Abstract
To determine previous exposure and incidence of rickettsial infections in western Kenya during 2007–2010, we conducted hospital-based surveillance. Antibodies against rickettsiae were detected in 57.4% of previously collected serum samples. In a 2008–2010 prospective study, Rickettsia felis DNA was 2.2× more likely to be detected in febrile than in afebrile persons.
Keywords: rickettsial infections, Rickettsia felis, rickettsia, Kenya, fever of unknown origin
Rickettsioses are a major human health problem in many parts of the world, including sub-Saharan Africa (1,2). Awareness of rickettsiae as causes of public health problems has been increasing; several novel or emerging diseases caused by these pathogens have been recognized. In Kenya, recent reports have documented human infections with Rickettsia conorii (3,4) and R. felis (5) and tick infection with R. africae (6). Our objectives were to assess previous human exposure to rickettsiae and to determine the incidence of rickettsial infections among febrile and afebrile persons in western Kenya.
The Study
The study was conducted among patients visiting the Lwak Mission Hospital, a rural health care facility in western Kenya in the Asembo area, Rarieda District, in western Kenya (Figure 1). Lwak Mission Hospital serves as the field clinic for population-based infectious disease surveillance conducted by the Kenya Medical Research Institute and the US Centers for Disease Control and Prevention as described (7). The study was conducted with ethical approval from these institutions (protocol nos. 932 and 4566, respectively). To assess previous exposure to rickettsiae, we examined a randomly selected subset of 357 serum specimens collected January 2007 through October 2008 from patients participating in population-based infectious disease surveillance. Samples were screened at a dilution of 1:128 for IgG against spotted fever group (SFG) and typhus group (TG) rickettsiae by using an indirect fluorescence antibody assay (Fuller Laboratories, Fullerton, CA, USA).
Figure 1.
Locations of villages (brown shading in inset map) in Asembo area of western Kenya where the study was conducted, January 2007 through October 2008. Used with permission of Kenya Medical Research Institute/Centers for Disease Control and Prevention Research and Public Health Collaboration.
Blood specimens were collected from the first 2 outpatients ≥5 years of age and the first 2 outpatients <5 years of age seen each day for acute febrile illness (recorded axillary temperature >38.0°C without an obvious cause, defined as cough, difficulty breathing, chest pain, signs of meningitis, or bloody diarrhea) from November 2008 through February 2010. A positive malaria smear was not an exclusion criterion. During the same period, blood specimens were also collected from controls: a group of outpatients who did not have febrile, respiratory, or diarrheal illness during the preceding 2 weeks and asymptomatic persons who accompanied patients to the clinic.
To detect rickettsial DNA, we performed 3 quantitative PCRs: a genus-specific assay selective for a 74-bp segment of the citrate synthase (gltA) gene, a group-specific assay that detects a 128-bp segment of the outer membrane protein (ompB) gene for tick-borne rickettsiae, and a species-specific assay that detects a 129-bp segment of the ompB gene for R. felis (5,8). To identify which Rickettsia sp. was present in the positive specimens, we PCR amplified and sequenced segments of 4 rickettsial genes—17-kDa, ompB, and 2 R. felis plasmid genes (pRF and pRFδ)—by using primers and procedures as described (5).
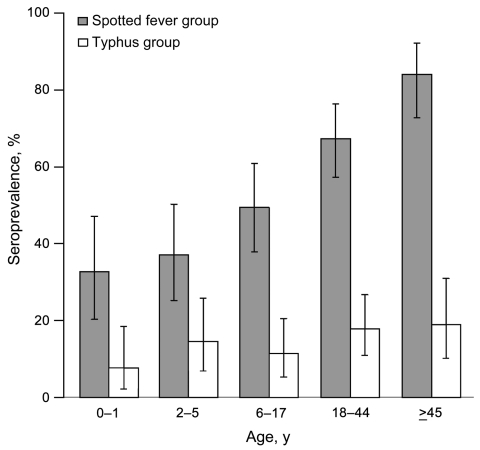
Overall, 205 (57.4%) of 357 specimens had antibodies against rickettsiae. Of 357 serum specimens tested, 200 (56.0%, 95% exact binomial CI 50.7%–61.2%) had detectable IgG against SFG antigen preparation. Antibodies against TG antigen preparation were detected in 52 (14.5%, 95% CI 11.0%–18.6%) of 357 specimens tested; 47 (90.4%) of these specimens that reacted to TG antigens were also positive for SFG antigens, and 5 (1.4%) of the 357 specimens were positive for TG antigens alone. Presence of antibodies against SFG or TG antigens was not associated with patient sex (p>0.05). In addition, patient age was not significantly associated with TG seropositivity (χ2 for linear trend 3.41, df 1, p = 0.065). However, an incremental linear association was demonstrated between age and IgG seropositivity to SFG (χ2 for linear trend 45.46, df 1, p<0.001) (Figure 2).
