration of two intranasal doses was necessary to achieve higher brain radioactivity than that achieved by intravenous dendriplexes or intranasal naked siRNA.
Conclusion
The increased radioactivity within the olfactory bulb suggested that the combination above mentioned favored the mediation of a direct brain delivery.
Keywords: nucleic acids, gel, dendrimers, mucosa, olfactory bulb
Introduction
While nasal delivery has probably been the most successful of the alternative transmucosal routes as a portal of entry into the systemic circulation for substances that cannot be given orally,1 research into whether the intranasal route might deliver potentially therapeutic amounts of large molecules such as proteins and nucleic acids to the central nervous system (CNS) was first described only a little over a decade ago.2–4 The delivery of most therapeutics to the brain parenchyma is impaired by the blood–brain barrier (BBB). Together with a low rate of pinocytosis and the presence of efflux pumps (eg, P-glycoprotein), the BBB presents low paracellular permeability, due to the tight junctions between endothelial cells, which induce a high transendothelial electrical resistance (1500–2000 Ω · cm2 compared to 3–30 Ω · cm2 across most peripheral microvessels). Excepting the diffusion of lipid-soluble molecules of molecular weight lower than 400 Da, only glucose and amino acids internalized by carrier-mediated transport or insulin and transferrin internalized by receptor-mediated transport, gain entry to the CNS by the BBB.5
The direct delivery of intranasally administered molecules occurs by neuronal uptake and is a noninvasive and simpler alternative route than parenteral administration that bypasses the BBB, avoids fast systemic clearance, and limits potential secondary effects.6–8 Neuronal uptake can occur at the olfactory or the trigeminal nerve systems, exposed at the olfactory and respiratory epitheliums of the nose, respectively. This makes the nose-to-brain pathway the most direct method for noninvasive entry into the brain. Direct delivery to the brain of small molecules and biologics such as proteins and gene vectors has now been documented in numerous animal and clinical studies.3,9,10 This pathway is presumably involved in the increased cerebrospinal fluid levels of molecules that do not cross the BBB, such as peptides melanocortin,4–10 and insulin11 in humans. Also intranasal delivery of oxytocin showed a wide range of effects on human behavior and may be of clinical benefit for social disorders such as anxiety, psychological stress, and autism.12,13
Although the potential of RNA interference-based therapies against diseases affecting the CNS such as spinocerebellar ataxias, Parkinson’s disease, Alzheimer’s disease, and brain tumors has been successfully revealed in vitro and on in vivo models,14 the BBB penetration of high molecular weight and negatively charged small interfering RNA (siRNA) is impaired. Because of this, the delivery of siRNA to the CNS has been mostly based on invasive administrations of naked or electrostatically complexed siRNA with cationic lipids or polymers. For instance intraventricular injection,15,16 perilesional stereotactic injections,17 direct injection in the hippocampus18 or in the right striatum,19 convection-enhanced delivery,20 and local electroporation21 have been used to knock down different exogenous or endogenous genes. Other approaches less explored, that do not involve direct neuronal uptake, rely on the intravenous administration of siRNA or its complexes coupled to ligands targeted to receptors on the brain capillary endothelial cell, such as the transferrin receptor,22 the nicotinic acetylcholine receptor,23 the low-density lipoprotein receptor,24,25 or the rabies virus glycoprotein peptide.26 One research project has shown the presence of fluorescein isothiocyanate-labeled siRNA and the reduced expression of αB-crystallin in the olfactory bulb after intranasal administration of siRNA/commercial cationic lipid complexes.27
We have previously shown that degradation of naked siRNA by RNAses can be reduced and its endocytic uptake increased after electrostatic complexation with cationic dendrimers. 28 We showed that electrostatic complexes between siRNA/poly(amidoamine) generation 7 dendrimers (G7) (siRNA dendriplexes) prepared in low ionic strength medium inhibited the expression of enhanced green fluorescent protein both in human glioblastoma cells and in macrophages in vitro.
In this work, we compared the brain radioactivity after intranasal administration of radiolabeled siRNA dendriplexes within in situ-forming mucoadhesive gel, with that after intranasal naked siRNA in gel, and intravenous siRNA dendriplexes. The route and formulation that provide the highest brain radioactivity would be the most likely to achieve brain silencing. In humans, the olfactory epithelium is located in the roof of the nasal cavity, making access by powders or instillations difficult.29 Hence to prevent drainage from the site of application and to overcome the nasal mucociliary clearance, siRNA dendriplexes were included within in situ-forming mucoadhesive gels. The rheological properties of blends of the thermosensitive poloxamer (23% w/w) with the mucoadhesive chitosan (1% w/w) or carbopol (0.25% w/w) were determined in the absence or presence of siRNA dendriplexes both in liquid and gel states. After testing the release from gels, in vitro silencing activity, and toxicity in nasal mucosa, the best-suited formulation was intranasally administered to rats. After one or two intranasal doses, the regional brain distributions and blood levels of radioactivity were determined.
Materials and methods
Materials
Poly(amidoamine) G7 dendrimers (MW: 116488.71 g/mol, 512 amine end groups) and benzethonium hydroxide 1M were purchased from Sigma-Aldrich, MO. Enhanced green fluorescent protein siRNA sense sequence 5-GCACGACUUCUUCAAGUCCdTdT-3 and 5-GGACUUGAAGAAGUCGUGCdTdT-3 (antisense) was synthesized by Bioneer and purified by high-performance liquid chromatography. Lipofectamine™ 2000 was obtained from Gibco, (Gibco Life Technologies, New York, NY) T4 polynucleotide kinase was from Thermo Scientific (Waltham, MA). Scintillation cocktail Optiphase “HiSafe” 3 and γ-[32P]-ATP 3000 Ci/mmol, 3.33 μM were from PerkinElmer (Waltham, MA). Ketamina 50 was from Holliday-Scott SA (Buenos Aires, Argentina) and acepromazine Seda-T from Zoovet Laboratories (Santa Fe, Argentina). Polyacrilic acid (carbopol 974P NF) was from Lubrizol (Wickliffe, OH), poloxamer 407 (Pluronic® F127) from BASF, (Buenos Aires, Argentina), and Protasan UP CL113 (chitosan with 75%–90% of deacetylation degree, molecular weight: 50,000–150,000 g/mol) was from NovaMatrix, Sandvika, Norway. All other chemicals and reagents were of analytical grade from Anedra (San Fernando, Argentina).
siRNA dendriplexes and lipoplexes preparation
siRNA dendriplexes were prepared as stated in Perez et al.30 Briefly, an aliquot of G7 from methanol stock was diluted in 10 mM Tris-HCl pH 7.4 buffer (Tris-HCl buffer) and siRNA was added to give a 10/1 (N/P, the available amino groups in dendrimer to the RNA phosphate groups ratio) ratio. The mixture was incubated 20 minutes at room temperature prior to use. SiRNA lipoplexes were prepared according to the manufacturer’s instructions; an aliquot of Lipofectamine 2000 was diluted in Tris-HCl buffer, and siRNA was added at a 3.6:1 (w/w) ratio.
