Abstract
Background and Aims
Formation of calcium oxalate crystals is common in the plant kingdom, but biogenic formation of calcium sulfate crystals in plants is rare. We investigated the morphologies and elemental compositions of crystals found in phyllodes and branchlets of Acacia robeorum, a desert shrub of north-western Australia.
Methods
Morphologies of crystals in phyllodes and branchlets of A. robeorum were studied using scanning electron microscopy (SEM), and elemental compositions of the crystals were identified by energy-dispersive X-ray spectroscopy. Distributional patterns of the crystals were studied using optical microscopy together with SEM.
Key Results
According to the elemental compositions, the crystals were classified into three groups: (1) calcium oxalate; (2) calcium sulfate, which is a possible mixture of calcium sulfate and calcium oxalate with calcium sulfate being the major component; and (3) calcium sulfate · magnesium oxalate, presumably mixtures of calcium sulfate, calcium oxalate, magnesium oxalate and silica. The crystals were of various morphologies, including prisms, raphides, styloids, druses, crystal sand, spheres and clusters. Both calcium oxalate and calcium sulfate crystals were observed in almost all tissues, including mesophyll, parenchyma, sclerenchyma (fibre cells), pith, pith ray and cortex; calcium sulfate · magnesium oxalate crystals were only found in mesophyll and parenchyma cells in phyllodes.
Conclusions
The formation of most crystals was biologically induced, as confirmed by studying the crystals formed in the phyllodes from seedlings grown in a glasshouse. The crystals may have functions in removing excess calcium, magnesium and sulfur, protecting the plants against herbivory, and detoxifying aluminium and heavy metals.
Keywords: Acacia robeorum, biomineralization, calcium oxalate, calcium sulfate, crystal sand, druses, Leguminosae, magnesium oxalate, Mimosoideae, prisms, raphides, styloids
INTRODUCTION
Mineral formation in plants is common (Franceschi and Nakata, 2005). In the biomineralization process, calcium is the predominant cation in most organisms, and calcium-bearing minerals comprise about 50 % of known biominerals (Weiner and Dove, 2003). The most abundant minerals formed by plants are silica, calcium carbonate and calcium oxalate (Bouropoulos et al., 2001). Calcium is an essential plant nutrient, which performs many fundamental functions in cellular metabolism, and the divalent cation of calcium is a counter-cation for inorganic and organic anions in the vacuole (Marschner, 1995; White and Broadley, 2003). The calcium concentration of plants varies between 1 and >50 mg g−1 dry mass (DM) depending on the species, growing conditions and plant organ (Hawkesford et al., 2011). Calcium deficiency is rare in nature, but excess soil calcium excludes calcifuge plant communities from calcareous soils (White and Broadley, 2003). Excess calcium is often precipitated in the form of calcium salts such as oxalate, carbonate, sulfate, phosphate, silicate, citrate, tartrate and malate (Franceschi and Horner, 1980; Pritchard et al., 2000; Weiner and Dove, 2003).
Calcium oxalate is the most frequently reported calcium precipitate in higher plant families. Calcium oxalate occurs in most plant tissues and organs such as roots, bark, stems, leaves, flowers, fruits and seeds (Bouropoulos et al., 2001), and it has been observed as an intra- and extracellular deposit (Franceschi and Nakata, 2005). In many plant species, considerable resources of carbon and calcium are invested in crystal formation. Plants accumulate crystals in the range of 3–80 % of their dry weight, with up to 90 % of the total calcium in a plant being present in the form of calcium oxalate (Franceschi and Nakata, 2005). The roles of calcium oxalate are largely unknown and probably variable (Prychid and Rudall, 1999). Based on the prevalence of crystals, their spatial distribution, and the variety of crystal shapes and sizes, a number of roles for crystal formation have been proposed, i.e. roles in cellular ion balance (sodium and/or potassium), in plant defence against herbivory, in tissue rigidity and support, in detoxification of oxalic acid or aluminium and/or heavy metals, in light gathering and reflection, and in bulk calcium regulation (Zindler-Frank, 1976; Franceschi and Horner, 1980; Nakata, 2003; Franceschi and Nakata, 2005).
The formation of calcium sulfate crystals is rare in plants, and there are only a few reports on calcium sulfate formation in pith (Arnott and Pautard, 1970), in ray cells of the secondary xylem (Miller, 1978), in mesophyll vacuoles and secretions of salt glands (Storey and Thomson, 1994), on the surfaces of conifer needles as a symptom of acid rain treatments (Huttunen et al., 1990; Fink, 1991), and in substomatal cavities of longleaf pine (Pritchard et al., 2000). Very little is known about the role of calcium sulfate crystals in plant function.
