Abstract
We describe a case of microsporidial myositis in a healthy man from Thailand. The small subunit rRNA sequence of this microsporidium is novel and has a close phylogenetic relationship with Endoreticulatus, a genus of lepidopteran microsporidia. Myositis could be caused by more genera of microsporidia than previously known.
Keywords: microsporidia, myositis, Endoreticulatus, lepidoptera, parasites, Thailand
Microsporidia are obligate intracellular eukaryotes of broad host range (1). At least 15 species have been implicated in human infections. Although few microsporidiosis cases have been reported, they have emerged as opportunistic infections in patients with HIV infection (1). In addition, microsporidiosis has increasingly been diagnosed in other immunocompromised patients, including those who have received an organ transplant, and in immunocompetent persons (1). Various clinical manifestations, ranging from localized (diarrhea, cholangitis, sinusitis, keratitis) to disseminated (myositis, osteomyelitis, encephalitis) infection have been observed, depending on the causative agent and host immune status (1). We report microsporidial myositis caused by a suspected new species of Microsporidium in an otherwise healthy man.
The Study
A 43-year-old man (a welder living in Lopburi Province, central Thailand) sought treatment at King Chulalongkorn Memorial Hospital, Bangkok, on November 30, 2010. He had experienced difficulty in swallowing for 2 weeks. Fifteen months before hospital admission, he experienced a low-grade fever and generalized muscle pain, especially in his lower back and both thighs. Despite several courses of medication, hospital, his condition had not improved.
Ten months before admission, weakness of lower and upper limbs developed in his proximal muscle, primarily on the left side. Two months before admission, his entire left leg was swollen, and he was confined to bed. During this illness, he had lost ≈29 pounds. He reported no travel abroad and had not taken any herbal medications. He did not smoke, drank alcohol occasionally, and did not use illicit drugs. He had no history of recurring infections during childhood and early adulthood.
Physical examination showed no keratoconjunctivitis, but indicated engorged jugular veins and right ventricular heave with loud pulmonic valve closure sound. Abdominal examination showed no hepatosplenomegaly. Non-pitting edema of the entire left leg was observed. Neurologic examination showed hyporeflexic proximal muscle weakness of grade III–IV/V in all limbs. Complete blood count showed the following: hematocrit 43.5%, leukocyte count 5,230/mm3 (79% neutrophils, 14% lymphocytes, 4% eosinophils, and 3% monocytes), platelet count 209,000/mm3. Liver function test showed the following results: total bilirubin 0.34 mg/dL, alkaline phosphatase 57 U/L (reference value 42 U/L–121 U/L), aspartate transaminase 268 U/L (4 U/L–36 U/L), alanine transaminase 101 U/L (4 U/L–36 U/L), albumin 3.1 g/dL, and creatine phosphokinase 4,308 U/L (60 U/L–400 U/L). Test results were negative for antibody against nuclear antigen and HIV (2 tests, 1 month apart). CD4 cell count was 368/μL (24%), and serum protein electrophoresis showed polyclonal gammopathy.
Chest radiograph showed pulmonary hypertension without significant pulmonary infiltration. Severe pulmonary hypertension without left ventricular dysfunction or left-to-right shunt was evident on electrocardiogram. Computed tomographic scan showed an enlarged pulmonary trunk (4 cm in diameter) and right and left main pulmonary arteries (thrombosis was not shown in the lumens). Electromyograph showed irritative myopathy without evidence of large-fiber sensory and motor polyneuropathy.
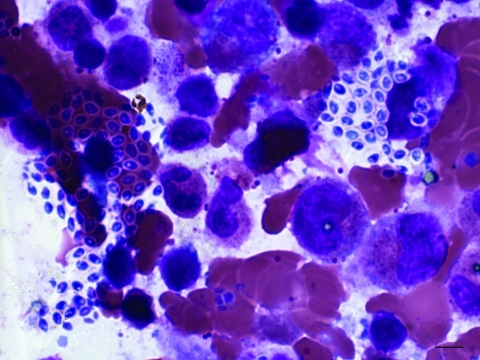
A biopsy of left deltoid and both vastus lateralis muscles showed necrotizing granulomatous inflammation without any organisms by Gram, acid-fast bacilli, Wright, and Gomori methenamine silver staining. Bone marrow biopsy showed normal cellularity with increased plasma cells and histiocytes and focal aggregation of microsporidial spores with characteristic belt-like stripe (Figure 1). A 24-hour urine sample (centrifuged) yielded characteristic microsporidial spores on modified trichrome stain, 1.0–1.5 μm × 1.2–2.2 μm, similar to those observed in the bone marrow sample. Because of an overgrowth of Candida yeast, we did not isolate the organism from the urine sample.