In situ-forming gel preparation
In situ-forming gel containing 23% w/w poloxamer and 0.25% w/w carbopol (PxCBP) was prepared as stated by Jones et al.31 Briefly, the required mass of each polymer was dissolved in distilled water (at 4°C) and proper quantities of propylene glycol and glycerol were added up to a 0.85% w/w final concentration each. The mixture was stirred in an Ultra-Turrax® (IKA® T10 basic; San Pablo, Brazil), the pH was adjusted to 5.5 with triethanolamine, and the mixture was stored at 4°C prior to further use.
In situ-forming gel containing 23% w/w poloxamer and 1% w/w chitosan (PxChi) was prepared as stated by Gratieri et al.32 Firstly, 30% w/w poloxamer solution was prepared by dissolving the required amount of poloxamer in distilled water (at 4°C) under agitation in Ultra-Turrax. The solution was stored at 4°C prior to further use. A 4% chitosan solution was prepared by dissolving the required amount of chitosan in 0.1% w/v acetic acid under agitation and the pH was adjusted to 5.5 with NaOH. Then, 23 mL of this chitosan solution was added to 77 mL of poloxamer solution (at 4°C), glutaraldehyde was added up to 0.25% w/w, and the mixture was stirred in Ultra-Turrax and stored at 4°C prior to further use.
In situ-forming gels containing siRNA dendriplexes or siRNA lipoplexes were obtained by adding 50 μL of the complexes to 1500 μL of in situ-forming gels at 4°C, where the final concentrations were 0.47 nmol/mL siRNA, 0.36 nmol/mL G7, and 0.65 μg/mL Lipofectamine 2000.
Rheological characterization of in situ-forming gels
Rheological studies were performed with an AR-G2 controlled stress rheometer (TA Instruments, New Castle, DE) equipped with cone/plate geometry of 40 mm diameter and an angle of 2°. Measurements were done on in situ-forming gels without or with dendriplexes. Samples were carefully applied to the lower plate of the rheometer and allowed to equilibrate for at least 5 minutes prior to analysis. Each preparation was tested three times.
Measurement of gelation temperature
The gelation temperatures (Tsol/gel) of in situ-forming gels were measured using oscillatory rheometry as previously reported.33 The samples were subjected to a constant shear stress value and frequency (chosen in the linear viscoelastic region, previously determined as 1 Pa and 3.14 rad/s, respectively). The temperature was increased in a stepwise manner from 4°C to 36°C (heating rate: 1°C/minute) and the elastic modulus (G′) was measured. The phase transition from liquid to gel (Tsol/gel) was inferred from the inflexion point of G′.
Measurement of flow properties
The flow curves of in situ-forming gels were determined at 10°C and 25°C over a range of shear rates (0.1–100 1/s). The flow curves were modeled using the Oswald-de-Waele equation (power law)34 τ = kγ n, where τ is the shear stress (Pa), k is the consistency index ((Pa s)n), γ is the rate of shear (1/s), and n is the flow behavior index (dimensionless). n is a measurement of the deviation from the Newtonian behavior, if n = 1 this indicates Newtonian behavior.
Measurement of viscoelastic properties
The frequency of samples subjected to a constant temperature of 10°C or 25°C and shear stress value (1 Pa) was increased in a stepwise manner from 0.1 to 100 rad/s and the elastic modulus (G′) and viscous modulus (G″) were measured.
Radiolabeling and purification
SiRNA was [32P] end-labeled using T4 polynucleotide kinase with 12.5 μM siRNA and 1.25 μM γ-[32P]ATP (50 μCi) as described previously35 and purified using Micro Bio-Spin P6 chromatography columns (Bio-Rad, Philadelphia, PA). In order to remove traces of free γ-[32P]ATP, the purification was repeated three times until scintillation counting of the radioactivity in the mixture remained constant before and after the spin column, thus indicating the complete absence of free γ-[32P]ATP. The radiolabeling efficiency and purity of the [32P]-siRNA was checked by electrophoresis in 20% polyacrylamide gel in TBE buffer (0.089 M Tris base, 0.089 M boric acid, 2 mM ethylenediaminetetraacetic acid) pH 8.3. The gel was cut horizontally in the middle and vertically between each lane, to determine the radioactivity corresponding to [32P]-siRNA (upper part of the gel lane) and [γ-32P]-ATP (lower part of the gel lane). Each fragment was disintegrated mechanically, scintillation solution was added and radioactivity was measured by liquid scintillation using a Wallac 1214 liquid scintillation counter (PerkinElmer).
The purity was calculated according to the following formula: [dpm [32P]-siRNA/(dpm [32P]-siRNA + dpm [γ-32P]-ATP)] × 100, where dpm [32P]-siRNA are disintegrations per minute (dpm) of the upper part of the gel lane and dpm [γ-32P]-ATP are the dpm of the lower part of the gel lane. The efficiency was calculated in the same way except that the aliquot submitted to electrophoresis was not purified. [32P]-siRNA was obtained with 70% of efficiency, 90%–95% of purity, and 50% of recovery.
Stability upon 100-fold dilution of [32P]-siRNA dendriplexes in Tris-HCl buffer was determined by polyacrylamide gel electrophoresis. Ten minutes after dilution, samples were run in a 20% polyacrylamide gel in TBE buffer pH 8.3. The gel was cut as stated above and siRNA released from dendriplexes was calculated as: [dpm [32P]-siRNA/(dpm [32P]-siRNA/G7 + dpm [32P]-siRNA)] × 100, where dpm [32P]-siRNA are the dpm of the lower part of the gel lane and dpm [32P]-siRNA/G7 are dpm of the upper part of the gel lane.
In vitro release from in situ-forming gels
The radioactivity release from the in situ-forming gels containing naked [32P]-siRNA, [32P]-siRNA dendriplexes, or [32P]-siRNA lipoplexes was determined using 3-μm pore size polycarbonate membrane Transwell® systems (Costar®, Corning Incorporated, Corning, NY). 0.5 mL of the in situ-forming gels at 4°C were placed in the upper chambers, placed in 24-well plates and incubated at 35°C for 10 minutes. Then 1 mL of simulated nasal electrolyte solution (0.8 g/L NaCl, 3 g/L KCl, and 0.45 g/L CaCl2, pH 6.8)36 was added to the lower chambers and the plates were placed into an oven at 35°C. At predetermined time points aliquot of 10 μL was taken from of the lower chambers, was mixed with 1.5 mL of scintillation cocktail, and the simulated nasal electrolyte solution was refilled. Radioactivity in each aliquot was determined using a Beckman LS 6000 scintillation counter (Pegasus Scientific, Rockville, MD), set to 1 minute counting time. The percentage of radioactivity release was calculated as follows = (dpma × 100)/dpmi × 100), where dpma are dpm of each time point lower chamber aliquot and dpmi are dpm in the upper chamber at the beginning.