Here, we investigated the occurrence of calcium crystals in Acacia robeorum, a desert shrub from north-western Australia. Acacia is a large and diverse genus that plays an important global role in nutrient, water and carbon cycling, especially in dry ecosystems. One key feature of most Australian Acacia species is their phyllodes, a type of foliar organ that is morphologically distinct from an ordinary leaf (Boke, 1940). A. robeorum is a hardy species that tolerates growth in a range of soil types and may hold potential for growth on calcium-rich substrates associated with mine site restoration. In a previous study, a very high leaf mass per unit leaf area (LMA) was found for A. robeorum (He et al., 2011), and concentrations of calcium, magnesium and sulfur in the phyllodes [the functional equivalent of leaves for A. robeorum (Fig. 1)] were considerably higher than the ‘sufficient concentrations for adequate growth’ (Kirkby, 2011). It was hypothesized that the high LMA and high calcium, magnesium and sulfur concentrations were due to the formation of crystals containing these elements, and this hypothesis is tested here using microscopic techniques. In this study, morphologies and elemental compositions of the crystals are presented, possible causes of the formation of the crystals are discussed and potential functions of the crystals are proposed.
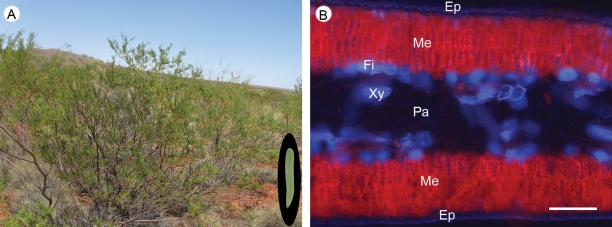
Fig. 1.
Phyllodes are the functional equivalent of leaves for Acacia robeorum. (A) A shrub of A. robeorum in its natural habitat, a phyllode of the plant is shown in the oval inset at the bottom right. (B) UV autofluorescence of a cross-section of the phyllode showing the presence of chlorophyll a (red); xylem and phloem fibre cells appear in blue. Cells with chlorophyll are referred to as mesophyll cells, and cells without chlorophyll (except epidermis and vascular tissues) are referred to as parenchyma cells. Abbreviations: Ep, epidermis; Fi, fibre; Me, mesophyll; Pa, parenchyma; Xy, xylem. Scale bar in B = 100 µm.
MATERIALS AND METHODS
Plant materials
Plants of Acacia robeorum Maslin (Leguminosae: Mimosoideae) are diffuse, spreading, openly branched, multi-stemmed shrubs, 2–3 m tall, phyllodes short and narrow [15–25(–35) × (1–)2–3(–4) mm] (Maslin and van Leeuwen, 2008). Study samples were collected from the plants' natural habitat, a rocky sand plain in the Great Sandy Desert in north-western Australia (21 °83′S, 122 °16′E). Six mature plants of similar size were selected, and ten healthy branches were cut from the centre of the crown of each plant and immediately placed in self-sealed plastic bags. The fresh collected samples were placed in a coolbox half-filled with ice and then transported to the laboratory.
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS)
Fresh, healthy and intact mature phyllodes (the youngest fully expanded phyllodes without any sign of damage due to insect bites or disease) were fractured by hand, and the branchlets from which the phyllodes were sampled were cut with a double-edged razor blade. Both were fixed in formalin/acetic acid/alcohol [FAA; 5 % (v/v) formalin, 5 % (v/v) acetic acid and 70 % (v/v) ethanol]. Due to the effects of acetic acid, calcium salts of phosphate and carbonate (if there were any) were removed (Berg, 1994; Lersten and Horner, 2011). The FAA-fixed samples were dehydrated in an ethanol series (70, 95 and 100 % dry ethanol), critical point-dried, mounted on SEM aluminium stubs with double-sided carbon tape and coated with gold. Images were captured with a Zeiss 1555 field-emission variable-pressure scanning electron microscope (Carl Zeiss, Oberkochen, Germany) at 5–10 kV. Crystals in SEM images were measured using Image J to obtain their sizes. Qualitative X-ray microanalyses were performed on certain crystals using an Si (LI) EDS machine (Oxford Instruments, Oxford, UK) on the same variable-pressure microscope at 20 kV.
