Abstract
Nicotine is reinforcing because it activates dopaminergic (DAergic) neurons within the ventral tegmental area (VTA) of the brain's mesocorticolimbic reward circuitry. This increase in activity can occur for a period of several minutes up to an hour and is thought to be a critical component of nicotine dependence. However, nicotine concentrations that are routinely self-administered by smokers are predicted to desensitize high-affinity α4β2 neuronal nicotinic acetylcholine receptors (nAChRs) in seconds. Thus, how physiologically relevant nicotine concentrations persistently activate VTA DAergic neurons is unknown. Here we show that nicotine can directly and robustly increase the firing frequency of VTA DAergic neurons for several minutes. In mouse midbrain slices, 300 nM nicotine elicited a persistent inward current in VTA DAergic neurons that was blocked by α-conotoxin MII[H9A;L15A], a selective antagonist of nAChRs containing the α6 subunit. α-conotoxin MII[H9A;L15A] also significantly reduced the long-lasting increase in DAergic neuronal activity produced by low concentrations of nicotine. In addition, nicotine failed to significantly activate VTA DAergic neurons in mice that did not express either α4 or α6 nAChR subunits. Conversely, selective activation of nAChRs containing the α4 subunit in knock-in mice expressing a hypersensitive version of these receptors yielded a biphasic response to nicotine consisting of an acute desensitizing increase in firing frequency followed by a sustained increase that lasted several minutes and was sensitive to α-conotoxin MII[H9A;L15A]. These data indicate that nicotine persistently activates VTA DAergic neurons via nAChRs containing α4 and α6 subunits.
Introduction
Complications from long-term exposure to cigarette smoke accounts for ∼5 million deaths, making nicotine addiction the predominant preventable cause of mortality worldwide (Centers for Disease Control and Prevention, 2010). Understanding the molecular mechanisms underlying the addictive nature of nicotine should yield new therapeutic targets aimed at facilitating smoking cessation. Nicotine, like all drugs of abuse, acts upon the mesocorticolimbic “reward” circuitry of the brain to initiate dependence (Tapper et al., 2006). Within this circuit, dopaminergic (DAergic) neurons originating in the ventral tegmental area (VTA), project to the nucleus accumbens (NAcc), as well as the prefrontal cortex and hippocampus, among other regions (Laviolette and van der Kooy, 2004; Placzek et al., 2009; De Biasi and Dani, 2011). Nicotine dependence is initiated by activation of the VTA, ultimately resulting in DA release in the NAcc (Di Chiara and Imperato, 1988). Short-term exposure to nicotine can increase DAergic neuron activity and DA release in NAcc for several minutes to more than an hour (Brodie, 1991; Mansvelder et al., 2002; Pidoplichko et al., 2004; Dong et al., 2010; De Biasi and Dani, 2011). Dopamine release in response to nicotine can occur via at least two mechanisms: 1) an increase in baseline DAergic neuron firing frequency and 2) a switch from tonic- to burst-firing mode (Mameli-Engvall et al., 2006). Within the VTA, DAergic neurons receive local inhibitory input from GABAergic interneurons (Mansvelder et al., 2002). In addition cortical and thalamic projections provide glutamatergic, excitatory input into the VTA (Mansvelder and McGehee, 2000; Pidoplichko et al., 2004). It is noteworthy that neuronal nicotinic acetylcholine receptors (nAChRs) that are activated by nicotine, as well as the endogenous neurotransmitter acetylcholine, are robustly expressed in both DAergic and GABAergic VTA neurons in addition to glutamatergic terminals (Klink et al., 2001; Champtiaux et al., 2002; Wooltorton et al., 2003).
The mechanism by which nAChR activation leads to long-lasting DAergic neuronal activity is unclear. Although nicotine can directly activate DAergic neurons via high-affinity nAChRs (i.e., receptors containing α4 and β2 subunits, denoted α4β2* nAChRs; the asterisk indicates that additional unidentified subunits may also be present), nAChRs containing solely α4 and β2 subunits desensitize within seconds to a few minutes of exposure to pathologically relevant concentrations of drug (Pidoplichko et al., 1997, 2004; Fisher et al., 1998). In addition, the nAChR α6 subunit is robustly expressed in DAergic neurons, where it can potentially coassemble with both α4 and β2 subunits, but its functional role in activation of VTA DAergic neurons by nicotine is unclear (Drenan et al., 2008; Pons et al., 2008; Gotti et al., 2010). Thus, whether the long-lasting activity of DAergic neurons in response to nicotine is cell autonomous and involves nAChRs containing the α6 subunit are open questions.
In this study, we investigated how exposure to pathologically relevant nicotine concentrations can induce long-lasting increases in VTA DAergic neuron activity. Our data indicate that nAChR containing α6 and α4 subunits can directly drive increases in VTA DAergic neuron activity for extended periods.
Materials and Methods
Animals.