Then, the silencing activity of the released material from in situ PxCBP forming gel containing siRNA dendriplexes in culture medium (Dulbecco’s modified Eagle’s medium; Gibco) was tested after 150 minutes of incubation, on enhanced green fluorescent protein-expressing J774 cells and compared with the silencing activity of siRNA dendriplexes not incorporated in gels, as previously described in Perez et al 2009 and 2011.28,30
Animals
Male Sprague-Dawley rats (8–10 weeks, ≈300 g) were purchased from Facultad de Veterinaria, Universidad de Buenos Aires and housed in a temperature- and humidity-controlled room with a 12-hour light/dark cycle, with free access to food and water. All animals used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). The protocol was approved by the Ethics Committee of the Cardiovascular Research Center, National Research Council (CCT-La Plata CONICET, Argentina).
Histopathology of nasal tissues after repeat administration
Animals were anesthetized by intraperitoneal injection of a mixture of ketamina and acepromazine at a dose of 45 and 0.5 mg/kg, respectively, and dendriplexes in Tris-HCl buffer or within in situ-forming PxCBP gel were administered into the nostrils once a day for 3 consecutive days. Animals were placed in the supine position and a total volume of 60 μL (30 μL to each nostril in aliquots of 10 μL using a p10 micropipette) was administered. Control groups received three doses of Tris-HCl buffer or one dose of 0.1% w/v sodium dodecyl sulfate. Three hours after the last dose, the animals were sacrificed by cervical dislocation, and the heads were removed and fixed in Bouin solution for 24 hours followed by immersion in 70% v/v ethanol. After fixation, the heads were decalcified for 4 days in a solution of formaldehyde/formic acid. Next, the heads were embedded in paraffin and sectioned in four 2–3 mm thick sections according to Jansson et al.37 Then, 5 μm-thick cross-sections of sections containing respiratory and olfactory epithelium were made. Sections were mounted on cut edge glass slides and stained with hematoxylin and eosin or hematoxylin and periodic acid–Schiff reaction for microscopic examination. Toxicological evaluation was done by a trained toxicologist using the criteria of histopathology described by Bindseil et al.38 The presence of epithelial eosinophilia, luminal exudates, and leukocytic margination (inflammation signs), desquamated epithelial cells, hyperemia (excess of blood), and osteoblast activation (inflammation signs) was examined. The microscopic findings were graded between 0 and 3 (0 = absent, + = minimal, ++ = moderate, +++ = severe histological change).
Nasal absorption and brain distribution
Groups of male Sprague-Dawley rats (n = 3 per group) were anesthetized as stated above and then received intravenously or intranasally 28 pmol [32P]-siRNA (22 pmol G7 or 1.4 μg Lipofectamine 2000) corresponding to 10 μCi per dose per animal, as shown in Table 1. After 90 or 180 minutes, rats were euthanized and samples of blood and brains were collected, washed with cold saline, blotted dry, and their weight determined. For scintillation counting, blood and tissue samples were dissolved in 0.5 mL benzethonium hydroxide at 37°C, sonicated for 30 minutes, and incubated overnight at 37°C. Then, 100 μL of 100 vol H2O2, 40 μL of 1 M HCl, and 5 mL of scintillation cocktail was added to each sample. The activity of [32P] in each sample was determined using a Beckman LS6000 scintillation counter, set to 1 minute counting time. The activity of the injected dose was calculated based on the scintillation count of a small sample of the injected solution.
Table 1.
Samples, dosage, routes and number of doses used in the different experimental groups
| Experimental group | Formulation | Route of administration | Number of dosesa | Timeb (minutes) |
|---|---|---|---|---|
| siRNA/G7 | Buffer | Intravenous | 1 | 90 |
| siRNA/G7 | Buffer | Intranasal | 1 | 90 |
| siRNA/G7 | Buffer | Intranasal | 2 | 180 |
| siRNA/Lipofectamine™ 2000 | Buffer | Intranasal | 2 | 180 |
| siRNA/G7 | PxCBP | Intranasal | 1 | 90 |
| siRNA/G7 | PxCBP | Intranasal | 2 | 180 |
| siRNA/Lipofectamine 2000 | PxCBP | Intranasal | 2 | 180 |
| Naked siRNA | PxCBP | Intranasal | 2 | 180 |
Notes:
The two doses were administered with a 90-minute interval;
time between first administration and sacrifice.
Abbreviations: siRNA, small interfering RNA; G7, generation 7 dendrimers; PxCBP, poloxamer/carbopol gel.
Statistical analysis
Statistical analyses were performed by one-way analysis of variance, followed by Dunnett’s test using Prisma software (v 4.00; Graphpad Software Corporation, San Diego, CA). Significance levels are shown in the Figure legends.
Results and discussion
In situ-forming gels
The aim of the present work was to explore the intranasal administration of siRNA dendriplexes within in situ-forming mucoadhesive gel as a means to increase brain radioactivity. For this, an in situ-forming gel was prepared by blending a thermosensitive polymer with mucoadhesive agents. Poloxamers, thermosensitive amphiphilic block copolymers of poly (ethylene oxide)–poly (propylene oxide)–poly ( ethylene oxide) (PEO–PPO–PEO) are free-flowing liquids at room temperature that allow easily reproducible administration into the nose. Once on the mucosa and in response to physiological temperatures, Tsol/gel is undergone.31,32,39,40 The Tsol/gel is inversely proportional to poloxamer concentration and for 15%–23% w/w solutions is close to body temperature (20ºC–30ºC).31,41 Poloxamer gels, however, have weak mechanical strength and are not mucoadhesive. 42 On the other hand, chitosan7,43 and carbopol44 form highly viscous concentrated mucoadhesive solutions. This makes application difficult and impairs the accuracy and reproducibility of the dosing volume. However, the literature indicates that mixtures of carbopol at 0.10%–0.25% w/w or chitosan at 0.5%–1.5% w/w with poloxamer, lead to liquid solutions of low viscosity and easy application at room temperature.31,32 The solutions become mucoadhesive gels within the nose, increasing the time of residence of the loaded material.