Figure 2.
Age-stratified seroprevalence of IgG to rickettsiae among patients participating in population-based infectious disease surveillance, January 2007 through October 2008. Vertical lines indicate 95% binomial CIs.
A total of 699 febrile patients who sought care at Lwak Mission Hospital from November 2008 through February 2010 and 236 afebrile persons enrolled during this same period were tested for rickettsiae (Table 1). Overall, 50 (7.2%, 95% CI 5.4%–9.3%) of the febrile patients and 8 (3.4%, 95% CI 1.5%–6.6%) of the afebrile persons had positive rickettsiae results according to the genus-specific gltA assay. Univariate logistic regression indicated that febrile patients were more likely than afebrile persons to have positive PCR results (odds ratio 2.20, 95% CI 1.03–4.70, p = 0.04). According to the ompB assay, all specimens tested were negative for tick-borne rickettsiae. BLAST searches (www.ncbi.nlm.nih.gov/blast/Blast.cgi) for homologous sequences determined that the segments amplified from the 3 genes had 100% nt homology with R. felis URRXWCal2 (Table 2).
Table 1. Demographic characteristics of participants in hospital-based survey, western Kenya, 2008–2010.
| Characteristic | No. (%) febrile, n = 699 | No. (%) afebrile, n = 236 | p value* |
|---|---|---|---|
| Age group, y | <0.001 | ||
| 0–1 | 61 (8.7) | 15 (7.0) | |
| 2–5 | 345 (49.4) | 30 (14.1) | |
| 6–17 | 214 (30.6) | 63 (29.6) | |
| 18–44 | 61 (8.7) | 72 (33.8) | |
| >45 | 18 (2.6) | 33 (15.5) | |
| Missing | 0 | 23 | |
| Sex | <0.001 | ||
| M | 352 (50.6) | 73 (30.4) | |
| F | 344 (49.4) | 163 (69.1) | |
| Missing | 3 | 0 |
*By χ2 test.
Table 2. Genetic sequence analysis results of rickettsial DNA amplified from 17-kDa, ompB, and pRF genes from 21 human specimens, Lwak Mission Hospital, Western Kenya, 2008–2010*.
| DNA no. | 17-kDa gene sequence | ompB gene sequence† | pRF gene sequence | pRFδ gene sequence |
|---|---|---|---|---|
| 1 | + | + | – | – |
| 2 | + | + | + | – |
| 3 | + | + | – | – |
| 4 | + | + | – | – |
| 5 | + | + | – | – |
| 6 | + | + | – | – |
| 7 | + | + | – | – |
| 8 | + | + | – | – |
| 9 | + | – | – | – |
| 10 | + | + | + | – |
| 11 | + | – | – | – |
| 12 | + | – | – | – |
| 13 | + | – | – | – |
| 14 | + | + | – | – |
| 15 | + | + | + | – |
| 16 | + | + | – | – |
| 17 | + | – | – | – |
| 18 | + | + | + | – |
| 19 | + | + | + | – |
| 20 | + | – | + | – |
| 21 | + | + | + | – |
*omp, outer membrane protein; pRf, R. felis plasmid; +, positive; –, negative. †Rickettsia DNA from fleas, dogs, and cats had 93%–99% nt sequence homology with R. felis.
In addition to fever, the most common clinical manifestations among patients with positive PCR results for rickettttsiae were headaches (100%), chills (93.8%), muscle aches (68.8%), and joint pains (68.8%). Rash was reported for 4.4% of rickettsiae-positive patients. Among febrile patients, no statistically significant associations were found between specific signs or symptoms and positive PCR results for rickettsiae (p>0.05). Samples from all febrile patients were Giemsa stained and examined; malaria parasites were detected in 79.2% and 73.4% of samples from patients who had PCR-positive and PCR-negative results for rickettsiae, respectively.
Conclusions
The 2007–2008 serosurvey found prevalence of IgG against rickettsiae to be high. Other countries in Africa have reported similar (28%–58%) seroprevalence (9,10). The finding that prevalence of IgG to SFG rickettsiae increased with age can, in part, be explained by cumulative exposure to the pathogen and lifelong persistence of IgG. The high number of patients seropositive for SFG and TG rickettsiae may be attributed to cross-reactivity between SFG and TG rickettsial antigens (11), although results from studies in animal models show that cross-reactivity between SFG and TG rickettsiae is not consistent (12). Znazen et al. (13) speculate that antibodies against R. felis may be the major cause of cross-reactions to TG-rickettsiae–specific and SFG-rickettsiae–specific antigens.