Adult (8–10 weeks), C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), were used in all experiments, in addition to α4 knockout (KO) and α6 KO homozygous mice and L9′A heterozygous mice. L9′A, α4 KO, and α6 KO lines have been back-crossed to the C57BL/6J strain for at least nine generations. The genetic engineering of L9′A, α4 KO, and α6 KO lines has been described previously (Ross et al., 2000; Champtiaux et al., 2003; Tapper et al., 2004). Animals were housed four per cage up until the start of each experiment. Animals were kept on a standard 12-h light/dark cycle with lights on at 7:00 AM and off at 7:00 PM. Mice had access to food and water ad libitum. All experiments were conducted in accordance with the guidelines for care and use of laboratory animals provided by the National Research Council (Institute of Laboratory Animal Resources, 1996) as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Drugs.
Stock solutions of nicotine hydrogen tartrate, dihydro-β-erythroidine (DHβE), 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX), atropine, bicuculline methylbromide (Sigma-Aldrich, St. Louis, MO), and tetrodotoxin (TTX; Tocris Bioscience, Ellisville, MO) were dissolved in distilled water then diluted with artificial cerebrospinal fluid (ACSF). ACSF solution contained 125 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4·H2O, 1.2 mM MgCl2·6H2O, 2.4 mM CaCl2·2H2O, 26 mM NaHCO3, and 11 mM d-Glucose. α-conotoxin MII[H9A;L15A] was synthesized as described previously (McIntosh et al., 2004).
Slice Preparation and Electrophysiological Recordings.
Mice were deeply anesthetized with sodium pentobarbital (200 mg/kg i.p.) and then decapitated. Brains were quickly removed and placed in an oxygenated ice-cold high-sucrose ACSF containing kynurenic acid (1 mM; Sigma-Aldrich). Brain slices (180–200 μm) were cut using a Vibratome (VT1200; Leica Microsystems, Inc., Bannockburn, IL). The brain slices were incubated in oxygenated Earl's balanced salt solution supplemented with glutathione (1.5 mg/ml; Sigma-Aldrich), N-ω-nitro-l-arginine methyl ester hydrochloride (2.2 mg/ml; Sigma-Aldrich), pyruvic acid (11 mg/ml; Sigma-Aldrich), and kynurenic acid (1 mM) for 45 min at 34°C. Slices were transferred into oxygenated ACSF at room temperature for recording. High-sucrose ACSF solution was identical to ACSF except that it contained 250 mM sucrose instead of 125 mM NaCl. Single slices were transferred into a recording chamber continually superfused with oxygenated ACSF. The junction potential between the patch pipette and bath ACSF was nullified just before obtaining a seal on the neuronal membrane. Action potentials and currents were recorded at 32°C using the whole-cell configuration of a patch-clamp amplifier (Multiclamp 700B; Molecular Devices, Sunnyvale, CA). Action potentials were also measured in cell-attached mode. Action potentials were obtained using a gap-free acquisition mode and Clampex software (Molecular Devices). Ih currents were elicited every 5 s by stepping from −60 mV to a test potential of −120 mV for 1 s by using Clampex. Input resistances were calculated using steady-state currents elicited by 5-mV hyperpolarizing pulses. Signals were filtered at 1 kHz using the amplifier's four-pole, low-pass Bessel filter, digitized at 10 kHz with an Digidata 1440A interface (Molecular Devices) and stored on a personal computer. Potential DAergic neurons were selected for recording, initially based on neuroanatomical region and soma shape. Action potential frequency (< 8 hz) and Ih expression were also used for identification of neuronal identity. In addition, at the end of recording, the neuron cytoplasm was aspirated into the recording pipette, and the contents were expelled into a microcentrifuge tube containing 75% ice-cold ethanol and stored at −20°C for at least 2 h before single-cell reverse transcription-polymerase chain reaction experiments to verify expression of TH. Non–TH-expressing neurons were excluded from analysis. DAergic neurons from the posterior portion of the VTA were recorded because previous data indicate that these are most sensitive to activation by nicotine (Zhao-Shea et al., 2011). Pipette solution contained 121 mM KCl, 4 mM MgCl2·6H2O, 11 mM EGTA, 1 mM CaCl2·2H2O, 10 mM HEPES, 0.2 mM GTP, and 4 mM ATP. Pipette solution was made using sterile-filtered diethyl pyrocarbonate-treated distilled water. Drugs were applied onto slices by gravity superfusion. Action potentials were recorded in the presence of a cocktail of inhibitors including atropine (1 μM) to block muscarinic receptors, bicuculline (20 μM) to block GABAA receptors, and CNQX (10 μM) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Whole-cell responses to nicotine under voltage clamp were recorded in the presence of the blockers above in addition to TTX (0.5 μM).
Data Analysis.
Summary data are presented as means ± S.E.M. For multiple comparisons, statistical significance was determined using Prism 5 (GraphPad Software, San Diego, CA) by one-way ANOVA followed by Tukey post hoc tests. A paired t test was used to analyze differences between control and drug group. Results were considered significant at p < 0.05.
Results
Nicotine Directly Activates VTA DAergic Neurons for Several Minutes.
