Abstract
Introduction
Little is known about the optimum timing of surgery for dense congenital bilateral cataracts. We examined the critical period for deprivation amblyopia in a cohort of patients with dense bilateral congenital cataracts.
Methods
Thirty-seven infants with dense bilateral congenital cataracts extracted by 31 weeks of age were enrolled prospectively. Visual acuity outcome was assessed at ≥5 years of age. We statistically evaluated which of 4 models provided the best fit to the data: 1) no change in visual acuity outcome with delay in surgery, 2) linear decline of outcome with delay, 3) a bilinear model in which there is a critical age after which outcome depends on delay, and 4) a bilinear model in which there is a critical age before which outcome depends on delay. In addition, we reviewed medical records for associated adverse outcomes, including strabismus, nystagmus, secondary membrane formation, and glaucoma.
Results
A bilinear model with a critical age of 14 weeks fit the data better than a linear model (χ2=14.7; p<0.0006). During weeks 0-14, mean visual acuity decreased by 1 line with each 3 weeks delay in surgery. From 14-31 weeks, visual acuity was independent of age at surgery, averaging 20/80. Surgery after 4 weeks was associated with a higher prevalence of strabismus and nystagmus than surgery before 4 weeks while surgery during the first 4 weeks was associated with a higher prevalence of secondary membrane formation and glaucoma.
Conclusion
We did not find a latent period for treatment of children with dense bilateral congenital cataracts. Deprivation amblyopia may be minimized with early surgery for bilateral cataracts.
Introduction
Dense congenital and infantile cataracts, if not treated promptly, lead to profound and irreversible vision loss. Animal models of unilateral visual deprivation have shown that few cortical neurons can be driven by the deprived eye and that, during an early critical period, these changes are reversible. Extraction of dense congenital unilateral cataracts by 6-8 weeks of age, along with optical correction and occlusion therapy, can result in near-normal visual acuity and, in some cases, fusion and stereopsis, consistent with a peak of recovery potential within this age range1-3.
On the other hand, little is known about the optimum timing of extraction for dense congenital bilateral cataracts. Maurer and Lewis4 reported that children treated by 5 months of age achieve a mean recognition acuity of about 20/80 when tested at ≥3 years of age but they also report little correlation between age at surgery and recognition acuity outcome. Likewise, our study of a small number of patients with congenital bilateral cataracts in 1998 showed no significant difference in visual acuity outcomes between those operated at ≤8 weeks of age (N=6) versus 12-30 weeks of age (N=6)1. Lambert et al 5 reported a linear trend for worse recognition acuity outcome at 4 to 6 years of age with increasing age at surgery, but this was not statistically significant and the correlation was low (r=0.28; i.e., the linear model accounted for only 8% of the variance in the visual acuity outcome). However, there has been no detailed analysis of age at surgery versus long-term visual acuity outcome, particularly within the first weeks of life.
Timing of surgical intervention for bilateral congenital cataracts has both important clinical and basic science implications since little is known about the critical period for bilateral deprivation amblyopia in humans. Additionally, this information can contribute to defining the risk-benefit ratio for very early surgery for congenital bilateral cataracts. An association between very early surgery for congenital cataracts and a higher risk of glaucoma has been reported6-10{ , but it is unclear if early surgery itself increases the risk or if eyes with congenital cataracts are at higher risk for glaucoma than those with acquired cataracts. Infants who have IOLs implanted under one month of age have a higher risk for glaucoma as well as secondary membranes9,11. On the other hand, the risk for strabismus and nystagmus may increase if surgery is delayed beyond 2-3 months of age.9, 12, 13
While most surgeons would agree that patients presenting with dense bilateral cataracts after three months of age should be operated on without delay14, it is less clear if immediate surgery is necessary to achieve optimal visual acuity outcomes in patients presenting prior to one month of age. An accurate assessment of the visual acuity and adverse event outcomes as a function of age at surgery is needed to determine the best timing for surgical intervention in these infants.