As mentioned above, a gel intended for nasal delivery has to possess mechanical properties other than mucoadhesivity. The viscosity or consistency of the liquid has to be low enough to ensure ease of administration. The viscosity of the gel phase, on the other hand, has to be similar to that of mucus in physiological conditions. Mucus is a non-Newtonian gel. At low shear rates, it is an elastic solid capable of recovering its original shape, while at high shear rates is a viscous liquid that can be irreversibly deformed. The viscosity of the nasal mucus ranges between 46, 5, and 1.3 Pa.s at shear frequencies of 1, 50, and 100 rad/s, respectively.45 A mucoadhesive gel of a viscosity higher than that of mucus could not be removed by the waving movement of cilia; it would remain at the administration site for long periods, leading to infectious and inflammatory processes.46 On the other hand, a mucoadhesive gel of low apparent viscosity would drain from the site of administration. Finally, the elastic properties of a gel have to predominate over its viscous properties to allow for its deformation but at the same time to impair its breaking by ciliary beating. Cilia beat at 1000 strokes per minute (around 17 Hz) and the elastic modulus of the mucus is 1 Pa, high enough to be removed by mucociliary clearance without being broken.
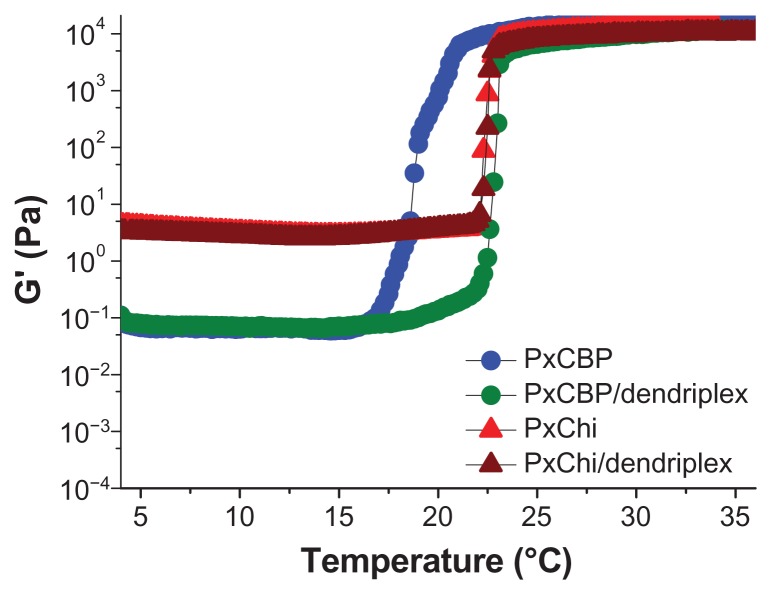
The Tsol/gel of each formulation was determined from the inflexion point of the elastic modulus G′ (a parameter measuring the material’s tendency to recover its original shape following a stress-induced deformation), as a function of temperature. Our results showed that the Tsol/gel of PxChi was 23°C while that of PxCBP was 19°C. The addition of dendriplexes neither modified the curve shape nor the Tsol/gel of PxChi but increased by 4°C the Tsol/gel of PxCBP (Figure 1).
Figure 1.
Variations of the elastic modulus, G′, as a function of temperature for the in situ-forming gels with or without dendriplexes.
Abbreviations: PxCBP, poloxamer/carbopol gel; PxChi, poloxamer/chitosan gel.
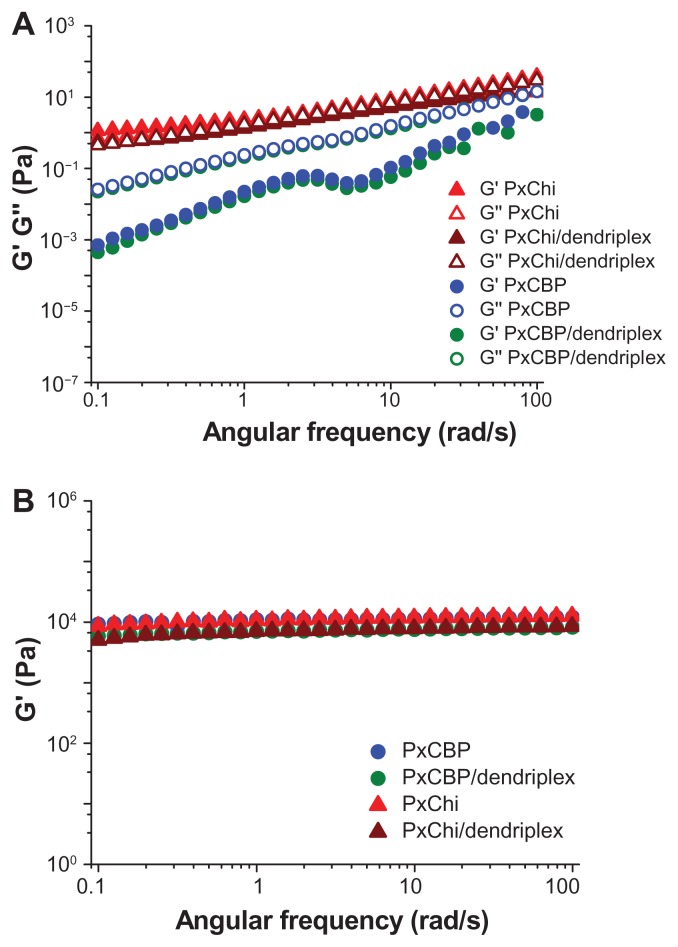
At 10°C, PxCBP exhibited a Newtonian flow (Figure 2A) and viscoelastic behavior typical of liquids. Its viscous modulus G″ (a parameter measuring the extent to which the material resists the tendency to flow), increased as a function of the oscillation frequency, and was higher than G′ (Figure 3A). In contrast, PxChi exhibited a non-Newtonian pseudoplastic or shear thinning behavior (Figure 2A). The similar values of G″ and G′ indicated that PxChi possessed both viscous properties typical of liquids and the elastic properties of solids (Figure 3A). Flow curves were adjusted to the power law model and the flow behavior (n) and consistency (k) indexes of each formulation were derived (Table 2). As expected, PxCBP had a flow index close to 1 and its viscosity was 0.25 Pa.s, whereas PxChi had a flow index lower than 1 and consistency higher than that of PxCBP. The addition of dendriplexes did not modify these properties.
Figure 2.
Flow curves (shear stress as function of shear rate) of in situ-forming gels with or without dendriplexes at (A) 10°C and (B) 25°C.
Note: “Mod” indicates experimental data fitted to the Oswald–de Waele equation.
Abbreviations: siRNA, short interference RNA; PxCBP, poloxamer/carbopol gel; PxChi, poloxamer/chitosan gel.
Figure 3.
Elastic (G′) and viscous (G″) modulus as a function of angular frequency of in situ-forming gels with or without dendriplexes at (A) 10°C and (B) 25°C.