To examine the effects of short-term nicotine exposure on VTA DAergic neuron firing rates, we recorded action potentials in current clamp mode from DAergic neurons in mesocortical slices containing the VTA at baseline and during and after 5-min bath exposure to 1 μM nicotine. DAergic neurons were identified by a slow firing frequency (<8 Hz; Fig. 1A), expression of a hyperpolarizing activated current Ih (Fig. 1B), and expression of tyrosine hydroxylase (Fig. 1C), as described previously (Zhao-Shea et al., 2011). In addition, action potentials were recorded in the presence of a cocktail of inhibitors to block muscarinic receptors, GABAA receptors, and glutamatergic signaling (see Materials and Methods) in an effort to facilitate isolation of nicotine effects directly mediated by nAChRs. A 5-mi application of nicotine elicited a long-lasting (∼12 min) reversible increase in firing frequency (Fig. 2, A and B). On average, nicotine elicited a significant 2.32 ± 0.4-fold increase in firing frequency compared with baseline (p < 0.01, paired t test, n = 7; Fig. 2C). Nicotine exposure can elicit lasting increases on DAergic neuron firing rate for several minutes up to an hour. To test the hypothesis that nicotine concentrations experienced by smokers could directly increase DAergic neuron firing frequency, we again measured action potential frequency, but this time in response to a lower pathologically relevant nicotine concentration, 300 nM (Fig. 3A). In addition, we measured DAergic neuron activity using cell-attached recordings so as not to perturb the intracellular milieu. A 30 min bath application of nicotine resulted in a persistent and significant increase in DAergic neuron firing that returned to baseline after washout (Fig. 3, A and B). Overall nicotine elicited a statistically significant increase in firing frequency (repeated-measures one-way ANOVA, F3,23 = 6.45, p < 0.01, n = 6). Tukey post hoc tests revealed that DAergic neuron activity was significantly increased after 10- and 30-min nicotine exposure compared with firing frequency both at baseline and after nicotine washout (p < 0.05; Fig. 3B). It is noteworthy that there was no statistically significant difference between firing rates regardless of nicotine exposure time. These data indicate that a sustained single bolus of nicotine at pathologically relevant concentrations can persistently activate DAergic neurons for several (i.e., 30–60) minutes.
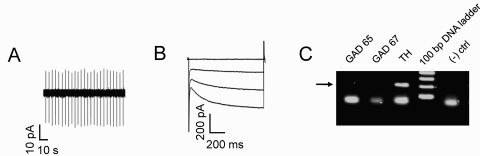
Fig. 1.
Characterization of VTA DAergic neurons. A, representative cell-attached recording from a putative DAergic neuron. DAergic neurons had characteristic low baseline firing frequencies (< 8 Hz) and B, expressed the hyperpolarizing activated cation current, IH. Currents were elicited by 20 -mV hyperpolarizing steps from a holding potential of −60 mV to −120 mV. C, at the end of each recording, the content of each neuron was aspirated into the patch pipette, and TH expression was verified by single-cell reverse transcription-polymerase chain reaction (RT-PCR). A representative DNA agarose gel is shown illustrating a typical result for a DAergic neuron. Only neurons that clearly expressed TH (arrow) and not GAD 65/67 were included in the analysis.
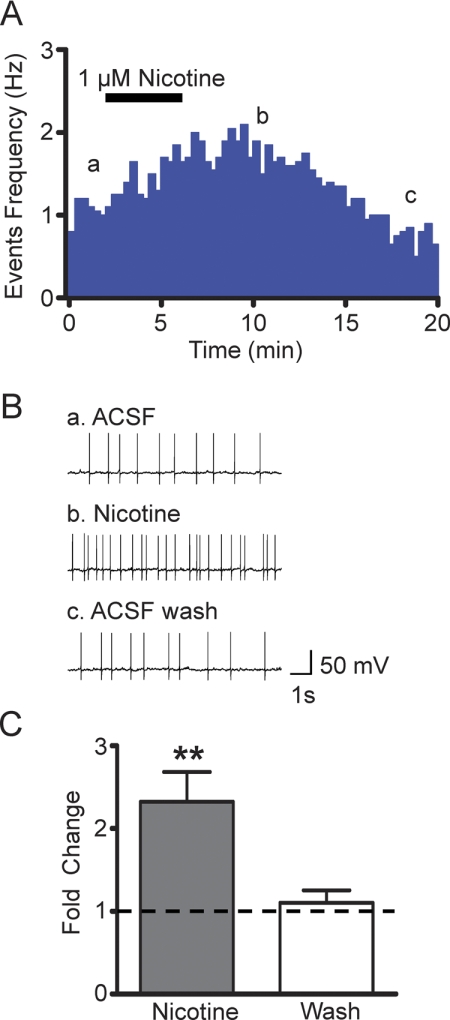
Fig. 2.