Methods
Participants
All infants with dense bilateral congenital cataracts diagnosed during the first week of life were referred to the Retina Foundation of the Southwest following cataract extraction for enrollment in a prospective study of visual acuity and stereoacuity development. Dense nuclear or complete cataracts were noted by the pediatric ophthalmologist prior to surgery in all participants. This cohort returned at 3-month intervals during infancy, 6-month intervals until age 5 years, and annual intervals thereafter for sensory testing in the laboratory. At each visit, we also conducted a standardized interview to determine compliance and asked the parents to sign medical record release forms so that we obtain medical records from their child’s pediatric ophthalmologist for review and identification of adverse outcomes. From this large ongoing prospective cohort, we identified 37 children who were age 5 years or older, had good to excellent contact lens/spectacle wear compliance throughout follow-up, and no secondary intraocular lens implantation. Patients with associated retinal anomalies, systemic disease, or neurological disorders were not eligible. Informed consent was obtained from one or both parents prior to the infant’s enrollment. This research protocol observed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the UT Southwestern Medical Center.
Visual Acuity
Best-corrected visual acuity was assessed monocularly using crowded HOTV or ETDRS optotypes, using the Amblyopia Treatment Study protocols15, 16 on the EVA system17.
Adverse outcomes
Medical records from all ophthalmologists who provided care during the follow-up period of 5 to 16 years were reviewed in order to identify onset of strabismus, nystagmus, secondary membranes, and glaucoma.
Data Analysis
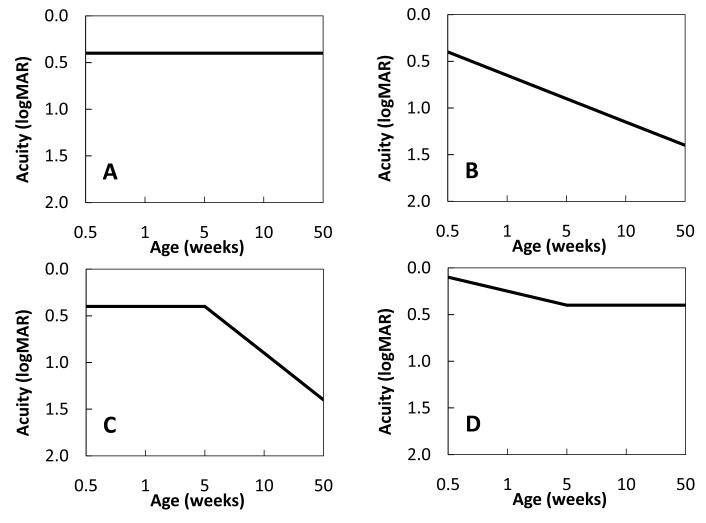
Four alternative models of the critical period were evaluated for best fit to visual acuity outcomes as a function of age at surgery. Two of the models are linear. First, it is possible that the age at surgery/optical correction will have no effect on the outcome variable (Fig 1A). This outcome is possible given our preliminary data showing no difference between groups of children with dense bilateral congenital cataracts who had surgery at ≤8 weeks of age versus 12-30 weeks1. On the other hand, our sample size was very small (N=6 per group), so we may have been unable to see trends in the data, particularly during the first 8-12 weeks of life. Therefore, a second linear model was used to represent the alternative hypothesis that the earlier the treatment is initiated, the better the outcome (Fig 1B). In addition, two bilinear models were considered. In the first bilinear model (Fig 1C), there exists an early window of time, beginning at birth, during which an optimal outcome can be attained but, once deprivation continues outside of this window, visual acuity outcomes decline progressively. This type of model provided the best fit to the data from patients with unilateral congenital cataracts 2. Another bilinear model considered was that duration of deprivation is associated with visual acuity outcome only during the first weeks of life, after which there is no further effect of duration (Fig 1D).
Figure 1.
Four models of the effects of bilateral deprivation on longterm logMAR recognition acuity outcome: A. age at surgery has no effect on longterm visual acuity outcome, B. the earlier the surgery the better the longterm visual acuity outcome, C. there is an early window of time, beginning at birth, during which an optimal outcome can be attained but, if surgery is delayed beyond this window, longterm visual acuity outcome declines progressively, D. delay in surgery is associated with progressive decline in visual acuity outcome only during the first weeks of life, after which there is no further effect of duration.
Linear and bilinear models of the critical period were compared using a statistical procedure18 to evaluate the effects of aging on the visual system. Briefly, a bilinear model was recast into a four-parameter nonlinear model by means of B-splines with only a single nonlinear parameter; thus, only a one-dimensional nonlinear optimization is required. The same approach was used to construct a linear model with the constraint that the slopes of both halves of the single straight line are equal. Linear and bilinear models were compared by constructing a likelihood ratio test under the assumption that the errors are distributed normally. Under this assumption, the least-square estimate equals the maximum likelihood estimate and χ2=2 [ log(likelihood ratio)] with 2 degrees of freedom. This model has been used successfully to evaluate the effects of aging on various visual functions2, 19, 20. After determining whether a linear or bilinear model provided a better fit to the data, the slopes of the best-fit models were examined to distinguish between the two models within that category; i.e., to distinguish model A vs. B or model C vs. D.