The identification of R. felis DNA sequences in febrile patients confirms the previous finding of this pathogen among febrile patients in Kenya (5). Our findings are also similar to those from northern Tanzania, where acute rickettsiosis was serologically confirmed for 8% of febrile hospital inpatients (14), and from rural Senegal, where 6% of febrile patients without malaria had positive R. felis test results (15). Our findings suggest that rickettsial infections should be considered in the differential diagnosis of febrile cases in western Kenya and that diagnostic capacity should be established. Clinicians in malaria-endemic areas should consider rickettsial co-infections in diagnostic protocols and treatment of patients with malaria and other febrile illnesses.
Acknowledgments
We thank Immaculate Amadi, Kabura Wamburu, and Sylvia Omulo for their assistance and all the reviewers for their helpful comments.
This research was supported by the US State Department Biosecurity Engagement Program, Wellcome Trust UK (grant no. 081828/B/06/Z), the US Centers for Disease Control and Prevention, and the US Department of Defense Global Emerging Infections Surveillance and Response System Program.
Biographies
Dr Maina is a researcher at the Kenya Medical Research Institute and a PhD student at Jomo Kenyatta University of Agriculture and Technology, Kenya. Her research interest is the epidemiology and diagnosis of emerging zoonotic diseases in multihost systems to identify reservoir hosts.
Dr Maina is a researcher at the Kenya Medical Research Institute and a PhD student at Jomo Kenyatta University of Agriculture and Technology, Kenya, at the time of this study. Her research interests are the epidemiology and diagnosis of emerging zoonotic diseases in multihost systems to identify reservoir hosts.
Footnotes
Suggested citation for this article: Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, et al. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis [serial on the Internet]. 2012 Feb [date cited]. http://dx.doi.org/10.3201/eid1802.111372
References
- 1.Parola P. Rickettsioses in sub-Saharan Africa. Ann N Y Acad Sci. 2006;1078:42–7. 10.1196/annals.1374.005 [DOI] [PubMed] [Google Scholar]
- 2.Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. 2011;17:996–1000. 10.1111/j.1469-0691.2011.03516.x [DOI] [PubMed] [Google Scholar]
- 3.Rutherford JS, Macaluso KR, Smith N, Zaki SR, Paddock SD, Davis J, et al. Fatal spotted fever rickettsiosis, Kenya. Emerg Infect Dis. 2004;10:910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshikawa H, Kimura M, Ogawa M, Rolain J, Raoult D. Laboratory-confirmed Mediterranean spotted fever in a Japanese traveler to Kenya. Am J Trop Med Hyg. 2005;73:1086–9. [PubMed] [Google Scholar]
- 5.Richards AL, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, et al. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16:1081–6. 10.3201/eid1607.091885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macaluso KR, Davis J, Alam U, Korman A, Rutherford JS, Resenberg R, et al. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am J Trop Med Hyg. 2003;68:551–3. [DOI] [PubMed] [Google Scholar]
- 7.Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, Aura B, et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS ONE. 2011;6:e16085. 10.1371/journal.pone.0016085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hyg. 2005;73:1083–5. [PubMed] [Google Scholar]
- 9.Kelly PJ, Mason PR, Mathewman LA, Raoult D. Seroepidemiology of spotted fever group rickettsial infections in humans in Zimbabwe. J Trop Med Hyg. 1991;94:304–9. [PubMed] [Google Scholar]
- 10.Kaabia N, Rolain JM, Khalifa M, Jazia EB, Bahri F, Raoult D, et al. Serologic study of rickettsioses among acute febrile patients in central Tunisia. Ann N Y Acad Sci. 2006;1078:176–9. 10.1196/annals.1374.126 [DOI] [PubMed] [Google Scholar]
- 11.Ormsbee R, Peacock M, Philip R, Casper E, Plorde J, Gabre-Kidan T, et al. Antigenic relationships between the typhus and spotted fever groups of rickettsiae. Am J Epidemiol. 1978;108:53–9. [PubMed] [Google Scholar]
- 12.Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4:e821. 10.1371/journal.pntd.0000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Znazen A, Rolain JM, Hammami N, Hammami A, Jemaa MB, Raoult D. Rickettsia felis infection, Tunisia. Emerg Infect Dis. 2006;12:138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhu M, Nicholson W, Roche A, Kersh G, Fitzpatrick K, Oliver L, et al. Spotted fever group and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. In: Abstracts of the 59th Annual Meeting of the American Society for Tropical Medicine and Hygiene; 2010. Nov 3–7; Atlanta, Georgia, USA. Abstract 1036. [Google Scholar]
- 15.Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, et al. Rickettsia felis–associated uneruptive fever, Senegal. Emerg Infect Dis. 2010;16:1140–2. 10.3201/eid1607.100070 [DOI] [PMC free article] [PubMed] [Google Scholar]