Nicotine activation of VTA DAergic neurons. A, representative events frequency histogram from a VTA DAergic neuron before, during, and after bath application of 1 μM nicotine (black bar). Current clamp (I = 0) responses were recorded from visually identified VTA DAergic neurons in C57BL/6J mesocortical coronal slices. Note that nicotine increases DAergic neuron firing frequency for several minutes during and after initial exposure. B, representative current recordings taken from a) baseline, b) 5-min after nicotine exposure, and c) 12-min after nicotine exposure. C, change in average DAergic neuron action potential frequency in response to nicotine (gray bar, 5 min application) and after several minute wash with ACSF (white bar) compared with baseline (dotted line). **, p < 0.01.
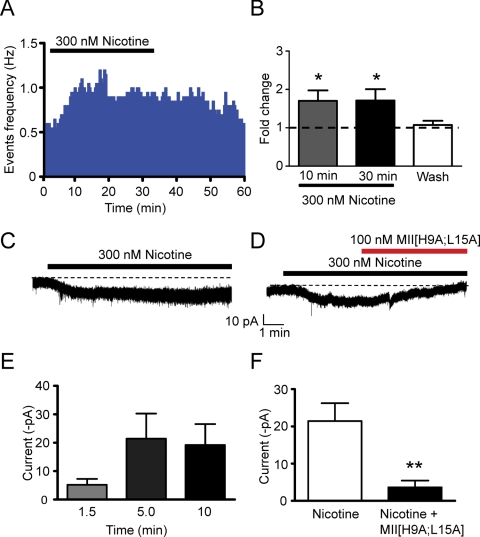
Fig. 3.
Direct and persistent activation of VTA DAergic neurons by nicotine. A, representative action potential firing frequency histogram from a VTA DAergic neuron before, during, and after 30-min bath application of 300 nM nicotine. Action potentials were recorded in cell-attached mode. B, change in average firing frequency compared with baseline (1 min before nicotine application, dotted line) 10 and 30 min after nicotine application and after 20-min washout. *, p < 0.05, one-way ANOVA, Tukey post hoc, n = 6. C, representative whole-cell response to 300 nM nicotine in a VTA DAergic neuron. The neuron was voltage-clamped at −60 mV and 300 nM nicotine was bath applied for 10 min (black bar). The dotted line represents baseline current extrapolated from the recording before nicotine application. Responses were recorded in the presence of a cocktail of inhibitors. D, representative whole-cell response to 300 nM nicotine (black bar) in the presence and absence of 100 nM α-conotoxin MII[H9A;L15A] (red bar). E, average current amplitude minus baseline during 1.5-, 5-, and 9-min exposure to 300 nM nicotine. n = 8 neurons. F, average current amplitude minus baseline after a 5-min exposure to 300 nM nicotine (white bar) and after an additional 5 min exposure of nicotine + 100 nM α-conotoxin MII[H9A;L15A]. **, p < 0.01, compared with nicotine-induced current, n = 5 neurons/treatment.
VTA DAergic Neurons Express Desensitization-Resistant nAChRs Containing α4 and α6 Subunits.
To test the hypothesis that nicotine persistently activated DAergic neurons directly via desensitization-resistant nAChRs, we recorded whole-cell responses to 300 nM nicotine exposure in VTA DAergic neurons under voltage clamp. Nicotine elicited a significant inward current that persisted over the course of 10-min exposure to drug (Fig. 3, C and E; average current amplitude, −21.5 ± 8.8 pA and −19.2 ± 7.4 pA after 5 and 10 min nicotine exposure, respectively, n = 8). Furthermore, there was a significant increase in RMS current noise after 10 min nicotine compared with baseline before drug perfusion (5.48 ± 0.64 pA compared with 4.26 ± 0.25 pA, respectively, p < 0.05 paired t test), presumably generated by an increase of randomly gating nAChR channels. As we previously had determined that nAChRs containing α4 and α6 subunits were expressed in VTA DAergic neurons (Zhao-Shea et al., 2011), we tested the sensitivity of nicotine-induced current to the α6* nAChR selective antagonist, α-conotoxin MII[H9A;L15A]. During the initial 5 min application of drug, nicotine elicited an inward current and this current was significantly attenuated during application of α-conotoxin MII[H9A;L15A] (Fig. 3, D and F, p < 0.01, paired t test, n = 5). Together, these data indicate that nicotine can persistently depolarize DAergic neurons via α6* nAChRs.