Risk for Adverse Outcomes
Risk for strabismus, nystagmus, secondary membrane formation, and glaucoma were analyzed using the z-test for differences in prevalence of each adverse outcome for children who had surgery during the first 4 weeks of life (the time period considered to be high risk for secondary membranes and glaucoma11) versus children who had surgery at 5-31 weeks of age. In cases where the child had surgery for one eye in each age group (N=2), they were excluded from analysis of prevalence of strabismus and nystagmus, since this determination is made on a per person basis not a per eye basis.
Results
Thirty-seven patients (74 eyes) met criteria for study inclusion. The mean age at surgery was 10 weeks ± 8.7 weeks (range = 0.4 to 31 weeks). In 54% of participants (20/37 children), the difference in age at surgery for the two eyes was 0-4 days in 30% (11/37 children), 5-11 days, in 14% (5/37 children), and 49 days in 2% (1/37 children). Fourteen children had both surgeries completed by 4 weeks of age and an additional two children had surgery on one eye completed by 4 weeks of age.
Visual Acuity
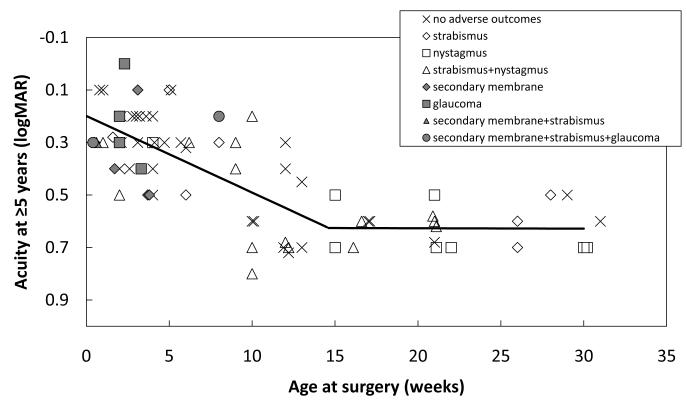
Visual acuity outcome was assessed at 5-16 years of age (mean = 10.6 years; SD = 3.8 years). Overall, the mean visual acuity outcome was 0.4 ± 0.2 logMAR (20/50 ± 2 lines) , with a range from 0.0 to 0.8 logMAR (20/20 to 20/125). Statistical comparison of linear and bilinear models of the critical period found that a bilinear model with a critical age of 14 weeks fit the data better than a linear model (χ2=14.7; p<0.0006). Consistent with model D in Figure 1, the slope of the best-fit function during the intial 14 weeks of life was 0.029 logMAR/week. That is, during weeks 0-14, mean visual acuity decreased by 1 line with each 3 weeks delay in surgery, from 0.2 logMAR (20/30) at 1 week to 0.6 logMAR ( 20/80) at 14 weeks (Figure 2). After 14 weeks, the slope of the best-fit fuction was near-zero (−0.0001 logMAR/week); i.e., visual acuity was independent of age at surgery between 14 and 31 weeks, averaging 0.6 logMAR (20/80).
Figure 2.
Longterm visual acuity outcome for 37 patients (74 eyes) treated for dense congenital bilateral cataracts along with the best-fit bilinear model of the critical period (solid line). Associated adverse outcomes are indicated by symbol type.
Adverse Outcomes
Adverse events are coded in Figure 2 by symbol type. Overall, 35% (13/37 children) developed strabismus that was treated surgically; all cases were alternating strabismus and none were treated with occlusion therapy. In addition, 32% (12/37 children) developed manifest nystagmus observable on clinical examination, 8% (6/74 eyes) developed a secondary membrane requiring re-operation, and 8% (6/74 eyes) developed glaucoma that required treatment with medication and/or surgery during the follow-up period.