Abbreviations: PxCBP, poloxamer/carbopol gel; PxChi, poloxamer/chitosan gel.
Table 2.
Parameters obtained from flow curves modeled using the Oswald-de-Waele equation (power law) of in situ-forming gels at 10°C and 25°C
| Sample | Temperature (°C) | Flow index (n) | Consistency index (k: Pa.sn) | R2 |
|---|---|---|---|---|
| PxCBP | 10 | 0.929 | 0.25 | 0.99 |
| PxCBP/dendriplex | 10 | 0.901 | 0.24 | 0.99 |
| PxChi | 10 | 0.201 | 55 | 0.98 |
| PxChi/dendriplex | 10 | 0.373 | 10 | 0.97 |
| PxCBP | 25 | 0.063 | 318 | 0.99 |
| PxCBP/dendriplex | 25 | 0.043 | 209 | 0.92 |
| PxChi | 25 | 0.059 | 351 | 0.70 |
| PxChi/dendriplex | 25 | 0.074 | 263 | 0.63 |
Abbreviations: PxCBP, poloxamer/carbopol gel; PxChi, poloxamer/chitosan gel.
At 25°C, both PxCBP and PxChi gels exhibited non-Newtonian pseudoplastic behavior, with flow index lower than 1 and high consistency (Figure 2B, Table 2). The addition of dendriplexes to PxChi caused a decrease in shear stress at shear rates above 40 1/s, this indicating a permanent damage in the gel structure. On the contrary, the addition of dendriplexes to PxCBP did not modify the shape of the curves and the calculated ηapp was 5 and 2.5 Pa.s at shear rates of 50 1/s and 100 1/s, respectively. The two gels presented high elastic modulus (G′ 1.104 Pa) and low viscous modulus (G″ < 102–103 Pa, data not shown) that remained constant up to a frequency of 100 rad/s (16 Hz) (Figure 3B).
Before being removed by ciliary beating, the gel’s mesh has to release the dendriplexes within a short time period. This was estimated in two ways. In the first, the radioactivity release when 32P-siRNA dendriplexes were incorporated within PxChi or PxCBP gels was determined. We observed that nearly 35% of the radioactivity was released from the PxCBP gel. A comparable amount of radioactivity was released from gels containing naked 32P-siRNA, while 55% of the radioactivity from gels containing free 32P-ATP was released after 150 minutes (Figure 4A). In contrast, there was no significant radioactivity release from PxChi gel during the same period of time (Figure 4B). Additionally the radioactivity released from PxCBP and PxChi gels containing 32P-siRNA lipoplexes was nearly 35% and 0%, respectively. Two of the primary mechanisms of drug retention are physical hindrance caused by the network of gel matrix and possible attractive forces between the drug and the polymer molecules.29 PxChi gel had a higher consistency index (higher viscosity) than the PxCBP gel that together with its positive charge could contribute to the higher retention within the mesh. In the second, we determined the silencing activity of the material released from PxCBP gel containing siRNA dendriplexes on enhanced green fluorescent protein-expressing J774 cells. We found that the silencing caused by the released material was nearly 50% of that produced by siRNA dendriplexes in buffer. In view of the absence of cell internalization and therefore lack of silencing activity of naked siRNA,28 these results suggested that at least part of the released material was dendriplexes.
Figure 4.
Release profile of radioactivity from in situ-forming (A) poloxamer/carbopol and (B) poloxamer/chitosan gels as function of time.
Abbreviations: PxCBP, poloxamer/carbopol gel; PxChi, poloxamer/chitosan gel; siRNA, short interference RNA; ATP, adenosine 5″-triphosphate.
Overall, these results showed that the Tsol/gel of both in situ-forming gels containing dendriplexes was suitable for nasal administration. However, while the PxChi liquid showed a non-Newtonian pseudoplastic behavior of high consistency (apparent viscosity) and difficult manipulation, the PxCBP liquid had a Newtonian behavior of low viscosity and easy manipulation. In the gel phase both formulations showed apparent viscosity similar to that of the mucus at low shear rates but higher than those of an aqueous solution (0.5 Pa.s). The irreversible deformation of PxChi gel at shear rate above 40 1/s and the nonrelease of radioactivity led us to discard this gel for further in vivo assays.
Assessment of local toxicity
Intranasal administration of dendriplexes can cause local irritation, epithelial or subepithelial toxicity, and ciliatoxicity. Histopathological screening of the rat nasal mucosa showed no important alterations after three doses of dendriplexes in buffer or within the gel (Figure 5A–F). On the contrary, the mucosa treated with SDS showed moderate and severe lesions (Table 3). In spite of the absence of similar reports in the literature, it was recently determined that 120 minutes after treatment with 0.1% to 5% w/v poly(amidoamine) dendrimers 0–3 generation, release of proteins and lactate dehydrogenase enzyme in the lavage of the nasal cavity was insignificant, as compared to that induced by sodium dodecyl sulfate.47
Figure 5.
Histopathology of rat nasal tissues after three intranasal doses of (A–C) dendriplexes in buffer or (D–F) dendriplexes within in situ-forming poloxamer/carbopol gel. (A and D) magnification ×40, (B and E) magnification ×100, (C and F) magnification ×400 (respiratory epithelium). Arrows showed luminal exudates (A and E) and normal lymphatic infiltration (E).
Table 3.
Histopathological evaluation of nasal mucosa after treatments
| Buffer | Dendriplex in buffer | Dendriplex within PxCBP gel | SDS | |
|---|---|---|---|---|
| Epithelial eosinophilia | 0 | 0 | 0 | 0 |
| Leukocytic margination | 0 | 0 | 0 | + |
| Luminal exudate | ++ | + | ++ | +++ |
| Desquamated epithelial cells | 0 | 0 | 0 | + |
| Hyperemia | + | 0 | + | ++ |
| Osteoblast activation | 0 | 0 | 0 | + |
Notes: The microscopic findings were graded between 0 and 3 (0 = absent, + = minimal, ++ = moderate, +++ = severe histological change).
Abbreviations: PxCBP, poloxamer/carbopol gel; SDS, sodium dodecyl sulfate.
Nasal absorption and brain distribution
After intranasal administration of [32P]-siRNA dendriplexes within in situ-forming mucoadhesive PxCBP gels, we found that part of the radioactivity was absorbed across the nasal mucosa, an important fraction was swallowed and aspirated (resulting in radioactivity in the esophagus and trachea) while a minimal fraction could have accessed the brain parenchyma.