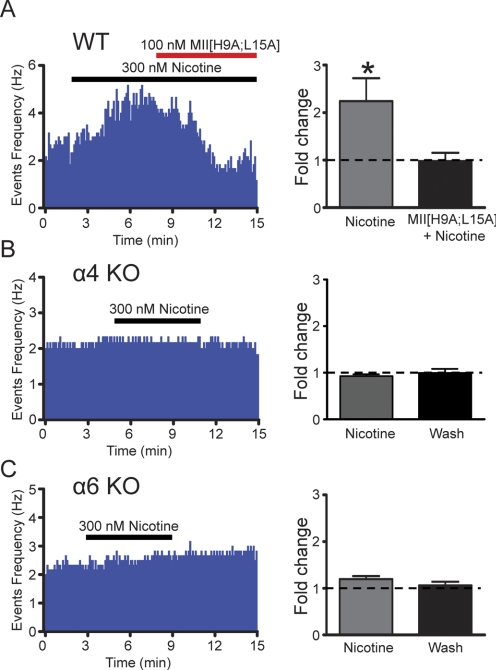
To determine whether these same receptors were responsible for our observed nicotine-induced increase in DAergic neuron firing frequency, we recorded VTA DAergic neuron activity in the cell-attached configuration in WT, α4 KO, and α6 KO mice. Average baseline firing frequency was not significantly different among mouse models (5.0 ± 1.8, 4.4 ± 1.1, and 4.0 ± 1.0 Hz in WT, α4 KO, and α6 KO mice, respectively; n = 6 neurons/genotype). In WT animals, 300 nM nicotine persistently and significantly increased DAergic neuron firing rate (Fig. 4A; p < 0.05, paired t test, n = 6). Application of α-conotoxin MII returned the increased firing of DAergic neurons in response to nicotine to baseline (Fig. 4A, right). It is noteworthy that in the presence of nicotine, four of six neurons decreased DAergic neuron firing frequency below baseline during application of α-conotoxin MII[H9A;L15A] suggesting, perhaps, a role for ACh in tonic activation of DAergic neurons in our slice prep. However, when α-conotoxin MII[H9A;L15A] was applied to a separate group of VTA DAergic neurons in the absence of nicotine, no significant change in baseline firing rate was observed (firing rate of DAergic neurons in the presence of α-conotoxin MII[H9A;L15A] = 103.4 ± 0.2% of control, n = 6) indicating that blockade of α6* nAChRs unmasked an inhibitory effect of nicotine in a portion of VTA DAergic neurons. In α4 KO mice, nicotine did not significantly increase DAergic neuron activity (Fig. 4B). Likewise, nicotine did not yield a significant increase in DAergic neuron firing in α6 KO mice (Fig. 4C; 1.20 ± 0.063-fold compared with baseline, n = 6) compared with WT. Together these data indicate that the persistent activation of VTA DAergic neurons arises mostly from nicotinic receptors containing α4 and α6 subunits.
Fig. 4.
The contribution of α4 and α6 subunits to nicotine-induced increases in DAergic neuron firing frequency. A, left, representative action potential firing frequency histogram from a VTA DAergic neuron in a WT midbrain slice. Nicotine (300 nM) was applied over the times indicated by the black bar. After 5 min of nicotine application, 100 nM α-contoxin MII[H9A;L15A] was applied concomitantly with nicotine. Right, change in average firing frequency compared with baseline (dotted line) 5 min after nicotine exposure and after an additional 5-min exposure to both nicotine and α-contoxin MII[H9A;L15A]. *, p < 0.05, compared with nicotine, n = 6. B, left, representative action potential firing frequency histogram from a VTA DAergic neuron in an α4 KO midbrain slice. Right, average firing frequency fold change compared with baseline (dotted line) 5 min after nicotine exposure and after an additional 5-min wash period. C, left, representative action potential firing frequency histogram from a VTA DAergic neuron in an α6 KO midbrain slice. Right, average firing frequency fold change compared with baseline (dotted line) 5 min after nicotine exposure and after an additional 5-min wash period. n = 6 neurons/genotype.
Selective Activation of α4* nAChRs Reveals Two nAChR Subtypes.
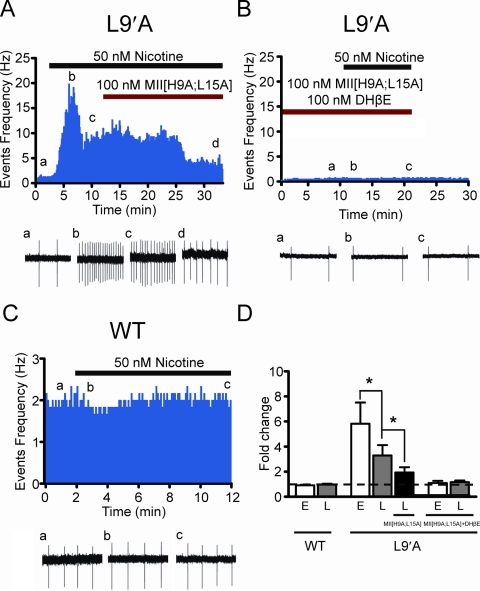
To test the hypothesis that selective activation of α4* nAChRs was sufficient to drive nicotine-induced activation of VTA DAergic neurons, we recorded effects of nicotine on DAergic neurons in VTA slices from mice expressing an α4 point mutant, L9′A, that renders α4* nAChRs 50-fold more sensitive to nicotine compared with wild-type DAergic neurons (Tapper et al., 2004). As reported previously, we did not observe differences in baseline firing frequency between L9′A and WT VTA DAergic neurons (Tapper et al., 2004). Bath application of 50 nM nicotine, a concentration that did not significantly increase firing of WT VTA DAergic neurons (Fig. 5, C and D), dramatically altered the firing frequency of VTA DAergic neurons in L9′A mice. The response to nicotine in L9′A DAergic neurons was biphasic, consisting of an early short-term phase that desensitized in the presence of agonist within ∼2 min followed by a sustained, late increase in firing frequency compared with baseline (early peak, p < 0.01 compared with baseline; late sustained phase, p < 0.01 compared with baseline, n = 7; Fig. 5, A and D). To determine whether α4* nAChRs mediating the persistent firing phase of the nicotine response in L9′A DAergic neurons also contained the α6 subunit, we coapplied α-conotoxin MII[H9A;L15A] with nicotine during the sustained firing response to nicotine. α-Conotoxin MII[H9A;L15A] significantly reduced the sustained firing frequency of L9′A VTA DAergic neurons (p < 0.05, n = 7; Fig. 5, A and D). As in WT VTA DAergic neurons, α-conotoxin MII[H9A;L15A] alone did not significantly modulate baseline firing frequency (firing rate of DAergic neurons in the presence of α-conotoxin MII[H9A;L15A] = 99.5 ± 0.15% of control, n = 4). In addition, coapplication of α-conotoxin MII[H9A;L15A] and the β2* competitive antagonist DHβE completely blocked both short-term and sustained activation of L9′A VTA DAergic neurons by nicotine (Fig. 5, B and D).