Surgery during the first 4 weeks was associated with a lower prevalence of strabismus compared with surgery at 5-31 weeks (21% vs. 48%; 3/14 vs. 10/21 children; z=1.75, p<0.05) and a lower prevalence of nystagmus (14% vs. 48%,2/14 vs. 10/21 children; z=2.38, p<0.01). On the other hand, surgery during the first 4 weeks of life was assosicated with a higher prevalence of secondary membranes than surgery after 4 weeks (20% vs. 0%; 6/30 vs 0/44 eyes; z=2.73, p<0.005) and glaucoma (17% vs. 2%; 5/30 vs. 1/44 eyes; z=2.09, p<0.025). If the analysis is limited only to surgeries during the first 14 weeks of life, similar results are obtained. Surgery during the first 4 weeks was associated with a lower prevalence of strabismus compared with surgery at 5-14 weeks (21% vs. 55%; 3/14 vs. 6/11 children; z=1.83,p<0.05) and a higher prevalence of secondary membranes than surgery at 5-14 weeks (20% vs. 0%; 6/30 vs. 0/24 eyes; z=2.74, p<0.005. In addition, there were trends for a higher prevalence of nystagmus (14% vs. 36%, 2/14 vs. 4/11 children; p=0.08) and a lower prevalence of glaucoma (17% vs. 4%; 5/30 vs. 1/24 eyes; p=0.06) with surgery at 5-14 weeks compared to very early surgery, but these did not reach statistical significance with the smaller sample size available for 5-14 weeks compared to 5-31 weeks.
Discussion
Deprivation amblyopia may be minimized with early surgery for dense congenital bilateral cataracts. During the first 14 weeks of life, there is a linear relationship between delay in surgery and longterm visual acuity outcome, with an average loss of one line of visual acuity for every three weeks of delay. After 14 weeks of age, up to 31 weeks of age, there is little additional adverse effect of further delay in surgery. In addition, surgery during the first 4 weeks of life was associated with a significantly lower prevalence of strabismus and nystagmus than surgery after 14 weeks.
The statistical analysis used to determine whether a linear model or bilinear model provided a better fit to the visual acuity outcome data has been shown to be relatively immune to missing regions of the age continuum if the underlying distribution is truly linear 18. In fact, when the underlying distribution is truly bilinear, missing regions of the age continuum bias the analysis toward rejection of the bilinear model. Thus, any sparseness or inhomogeneity of sampling along the age at surgery continuum would have biased the analysis toward the linear model. The bilinear model provides a good empirical fit to the data (r=0.75), accounting for 58% of the variance. It is possible that other nonlinear models may provide a better fit to the data. Nonetheless, the analysis presented here is sufficient to establish that the first 14 weeks after birth represent a period during which treatment effectiveness declines linearly with delay in surgery.
The best-fit model of the critical period for dense congenital bilateral cataracts differs significantly from the best-fit model described for dense congenital unilateral cataracts. For unilateral cataracts, there appears to be a window of time, beginning at birth and lasting 6-8 weeks, for cataract extraction and intiation of postoperative treatment to be maximally effective, followed by a period of sharply declining visual acuity outcomes with progresively longer delays1, 2, 21. Previous studies support the hypothesis that only the direct effects of visual deprivation are active in both unilateral and bilateral cases during the first 8 weeks of life but, at older ages, the unilateral cases experience the additional adverse effects of interocular competition on visual acuity development1, 4, 22. Consistent with this, we found that both the present bilateral cohort and a cohort with congenital unilateral cataracts with similar inclusion/exclusion criteria published previously1 had similar mean longterm visual acuity outcomes when surgery was performed before 8-14 weeks of age (0.35 ± 0.19 logMAR bilateral; 0.38 ± 0.22 logMAR unilateral; t = 0.41, p = 0.69) but the unilateral cases fared much worse than bilateral cases if surgery was delayed beyond 12-14 weeks (0.62 ± 0.08 logMAR bilateral; 0.89 ± 0.37 logMAR unilateral; t = 3.00, p<0.005). The finding of a significant relationship between delay in surgery during the first weeks of life and visual acuity outcome in children treated for bilateral cataracts suggests that delay may be an important factor in determining the direct effects of visual deprivation, even in unilateral cases.
The best-fit model suggests that surgery should be scheduled as early as possible to optimize visual acuity outcome, and the earliest surgeries (during the first 4 weeks of life) were associated with a lower prevalence of strabismus and nystagmus. On the other hand, the earliest surgeries were associated with higher prevalence of secondary membrane formation and glaucoma, which has been reported by others11. Despite the higher risk for secondary membrane formation and glaucoma with very early surgery, excellent longterm visual acuity outcomes were obtained with surgery during the first 4 weeks of life; no patient who had surgery on both eyes by 4 weeks of age had a visual acuity outcome poorer than 20/60 and the mean visual acuity was 0.29 logMAR (20/40). Thus, early surgery may minimize bilateral deprivation amblyopia without significantly increasing the risk for sight-threatening associated adverse events.