Figure 1.
Microsporidium species from bone marrow aspiration specimen (Wright stain) from a 43-year-old man, Thailand. The image shows focal aggregations of microsporidia-like microorganisms with a belt-like stripe (1.0–1.5 μm x 1.2-2.2 μm). Original magnification ×100.
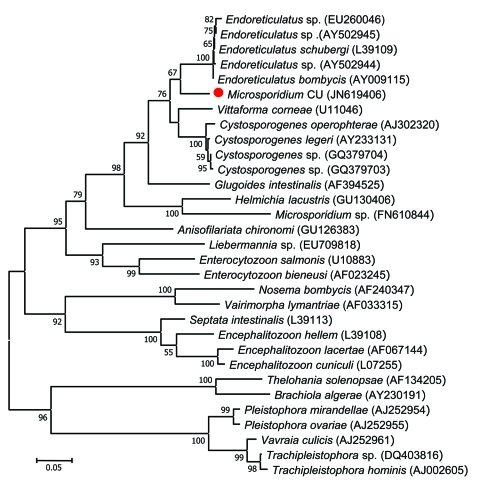
Analysis of the small subunit rRNA gene spanning 797 bp of DNA from microsporidial spores isolated from this patient showed a novel sequence (GenBank accession no. JN619406). Phylogenetic reconstruction using the maximum-likelihood method placed the patient’s Microsporidium sp. within the cluster of the genera Endoreticulatus, Cystosporogene, and Vittaforma (Figure 2) (2). This species has a close phylogenetic relationship with Endoreticulatus spp. Notably, the guanine-cytosine content of the SSU rRNA locus of microsporidia within the genus Endoreticulatus varied from 51.4% to 51.8%, with the base composition distance 0.001–0.029. However, the small subunit rRNA sequence of microsporidium from our patient (GenBank accession no. JN619406) contained 52.4% guanine-cytosine, and the base composition distance differed from Endoreticulatus spp. from 0.094–0.170, making it unlikely that the patient was infected with an organism from this genus.
Figure 2.
Phylogenetic tree inferred from the small subunit rRNA sequences of microsporidia in this study and those in the GenBank database by using the maximum-likelihood method as implemented in MEGA5.05 software (2). Red circle indicates a novel microsporidium identified in this study (Microsporidium CU) that caused myositis. GenBank accession numbers are listed in parentheses after each species. Bootstrap percentages >50% based on 1,000 replicates are shown on the branches. The tree is drawn to scale, with branch lengths measured in the number of nucleotide substitutions per site.
The patient was treated with albendazole (800 mg/d continuously). Fever subsided after 1 week of treatment, and weakness gradually improved. He was discharged on a regimen of oral albendazole (800 mg/1×/d) but died unexpectedly from aspiration pneumonia 1 month later. An autopsy could not be performed.
Conclusions
Microsporidia can cause either localized or disseminated infection in humans. The true prevalence of microsporidiosis in humans may be underestimated because of asymptomatic infection in most healthy persons, clinician unawareness, and difficulties in diagnosis. Myositis caused by microsporidia is rare; only 12 patients (including our patient) have been reported in the literature (Table A1; 3–12). Among these 12 patients, 9 were male and 3 were female (age range 4 months to 67 years). Patients were from all continents except Europe: United States (7 patients), Australia (2), Haiti (1), the Gambia (1), and Thailand (1). The patient described here is the first report from Asia. Trachipleistophora sp. (6 patients) is the most common causative agent of myositis, followed by Brachiola sp. (3 patients), Pleistophora sp., Tubulinosema acridophagus sp., and Microsporidium spp. (1 patient each). However, the species of microsporidia originally described in 2 reports (5,6) was changed from Pleistophora sp. to Trachipleistophora sp. upon reexamination of the ultrastructures by electron microscopy.
Although ultrastructural study of the microsporidium from this patient has not been performed, the SSU rRNA sequence clearly supports the finding that it is a novel species, closely related to Endoreticulatus spp. (Figure 2). Most patients in previous reports had disseminated infection or isolated myositis. The patient described here had pulmonary hypertension, likely caused by microsporidia; several reports have shown that these organisms can cause pulmonary infection (13).