Fig. 5.
Selective activation of α4* nAChRs reveals two receptor subtypes mediating nicotine-induced activation of VTA DAergic neurons. A, representative action potential firing frequency histogram from a VTA DAergic neuron in a L9′A midbrain slice. Nicotine (50 nM) was applied over the times indicated by the black bar. After 5 min of nicotine application, 100 nM α-contoxin MII[H9A;L15A] was applied concomitantly with nicotine (red bar). Representative cell-attached recordings corresponding to individual time points before, during, and after drug treatment are illustrated below the graph. B, representative action potential firing frequency histogram from a VTA DAergic neuron in a L9′A midbrain slice. Nicotine was applied in the presence of 100 nM α-contoxin MII[H9A;L15A] and 100 nM DHβE. Cell-attached recordings are shown below the histogram as in A. C, representative action potential firing frequency histogram from a VTA DAergic neuron from a WT animal. Nicotine (50 nM) was applied to the slice as in A. Cell-attached recordings are shown below the histogram as in A. D, average firing frequency fold change compared with baseline (dotted line) in response to 50 nM nicotine in DAergic neurons from WT and L9′A slices in experiments depicted in A to C. The early phase of the nicotine response (E) is defined as the peak fold change produced by nicotine compared with baseline within the first 2 to 3 min of application, whereas the late phase of the nicotine response (L) is defined as the fold change produced by nicotine compared with baseline after 5-min application of drug.
Discussion
Nicotine initiates dependence by activating the VTA, ultimately resulting in an increase in DA concentration in NAcc, a phenomenon both necessary and sufficient for reinforcement (Corrigall and Coen, 1991; Corrigall et al., 1992, 1994; Tsai et al., 2009). Although previous work has clearly implied that nAChR expression and activation in the VTA is critical for nicotine reward (Picciotto et al., 1998; Tapper et al., 2004), the mechanism by which nicotine may activate DAergic neurons for extended periods of time has remained elusive. It has been proposed that low nanomolar concentrations of nicotine that are routinely self-administered by smokers desensitize high-affinity nAChRs almost completely within seconds to a few minutes (Pidoplichko et al., 1997; Mansvelder et al., 2002). Here we show that a physiologically relevant concentration of nicotine elicited a persistent inward current in VTA DAergic neurons even in the presence of TTX and CNQX, which blocks action potential and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-dependent signaling, respectively, indicating that the whole-cell current was carried by nAChRs expressed in VTA DAergic neurons. In addition, nicotine-induced current was blocked by an α6 nAChR-selective antagonist. This inward current was sufficient to drive an increase in VTA DAergic neuron activity for more than 30 min. Expression of α4* nAChRs was critical for this effect of nicotine, because the drug failed to elicit an increase in firing frequency in α4 KO mice, further supporting our previous study, which found that bath application of up to 1 μM nicotine does not elicit an inward current in DAergic neurons from these animals (Zhao-Shea et al., 2011). Furthermore, nicotine failed to significantly increase DAergic neuron firing in α6 KO mice. It is noteworthy that selective activation of α4* nAChRs in VTA DAergic neurons from L9′A mice yielded a biphasic response to nicotine consisting of a rapid, robust increase in firing frequency that desensitized quickly followed by a sustained increase in firing frequency. The sustained increase in firing frequency was sensitive to blockade by an α6-selective antagonist, indicating that α4α6* nAChRs underlie the sustained increase in nicotine-induced firing. Both responses could be inhibited by pre-exposure of DHβE and α-conotoxin MII[H9A;L15A], which suggests that at least two distinct nAChR subtypes may contribute to nicotine's mechanism of action. Although we hypothesize that the short-term desensitizing nicotine response in L9′A DAergic neurons may be mediated by α4non-α6β2* nAChRs, this interpretation may be confounded by the L9′A α4 subunit mutation, which may confer slower desensitization properties to α4β2* nAChRs compared with WT (Labarca et al., 1995). In addition, DHβE is not truly specific for α4β2 nAChRs, because it can also block α6* nAChRs (Drenan et al., 2008), precluding identification of functional nAChRs containing only α4 and β2 subunits. Previously, we have shown that nAChRs containing α4 and α6 subunits are critical for activation of DAergic neurons in the VTA in response to rewarding nicotine doses (i.e., doses that condition a place preference) (Zhao-Shea et al., 2011). This is also supported by studies measuring nicotine-induced DA release from striatal synaptasomes, which indicate that nAChRs containing α4 and α6 subunits have the highest affinity for nicotine recorded to date, with an EC50 of ∼230 nM (Salminen et al., 2007), well within the range of nicotine blood concentrations achieved by smoking (Russell et al., 1980), as opposed to nAChRs containing (non-α4)α6β2 nAChRs, which have an EC50 of 1.52 μM nicotine.