Acknowledgments
This research was supported by a grant from the National Eye Institute (EY05236).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study was conducted at: Retina Foundation of the Southwest
This manuscript will be presented at the annual meeting of AAPOS at Washington, DC, 2008
The authors have no commercial interest.
Literature Cited
- 1.Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Invest Ophthalmol Vis Sci. 1998;39(9):1560–6. [PubMed] [Google Scholar]
- 2.Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37(8):1532–8. [PubMed] [Google Scholar]
- 3.Jeffrey BG, Birch EE, Stager DR, Jr., et al. Early binocular visual experience may improve binocular sensory outcomes in children after surgery for congenital unilateral cataract. Journal of AAPOS. 2001;5(4):209–16. doi: 10.1067/mpa.2001.115591. [DOI] [PubMed] [Google Scholar]
- 4.Maurer D, Lewis TL. Visual outcomes after infantile cataract. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. [Google Scholar]
- 5.Lambert S, Lynn M, Reeves R, et al. Is there a latent period for the surgical treatment of children with dense bilateral congenital cataracts? Journal of AAPOS. 2006;10:30–1. doi: 10.1016/j.jaapos.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Vishwanath M, Cheong-Leen R, Taylor D, et al. Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol. 2004;88(7):905–10. doi: 10.1136/bjo.2003.040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundvall A, Kugelberg U. Outcome after treatment of congenital bilateral cataract. Acta Ophthalmol Scand. 2002;80(6):593–7. doi: 10.1034/j.1600-0420.2002.800607.x. [DOI] [PubMed] [Google Scholar]
- 8.Lambert S, Amaya L, Taylor D. Detection and treatment of infantile cataracts. Int Ophthalmol Clin. 1989;29:51–65. doi: 10.1097/00004397-198902910-00015. [DOI] [PubMed] [Google Scholar]
- 9.Watts P, Abdolell M, Levin A. Complications in infants undergoing surgery for congenital cataract in the first 12 weeks of life: Is early surgery better? Journal of AAPOS. 2003;7:81–5. doi: 10.1016/mpa.2003.S1091853102420095. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi R, Wilson M, Jr, Golub R. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. Journal of AAPOS. 2006;10:117–23. doi: 10.1016/j.jaapos.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Lambert S, Buckley E, Plager D, et al. Unilateral intraocular lens implantation during the first six months of life. Journal of AAPOS. 1999;3:344–9. doi: 10.1016/s1091-8531(99)70043-1. [DOI] [PubMed] [Google Scholar]
- 12.Abadi RV, Forster JE, Lloyd IC. Ocular motor outcomes after bilateral and unilateral infantile cataracts. Vision Res. 2006;46(6-7):940–52. doi: 10.1016/j.visres.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Ye H, Deng D, Qian Y, et al. Long-term visual outcome of dense bilateral congenital cataract. Chin Med J. 2007;120:1494–7. [PubMed] [Google Scholar]
- 14.Harley R, Nelson L, Olitsky S. Harley’s Pediatric Ophthalmology. Lippincott Williams & Wilkins; New York: 2005. [Google Scholar]
- 15.Cotter S, Chu R, Chandler D, et al. Reliability of the electronic early treatment diabetic retinopathy study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003;136:655–61. doi: 10.1016/s0002-9394(03)00388-x. [DOI] [PubMed] [Google Scholar]
- 16.Holmes J, Beck R, Repka M, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 17.Moke P, Turpin A, Beck R, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol for children. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 18.Owsley C, Knoblauch K, Katholi C. When does visual aging begin? ARVO. Ft.; Lauderdale, FL: 1992. [Google Scholar]
- 19.Curcio C, Millican C, Allen K, Kslina R. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3728–96. [PubMed] [Google Scholar]
- 20.Lachenmayr B, Kojetinsky S, Ostermaier N, et al. The different effects of aging on normal sensitivity in flicker and light sense perimetry. Invest Ophthalmol Vis Sci. 1994;35:2741–8. [PubMed] [Google Scholar]
- 21.Birch E, Stager D, Leffler J, Weakley D. Effects of unequal competition are minimized by very early treatment of congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1998;39:1560–6. [PubMed] [Google Scholar]
- 22.Tytla M, Maurer D, Lewis T, Brent H. Contrast sensitivity in children treated for congenital cataract. Clin. Vision Sci. 1988;2:251–64. [Google Scholar]