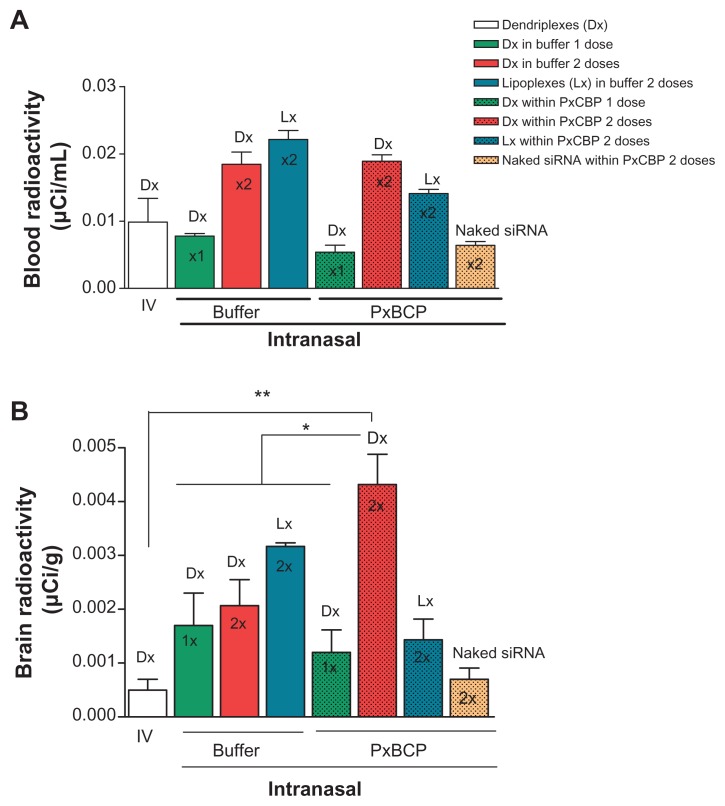
The radioactivity in blood after one or two doses of intranasal dendriplexes in buffer or gel, or naked siRNA in gel, was around 0.01–0.02 μCi/mL (0.1% of the injected dose [ID]). The same was found 90 minutes after the administration of intravenous dendriplexes (Figure 6A). Interestingly, gel electrophoresis analysis of the stability of dendriplexes after dilution showed that nearly 85% were disassembled after a 100-fold dilution. Therefore, since intravenously injected dendriplexes were submitted to a 250-fold dilution, the radioactivity in blood would correspond to that of naked siRNA or to its fragments. In this setting, our results were in agreement with those of van de Water et al,48 and Gao et al,49 where intravenously administered naked siRNA is rapidly eliminated by the kidneys, less than 0.1% of the ID remained in the rats’ blood after 1 hour. Similarly, the 0.1% ID in blood after intranasal administration would correspond to naked siRNA (because of the dilution suffered when entering the blood after mucosal absorption). Recently, Han et al,50 also reported that pDNAs up to 14.1 kb in size can be systemically absorbed after intranasal administration with a Tmax of 90 minutes.
Figure 6.
Scintillation measurement of radioactivity in (A) blood and (B) brain after one or two intravenous or intranasal doses of dendriplexes in rats (n = 3).
Notes: *P < 0.05; **P < 0.01 (statistically significant difference).
Abbreviations: IV, intravenous; DX, dendriplex; LX, lipoplex; siRNA, short interference RNA PxCBP, poloxamer/carbopol gel.
Intranasal naked siRNA within gel and intravenous dendriplexes delivered similar amounts of radioactivity to the brain (Figure 6B). However, intranasal dendriplexes both in gel and in buffer delivered significantly higher amounts of radioactivity to the brain than by the intravenous route. Moreover, the amounts of radioactivity in the brain after a single dose in buffer or in gel were comparable, but after a second dose of dendriplexes in buffer, the amount of radioactivity did not increase. Remarkably, a second dose in gel not only increased by 3.5-fold the amount of radioactivity in brain as compared to the first dose, but by twofold the delivered by dendriplexes in buffer. Additionally, the second dose of dendriplexes in gel delivered eightfold the amount of radioactivity in the brain than that delivered by two doses of naked siRNA in gel. Previously we determined that siRNA dendriplexes are taken up by caveolin and by caveolin- and clathrin-mediated endocytosis by glioblastoma cells and macrophages respectively; naked siRNA however is not taken up by any of these cell types.30 The lack of cellular uptake of naked siRNA could be the reason for its low level of brain radioactivity. The mediation of a direct brain delivery mechanism for naked siRNA could be discarded. On the other hand, two doses of lipoplexes in gels delivered lower amounts of radioactivity than two doses of lipoplexes in buffer. Clearly, in spite of finding that in vitro the radioactivities were released from the gel at the same rate, in vivo the delivery of radioactivity to the brain was higher from gels containing dendriplexes than when containing lipoplexes. Dendriplexes and lipoplexes share a size order of 150 nm. Shape and surface characteristics such as Z potential (25 mV dendriplexes vs 46.6 mV lipoplexes), or a potential release of naked siRNA from gels containing lipoplexes could explain the in vivo differences.8
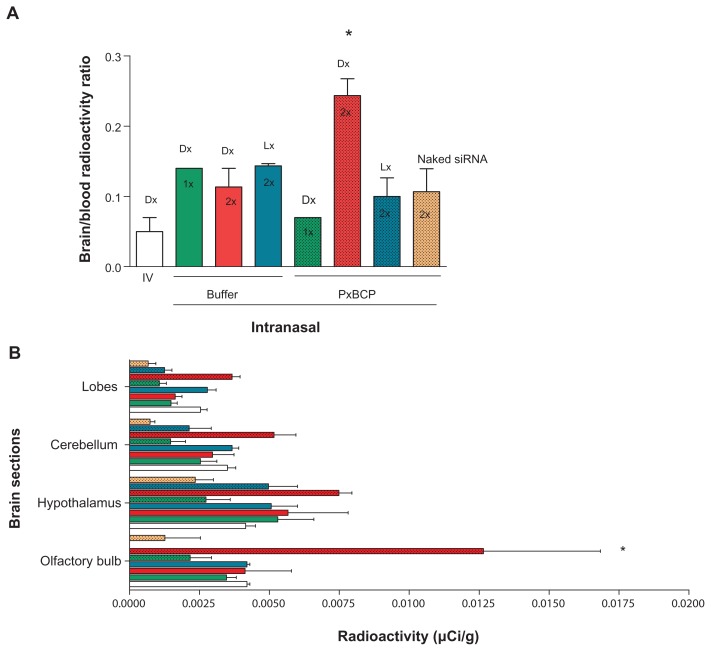
The brain/blood radioactivity ratio is a critical parameter to assess direct delivery to the parenchyma, since the radioactivity in the brain is the sum of that in parenchyma plus that in the brain blood.51 After two doses of intranasal dendriplexes in gel the brain/blood ratio was twofold higher than after intranasal dendriplexes in buffer and nearly fivefold higher than after intranasal naked siRNA in gel and than intravenous dendriplex administrations (Figure 7A).