The routes of microsporidial infection are still uncertain, but the species that can infect humans have been identified in water sources and in farm, domestic, and wild animals. Furthermore, Trachipleistophora spp. have been found only in human hosts. However, on the basis of the feature of bisporous sporophorous vesicles, T. anthropophthera was proposed to be related to Telomyxa spp., which are parasites of mayflies. T. anthropophthera was reported to be the causative agent of keratitis in an HIV-infected patient from Thailand (14). Brachiola spp. (formerly Nosema spp.) are known to be pathogens of several kinds of insects, including mosquitoes (10). Pleistophora spp. have been found in fish and reptiles, but P. ronneafiei has never been identified in any hosts other than humans. Microsporidium spp. that can infect humans are M. ceylonensis and M. africanum. Both species were reported from the patients with keratitis from Sri Lanka and Africa, respectively, although their natural hosts remain unknown.
To our knowledge, the microsporidium possessing a novel SSU rRNA sequence closely related to Endoreticulatus spp. has never been identified in patients with myositis. Notably, certain lepidopteran microsporidia can produce various responses in nontarget hosts, from refractory to severe infections. Although we have no evidence regarding the route or source of this patient’s infection, the close phylogenetic relationship of this microsporidium and lepidopteran microsporidia suggests that these non–blood-sucking insects could be natural hosts. Water or foods contaminated with lepidopteran insects could not be excluded as the source of infection. Likewise, recent reports that Trachipleistophora extenrec can cause myositis in anteaters (15) and that Tubulinosema sp. can cause myositis in an immunosuppressed patient (12) further support the role of insect microsporidia as human pathogens.
Biography
Dr. Suankratay is a professor in the Division of Infectious Diseases, Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. His research interests include clinical microbiology, invasive fungal infections, HIV, and tropical infectious diseases.
Table A1. Characteristics of 12 patients with microsporidial myositis*.
| Patient no. | Age/sex | Country | Year | Clinical features | Illness duration | Tissues and organs involved | HIV status | CD4 count/μL | Species | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 mo/M | United States | 1973 | Fever, weakness | 4 mo | Heart and striated muscle | NA | NA | Brachiola connori | NA | Died |
| 2 | 20 y/M | United States | 1985 | Fever, weakness, lympadenopathy, testicular atrophy, gynecomastia, sinusitis | 7 mo | Striated muscle | – | NA | Pleistophora raronneafiei | CTX | Survived >4 mo |
| 3 | 33 y/M | Haiti | 1993 | Fever, weakness, weight loss, co-infected with Norcardia sp. | 2 mo | Heart and striated muscle | AIDS | NA | Trachipliestophora sp. | PYM | Died |
| 4 | 35 y/M | The Gambia | 1996 | Fever, weakness, anemia | NA | Striated muscle | AIDS | 57 | Trachipliestophora sp. | CLI | NA |
| 5 | 34 y/M | Australia | 1996 | Fever, weakness, keratitis, sinusitis | 3 mo | Striated muscle, cornea, sinus | AIDS | 6 | T. hominis | NA | Partial recovery; died of HIV infection |
| 6 | 33 y/M | United States | 1996 | Fever, hemiparesis, seizures | 2 mo | Heart, striated muscle, brain, bone, viscera | AIDS | 2 | T. anthropophthera | ABZ, PYM, SDZ | Died |
| 7 | 8 y/F | United States | 1996 | Weakness, seizures, aphasia, dyspnea | 4 mo | Heart, striated muscle, brain, bone, viscera | AIDS | NA | T. anthropophthera | Agents for toxoplasmosis | Died |
| 8 | 31 y/M | United States | 1998 | Fever, weakness | 5 mo | Striated muscle | AIDS | 35 | B. vesicularum | PYM, SDZ, ABZ, ITZ | Complete recovery, died of viral pneumonitis |
| 9 | 67 y/F | United States | 2004 | Fever, weakness, co-infected with Pneumocystis sp.) | 6 wk | Striated muscle | – | NA | B. algerae | ABZ, CLI, ATQ, ITZ | Died |
| 10 | 47 y/M | Australia | 2005 | Weakness, co-infected with Burkholderia pseudomallei, MAC infections | NA | Striated muscle | AIDS | 30 | T. hominis | None | Suicide |
| 11 | 67 y/F | United States | 2011 | Fever, tongue lesions | 5 mo | Striated muscle | – | NA | Tubulinosema acridophagus | ABZ | Died of CLL |
| 12 | 43 y/M | Thailand | 2011 | Fever, weakness, pulmonary hypertension | 15 mo | Striated muscle, bone marrow, kidney, bladder | – | 368 | Microsporidium sp. | ABZ | Died of aspiration pneumonia |
*NA, not available, –, negative; CTX, cotrimoxazole; PYM, pyrimethamine; CLI, clindamycin; ABZ, albendazole; SDZ, sulfadiazine; ITZ, itraconazole; ATQ, atovaquone; MAC, Mycobacterium avium complex; CLL, chronic lyphocytic leukemia.