A number of mouse models have been used to gain insight into the potential composition of nAChRs critical for activation of DAergic neurons by nicotine and nicotine reinforcement. Mice that do not express nAChRs containing the α4, α6, or β2 subunit fail to self-administer nicotine (Picciotto et al., 1998; Maskos et al., 2005; Pons et al., 2008). Re-expression of each subunit in the VTA of the respective KO mouse rescues nicotine self-administration, indicating that activation of nicotinic receptors containing α4, α6, and/or β2 subunits, specifically in the VTA, are sufficient for nicotine reinforcement (Maskos et al., 2005; Pons et al., 2008). Although α4 and β2 nAChR subunits are expressed in both VTA DAergic and GABAergic neurons, expression of α6 subunits have predominantly been localized to DAergic neurons (Champtiaux et al., 2003; Grady et al., 2007; Drenan et al., 2008), although a recent report indicates they may also be expressed in GABAergic presynaptic boutons (Yang et al., 2011). More recently, Exley et al. (2011) examined intracranial nicotine self-administration in WT, α4 KO, and α6 KO mice. Both WT and α6 KO mice self-administered high doses of nicotine (50 nl of 4 mM nicotine/infusion or 100 ng/infusion) directly into the VTA, whereas α4 KO mice did not sustain nicotine self-administration for extended daily sessions (Exley et al., 2011). However, α6 KO mice self-administered less nicotine than WT when the nicotine concentration was decreased 10-fold. These data indicate that α6* nAChRs are critical for self-administration of lower nicotine doses and, in combination with the present study, would indicate that nAChRs containing both α4 and α6 subunits are expected to mediate nicotine reward in response to nM concentrations of the drug. However, the authors suggest that nAChRs containing both α4 and α6 subunits are not functionally expressed in VTA DAergic neuron soma; rather, they are expressed only at presynaptic terminals. This is based on firing patterns of DAergic neurons in vivo in response to intravenous nicotine: short-term intravenous nicotine failed to induce an increase in the number of spikes within a burst (SWB) in α4 KO VTA DAergic neurons, whereas nicotine did modestly increase SWB in both WT and α6 KO mice. Re-expression of the α4 subunit in the VTA rescued this effect of nicotine, indicating that α4(non-α6)* nAChRs are critical for nicotine-induced increases in SWB. However, nicotine increased the basal firing rate of VTA DAergic neurons in all three mouse lines and was abolished only in α4α6 double-KO animals, supporting our data, which clearly indicate that these receptors can directly contribute to nicotine-induced increases in DAergic neuron activity. This is also supported by immunoprecipitation studies in rat, which indicate that DAergic neurons within ventral midbrain express α4α6β2β3 nAChRs (∼21.4% of β2* nAChRs), whereas (non-α4)α6β2β3* nAChRs are undetectable (Gotti et al., 2010). One limitation to our study is that it provides little insight into the contribution of nAChR subtypes that are involved in nicotine burst activity because we observe little bursting in DAergic neurons within midbrain slices. This is probably because DAergic neuron bursting in response to concentrations of nicotine achieved by smoking requires other cholinergic and/or noncholinergic inputs that are severed in our slice preparation, and presumably require glutamatergic signaling, which we also block in our experiments (Mansvelder et al., 2002; Lodge and Grace, 2006; Ishibashi et al., 2009).
Desensitization-resistant nAChRs are an emerging subtype of receptor. Two classes of neurons have been found to express desensitization-resistant nAChRs that allow for long-lasting increases in neuronal activity in response to nicotine. These include horizontally oriented interneurons in the stratum oriens/alveus and pyramidal neurons in layer VI of the prefrontal cortex (Jia et al., 2009; Bailey et al., 2010). Although the precise subunit compositions of these receptors are unknown, the α2 subunit is a component of the desensitization-resistant nAChR in stratum oriens horizontally oriented interneurons (Jia et al., 2009), whereas expression of α5 nAChR subunits is required for desensitization-resistant nicotine-induced currents in layer VI pyramidal neurons (Bailey et al., 2010). These nAChRs also presumably contain α4 and β2 subunits, because currents were blocked with the α4β2 competitive antagonist dihydro-β-erythroidine. Our data indicate that the assembly of α6 subunits with α4 subunits may be sufficient to confer nAChRs with resistance to nicotine-induced desensitization.