Figure 7.
(A) Brain/blood radioactivity ratio and (B) brain distribution of radioactivity following one or two intravenous or intranasal doses of dendriplexes in rats.
Note: *P < 0.05 (statistically significant difference).
Abbreviations: IV, intravenous; DX, dendriplex; LX, lipoplex; siRNA, short interference RNA; PxCBP, poloxamer/carbopol gel.
We found as well that two doses of intranasal dendriplexes in gel delivered a significantly higher level of radioactivity in the olfactory bulb and hypothalamus than after intravenous or intranasal-in-buffer administrations (Figure 7B). The material delivered to these target tissues could be further distributed to distant zones of the CNS. Indeed, a number of studies have shown that after a primary accumulation of particulate material in the olfactory bulb, small enough particles could be transported via axons through the olfactory bulb into the olfactory cortex and then to the caudal pole of the cerebral hemisphere and into the cerebrum and the cerebellum.52–54 Probably the in situ-forming mucoadhesive gels maintain the structural stability of dendriplexes better than the liquid nonmucoadhesive buffer. Hence, opportunities for dendriplexes to be taken up by olfactory neurons would be higher for dendriplexes within gel than in buffer. Curiously, 180 minutes after two intranasal doses of lipoplexes in gels, the olfactory bulb showed no radioactivity, and there was only weak activity in the hypothalamus. Variations in the transcellular pathway (apical to basolateral transport through epithelial cells) followed in nose-to-brain delivery between dendriplexes and lipoplexes could explain this difference.
In general, the low concentrations of drugs found in the brain compared to other organs (often below 0.5% and can be as low as 0.01% ID/g brain) are nonetheless sufficient to result in a therapeutic effect.55 After two intranasal doses of dendriplexes within gel, the drainage to lungs and to the gastrointestinal system was not impaired (data not shown); however, we found 0.18% ID/g brain. Han et al,50 found 0.01% ID/g brain 60 minutes after an intranasal dose of pDNA. Also we found higher %ID/g brain than that obtained by Huang et al,56,57 (0.08–0.14%ID/g brain) who used intravenous administration of pDNA complexed with transferrinor lactoferrin-conjugated PEG-modified poly(amidoamine) dendrimers, but without covalent modification and with reduced systemic delivery.
In the current experimental design, the finding of radioactivity in the brain does not lead us to assume the presence of intact siRNA molecules or siRNA dendriplexes. However, this is the first work showing that the intranasal administration of in situ-forming mucoadhesive gel containing (nontargeted) siRNA dendriplexes increases the direct brain delivery of radiolabel above that achieved by parenteral administration of dendriplexes. Further studies will analyze the silencing activity of dendriplexes in vivo.
Conclusion
32P-siRNA complexation with dendrimers, incorporation of the dendriplexes into PxCBP gel, and administration of two intranasal doses were necessary to achieve higher brain radioactivity than that achived by intravenous dendriplexes or intranasal naked siRNA. The increased radioactivity within the olfactory bulb suggested that the combination abovementioned favors the mediation of a direct brain delivery.
Acknowledgments
This work was supported by the Secretaria de Investigaciones, Universidad Nacional de Quilmes. The authors are grateful to Prof Martinez from Departamento de Ciencia y Tecnología, Universidad Nacional de Quilmes for rheological analysis; Dr Claver and Dr Boviez from Cátedra de Histología, Facultad de Veterinaria, Universidad de Buenos Aires for histological evaluation of nasal mucosa and Dr Goldman and Dr Salgueiro from Cátedra de Radioisótopos, Facultad de Farmácia y Bioquímica, Universidad de Buenos Aires for siRNA radiolabel. MJM, ELR, and CMW are members of Carrera del Investigador Científico del Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lansley AB, Martin GP. Nasal drug delivery. In: Hillery AM, Lloyd AW, Swarbrick J, editors. Drug Delivery and Targeting. Boca Raton, FL: CRC Press; 2001. pp. 237–268. [Google Scholar]
- 2.Thorne RG, Emory CR, Ala TA, Frey WH., II Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 3.Frey WH, II, Liu JCX, Thorne RG, Fawcett JR, Ala TA, Rahman YE. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- 4.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. Epub Nov. 2011;15 doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59(2–3):141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 7.Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 8.Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379:146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Dhuria SV, Hanson LR, Frey WH., II Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 10.Baker H, Genter MB. The olfactory system and the nasal mucosa as portals of entry of viruses, drugs, and other exogenous agents into the brain. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker; 2003. pp. 549–573. [Google Scholar]
- 11.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptide: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 12.Ermisch A, Barth T, Rühle HJ, Skopková J, Hrbas P, Landgraf R. On the blood–brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp. 1985;19:29–37. [PubMed] [Google Scholar]
- 13.Kang YS, Park JH. Brain uptake and the analgesic effect of oxytocin – its usefulness as an analgesic agent. Arch Pharm Res. 2000;23:391–395. doi: 10.1007/BF02975453. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau RL, Davidson BL. RNAi therapeutics for CNS disorders. Brain Res. 2010;1338:112–121. doi: 10.1016/j.brainres.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Uno Y, Piao W, Miyata K, Nishina K, Mizusawa H, Yokota T. High-density lipoprotein facilitates in vivo delivery of α-tocopherol-conjugated short-interfering RNA to the brain. Hum Gene Ther. 2011;22(6):711–719. doi: 10.1089/hum.2010.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima H, Kubo T, Semi Y, et al. A rapid, targeted, neuron-selective, in vivo knockdown following a single intracerebroventricular injection of a novel chemically modified siRNA in the adult rat brain. J Biotechnol. 2012;157(2):326–333. doi: 10.1016/j.jbiotec.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Al-Jamal KT, Gherardini L, Bardi G, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108(27):10952–10957. doi: 10.1073/pnas.1100930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonoiu AC, Bergey EJ, Ding H, et al. Gold nanorod–siRNA induces efficient in vivo gene silencing in the rat hippocampus. Nanomedicine (Lond) 2011;6(4):617–630. doi: 10.2217/nnm.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng D, Cao N, Chen J, Yu X, Shuai X. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials. 2012;33(4):1170–1179. doi: 10.1016/j.biomaterials.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 20.Stiles D, Zhang Z, Ge P, et al. Widespread suppression of huntingtin with convection-enhanced delivery of siRNA. Exp Neurol. Epub Nov. 2011;19 doi: 10.1016/j.expneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Akaneya Y, Jiang B, Tsumoto T. RNAi induced gene silencing by local electroporation in targeting brain region. J Neurophysiol. 2005;93:594–602. doi: 10.1152/jn.00161.2004. [DOI] [PubMed] [Google Scholar]