Footnotes
Suggested citation for this article: Suankratay C, Thiansukhon E, Nilarantanakul V, Putaporntip C, Jongwutiwes S. Disseminated infection caused by a novel species of Microsporidium, Thailand. Emerg Infect Dis [serial on the Internet]. 2012 Feb [date cited]. http://dx.doi.org/10.3201/eid1802.111319
References
- 1.Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. 10.1016/j.actatropica.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 2.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Margileth AM, Strano AJ, Chandra R, Neafie R, Blum M, McCully RM. Disseminated nosematosis in an immunologically compromised infant. Arch Pathol. 1973;95:145–50. [PubMed] [Google Scholar]
- 4.Ledford DK, Overman MD, Gonzalvo A, Cali A, Mester SW, Lockey RF. Microsporidiosis myositis in a patient with acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:628–30. [DOI] [PubMed] [Google Scholar]
- 5.Chupp GL, Alroy J, Adelman LS, Breen JC, Skolnik PR. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin Infect Dis. 1993;16:15–21. 10.1093/clinids/16.1.15 [DOI] [PubMed] [Google Scholar]
- 6.Grau A, Valls ME, Williams JE, Ellis DS, Muntane MJ, Nadal C. Myositis caused by Pleistophora in a patient with AIDS. Med Clin (Barc). 1996;107:779–81. [PubMed] [Google Scholar]
- 7.Hollister WS, Canning EU, Weidner E, Field AS, Kench J, Marriott DJ. Development and ultrastructure of Trachipleistophora hominis n.g., n.sp. after in vitro isolation from an AIDS patient and inoculation into athymic mice. Parasitology. 1996;112:143–54. 10.1017/S0031182000065185 [DOI] [PubMed] [Google Scholar]
- 8.Vávra J, Yachnis AT, Shadduck JA, Orenstein JM. Microsporidia of the genus Trachipleistophora–causative agents of human microsporidiosis: description of Trachipleistophora anthropophthera n.sp. (Protozoa: Microsporidia.). J Eukaryot Microbiol. 1998;45:273–83. 10.1111/j.1550-7408.1998.tb04536.x [DOI] [PubMed] [Google Scholar]
- 9.Cali A, Takvorian PM, Lewin S, Rendel M, Sian CS, Wittner M, et al. Brachiola vesicularum, n.g., n.sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–51. 10.1111/j.1550-7408.1998.tb04532.x [DOI] [PubMed] [Google Scholar]
- 10.Coyle CM, Weiss LM, Rhodes LV III, Cali A, Takvorian PM, Brown DF, et al. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N Engl J Med. 2004;351:42–7. 10.1056/NEJMoa032655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curry A, Beeching NJ, Gilbert JD, Scott G, Rowland PL, Currie BJ. Trachipleistophora hominis infection in the myocardium and skeletal muscle of a patient with AIDS. J Infect. 2005;51:e139–44. 10.1016/j.jinf.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Choudhary MM, Metcalfe MG, Arrambide K, Bern C, Visvesvara GS, Pieniazek NJ, et al. Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg Infect Dis. 2011;17:1727–30. 10.3201/eid1709.101926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodqi M, Brazille P, Gonzalez-Canali G, Cornet M, Piketty C, Weiss L. Unusual pulmonary Enterocytozoon bieneusi microsporidiosis in an AIDS patient: case report and review. Scand J Infect Dis. 2004;36:230–1. [PubMed] [Google Scholar]
- 14.Pariyakanok L, Jongwutiwes S. Keratitis caused by Trachipleistophora anthropophthera. J Infect. 2005;51:325–8. 10.1016/j.jinf.2004.08.030 [DOI] [PubMed] [Google Scholar]
- 15.Vávra J, Kamler M, Modry D, Koudela B. Opportunistic nature of the mammalian microsporidia: experimental transmission of Trachipleistophora extenrec (Fungi: Microsporidia) between mammalian and insect hosts. Parasitol Res. 2011;108:1565–73. 10.1007/s00436-010-2213-3 [DOI] [PubMed] [Google Scholar]