Previous studies have identified potential mechanisms to account for how nicotine drives activation of VTA DAergic neurons. Using a biophysical approach in midbrain slices, Mansvelder et al. found that high-affinity nAChRs in VTA GABAergic interneurons rapidly desensitize to disinhibit DAergic neurons (Mansvelder and McGehee, 2002; Mansvelder et al., 2002). In addition, α7 nAChRs expressed in glutamatergic presynaptic terminals that innervate the VTA recover more rapidly from nicotine-induced desensitization, allowing for persistent increases in glutamate release and excitation of VTA DAergic neurons. In the context of this model, our data provide an additional mechanism. In a time on the order of seconds, nicotine activates high affinity α4β2* nAChRs expressed on both GABAergic and DAergic VTA neurons. Within seconds to a few minutes, nicotine desensitizes these receptors (Pidoplichko et al., 1997; Mansvelder et al., 2002). This is followed by nicotine-induced sustained activation of α4α6* nAChRs expressed in DAergic neurons. Together with a persistent glutamatergic drive mediated by recovery from desensitization of presynaptic α7 nAChRs, these two mechanisms allow nicotine to activate VTA DAergic neurons for several minutes up to an hour.
In summary, our data provide a mechanistic explanation for how nicotine concentrations achieved by smoking can increase and maintain VTA DAergic neuron activity. VTA DAergic neurons express functional α4α6* nAChRs that allow nicotine to depolarize and persistently activate VTA DAergic neurons for extended periods of time. Thus, this nAChR subtype is a prime candidate for therapeutics aimed at facilitating smoking cessation.
This study was supported by the National Institute on Alcohol Abuse and Alcoholism [Grant R01AA017656]; the National Institute of Mental Health [Grant R01MH53631]; and the National Institute on Neurological Disorders and Stroke [Grant R01NS030243]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- DAergic
- dopaminergic
- VTA
- ventral tegmental are
- NAcc
- nucleus accumbens
- DA
- dopamine
- nAChR
- neuronal nicotinic acetylcholine receptor
- KO
- knockout
- DHβE
- dihydro-β-erythroidine
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate
- TTX
- tetrodotoxin
- ACSF
- artificial cerebrospinal fluid
- TH
- tyrosine hydroxylase
- ANOVA
- analysis of variance
- SWB
- spikes within a burst.
Authorship Contributions
Participated in research design: Liu, Zhao-Shea, McIntosh, Gardner, and Tapper.
Conducted experiments: Liu and Zhao-Shea.
Contributed new reagents or analytic tools: McIntosh.
Performed data analysis: Liu and Tapper.
Wrote or contributed to the writing of the manuscript: Liu, Zhao-Shea, McIntosh, Gardner, and Tapper.
References
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. (2010) The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci 30:9241–9252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS. (1991) Low concentrations of nicotine increase the firing rate of neurons of the rat ventral tegmental area in vitro. Birkhauser Verlag; Basel [Google Scholar]
- Centers for Disease Control and Prevention (2010) Vital signs: current cigarette smoking among adults aged > or =18 years—United States, 2009. MMWR Morb Mortal Wkly Rep 59:1135–1140 [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23:7820–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. (2002) Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci 22:1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. (1991) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 104:171–176 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107:285–289 [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. (2011) Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34:105–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang T, Li W, Doyon WM, Doyon W, Dani JA. (2010) Route of nicotine administration influences in vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J Mol Neurosci 40:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, et al. (2008) In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron 60:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, et al. (2011) Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci USA 108:7577–7582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Pidoplichko VI, Dani JA. (1998) Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. J Physiol Paris 92:209–213 [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, et al. (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30:5311–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74:1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Ishibashi M, Leonard CS, Kohlmeier KA. (2009) Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine. Neuropsychopharmacology 34:2529–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yamazaki Y, Nakauchi S, Sumikawa K. (2009) Alpha2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits. Eur J Neurosci 29:1588–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376:514–516 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65 [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2006) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA 103:5167–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50:911–921 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33:905–919 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. (2002) Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol 53:606–617 [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, et al. (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107 [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. (2004) Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol 65:944–952 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177 [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404 [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. (2004) Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem 11:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. (2009) Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin 30:752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. (2008) Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 28:12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, et al. (2000) Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20:6431–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Jarvis M, Iyer R, Feyerabend C. (1980) Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J 280:972–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. (2007) Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol 71:1563–1571 [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032 [DOI] [PubMed] [Google Scholar]
- Tapper AR, Nashmi R, Lester HA. (2006) Neuronal nicotinic acetylcholine receptors and nicotine dependence, in Cell Biology of Addiction (Madras BK, Colvis CM, Pollock JD, Rutter JL, Shurtleff D, von Zastrow M. eds) pp 179–191, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23:3176–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ, Wu J. (2011) Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J Neurosci 31:2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. (2011) Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology 36:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]