- 22.Xia CF, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of brain cancer with receptor targeting and avidin-biotin technology. Pharm Res. 2007;24(12):2309–2316. doi: 10.1007/s11095-007-9460-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim SS, Ye C, Kumar P, et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010;18(5):993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, Bae KH, Yang H, et al. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22(12):2568–2572. doi: 10.1021/bc200406n. [DOI] [PubMed] [Google Scholar]
- 25.Kuwahara H, Nishina K, Yoshida K, et al. Efficient in vivo delivery of siRNA into brain capillary endothelial cells along with endogenous lipoprotein. Mol Ther. 2011;(12):2213–2221. doi: 10.1038/mt.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar P, Wu H, McBride JL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 27.Kim ID, Kim SW, Lee JK. Gene knockdown in the olfactory bulb, amygdala, and hypothalamus by intranasal siRNA administration. Korean J Anat. 2009;42:285–292. [Google Scholar]
- 28.Perez AP, Romero EL, Morilla MJ. Ethylendiamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int J Pharm. 2009;380(1–2):189–200. doi: 10.1016/j.ijpharm.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Charlton S, Jones NS, Davis SS, Illum L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur J Pharm Sci. 2007;30:295–302. doi: 10.1016/j.ejps.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Perez AP, Cosaka ML, Romero EL, Morilla MJ. Uptake and intracellular traffic of siRNA dendriplexes in glioblastoma cells and macrophages. Int J Nanomedicine. 2011;6:2715–2728. doi: 10.2147/IJN.S25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones DS, Bruschi ML, De Freitas O, Gremiao MPD, Lara EHG, Andrews GP. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int J Pharm. 2009;372:49–58. doi: 10.1016/j.ijpharm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75:186–193. doi: 10.1016/j.ejpb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Jones DS, Brown AF, Woolfson AD. Solute and solvent effects on the thermorheological properties of poly(oxyethylene)-poly(oxypropylene) block copolymers: implications for pharmaceutical dosage form design. J Appl Polym Sci. 2003;87:1016–1026. [Google Scholar]
- 34.Jones DS, Brown AF, Woolfson AD. Rheological characterization of bioadhesive, antimicrobial, semisolids designed for the treatment of periodontal diseases: transient and dynamic viscoelastic and continuous shear analysis. J Pharm Sci. 2001;90:1978–1990. doi: 10.1002/jps.1149. [DOI] [PubMed] [Google Scholar]
- 35.Malek A, Czubayko F, Aigner A. PEG grafting of polyethylenimine (PEI) exerts different effects on DNA transfection and siRNA-induced gene targeting efficacy. J Drug Target. 2008;16:124–139. doi: 10.1080/10611860701849058. [DOI] [PubMed] [Google Scholar]
- 36.Martinac A, Filipović-Griĉć J, Voinovich D, Perissutti B, Franceschinis E. Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm. 2005;291:69–77. doi: 10.1016/j.ijpharm.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Jansson B, Hägerström H, Fransén N, Edsman K, Björk E. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur J Pharm Biopharm. 2005;59(3):557–564. doi: 10.1016/j.ejpb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Bindseil E, Bechgaard E, Jorgensen L, Larsen R. Morphological examination of rabbit nasal mucosa after exposure to acetylsalicylic acid, glycofurol75 and ephedrine. Int J Pharm. 1995;119:37–46. [Google Scholar]
- 39.Collaud S, Peng QA, Gurny R, Lange N. Thermosetting gel for the delivery of 5-aminolevulinic acid esters to the cervix. J Pharm Sci. 2008;97:2680–2690. doi: 10.1002/jps.21181. [DOI] [PubMed] [Google Scholar]
- 40.Hartikka J, Geall A, Bozoukova V, et al. Physical characterization and in vivo evaluation of poloxamer-based DNA vaccine formulations. J Gene Med. 2008;10:770–782. doi: 10.1002/jgm.1199. [DOI] [PubMed] [Google Scholar]
- 41.Jeong B, Kim SW, Bae YH. Thermosensitive sol–gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 42.El Kamel AH. In vitro and in vivo evaluation of pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm. 2002;241:47–55. doi: 10.1016/s0378-5173(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 43.Soane RJ, Frier M, Perkins AC, Jones NS, Davis SS, Illum L. Evaluation of clearance characteristics of bioadhesive systems in humans. Int J Pharm. 1999;78:55–65. doi: 10.1016/s0378-5173(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 44.Tas C, Ozkan CK, Savaser A, Ozkan Y, Tasdemir U, Altunay H. Nasal absorption of metoclopramide from different Carbopol 981 based formulations: In vitro, ex vivo and in vivo evaluation. Eur J Pharm Biopharm. 2006;64:246–254. doi: 10.1016/j.ejpb.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Rubin BK, Druce H, Ramirez OE, Palmer R. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am J Respir Crit Care Med. 1997;155:2018–2023. doi: 10.1164/ajrccm.155.6.9196110. [DOI] [PubMed] [Google Scholar]
- 46.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Del Rev. 2005;57(11):1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Dong Z, Katsumi H, Sakane T, Yamamoto A. Effects of polyamidoamine (PAMAM) dendrimers on the nasal absorption of poorly absorbable drugs in rats. Int J Pharm. 2010;393:244–252. doi: 10.1016/j.ijpharm.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 48.van de Water FM, Boerman OC, Wouterse AC, Peters JGP, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–1397. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- 49.Gao S, Dagnaes-Hansen F, Nielsen EJB, et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther. 2009;17:1225–1233. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han In-K, Kim MY, Byun H-M, et al. Enhanced brain targeting efficiency of intranasally administered plasmid DNA: an alternative route for brain gene therapy. J Mol Med. 2007;85:75–83. doi: 10.1007/s00109-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 51.Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood–brain barrier? Questioning the direct transport theory. Drugs R D. 2007;8:133–144. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 52.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B, Feng WY, Wang M, et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res. 2007;118:233–243. doi: 10.1007/s12011-007-0028-6. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Liu Y, Jiao F, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology. 2008;254:82–90. doi: 10.1016/j.tox.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 55.van Rooy I, Cakir-Tascioglu S, Hennink WE, Storm G, Schiffelers RM, Mastrobattista E. In vivo methods to study uptake of nanoparticles into the brain. Pharm Res. 2011;28:456–471. doi: 10.1007/s11095-010-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang RQ, Ke WL, Qu YH, Zhu JH, Pei YY, Jiang C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J Biomed Sci. 2007;14(1):121–128. doi: 10.1007/s11373-006-9121-7. [DOI] [PubMed] [Google Scholar]
- 57.Huang RQ, Ke WL, Liu Y, Jiang C, Pei YY. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29:238–246. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]