Optical microscopy
Fresh, healthy and intact mature phyllodes were cut into small segments (about 3–5 mm long) using a double-edged razor blade and fixed in 2·5 % (v/v) glutaraldehyde in phosphate-buffered saline at pH 7·4, dehydrated in an ethanol series (50, 70, 90, 100 %, v/v), then infiltrated and embedded with glycol methacrylate (O'Brien and McCully, 1981). Cross-sections of embedded samples were cut at a thickness of 2 µm with dry glass knives, and sections were stained with 0·05 % (w/v) toluidine blue O in benzoate buffer at pH 4·4. The stained sections were photographed under transmitted light with a Zeiss Axioplan Microscope equipped with a Zeiss Axiocam digital camera (Carl Zeiss). Similar areas from unstained adjacent sections were imaged with an Olympus BX43 upright microscope (Olympus Corporation, Tokyo, Japan) between crossed polarizers, which cause the birefringent crystals to appear as very bright objects against the darker tissue background (Ilarslan et al., 2001). Xylem and fibrous cells also become birefringent due to their rich cellulose deposits (Bosca et al., 2006).
Analyses of phyllode element concentrations
Healthy and intact mature phyllodes were taken from each sampled branch and washed in deionized water to remove dust. Phyllodes from all ten branches of each plant were pooled and then oven-dried at 70 °C for 72 h. Dried samples were finely ground using a ball mill, digested in hot concentrated HNO3/HClO4 (3 : 1) (Zarcinas et al., 1987), and analysed for phosphorus, sulfur, sodium, magnesium, potassium, calcium, aluminium, iron, manganese, copper, zinc and molybdenum concentrations using an inductively coupled plasma atomic emission spectrometer (PerkinElmer, Inc., Waltham MA, USA). The ground samples were also used for total carbon and nitrogen analyses using a Vario MACRO Elemental CN Analyser (Elementar Analysensysteme GmbH, Hanau, Germany).
Data analysis
A one-sample t-test (α = 0·05) was performed for phyllode element concentrations using the SPSS 19·0 software package (SPSS Inc., Chicago, IL, USA).
RESULTS
Morphologies of crystals
Crystals of various morphologies formed in phyllodes and branchlets of A. robeorum, and sizes of the crystals varied greatly (Figs 2 and 3, Table 1). In phyllodes, we observed prismatic crystals (Fig. 2A, B), bundles of raphides with similar orientations (Fig. 2C, D), raphides with different orientations developing from the same nucleation site (Fig. 2E), solitary styloids (Fig. 2F), styloids as nucleation sites with a few or numerous raphides developing on them (Fig. 2G, H), styloid druses (Fig. 2I), and bundles of crystals which are intermediate forms of styloid and raphide (Fig. 2J). There were also platy aggregation clusters (Fig. 2K, L), tabular crystal druses (Fig. 2M), blocky and tabular crystal druses (Fig. 2N), platy crystal druses (Fig. 2O), crystal sand (Fig. 2P), tetrahedral crystal druses (Fig. 2Q) and spherical crystals (Fig. 2R).
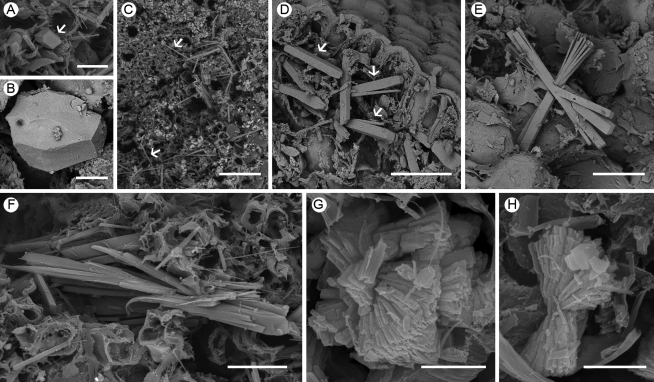
Fig. 2.
SEM images of various crystals in a phyllode of Acacia robeorum. (A) Several prismatic crystals (arrows) associated with phloem fibre cells (arrowheads). (B) A single hexagonal prismatic crystal. (C) Bundles of raphides with similar orientations (arrows) in the mesophyll. (D) A bundle of raphides with similar orientations (arrow) in the parenchyma. (E) Raphides with different orientations developing from the same nucleation site in the parenchyma. (F) A solitary styloid in the mesophyll. (G) A styloid with two raphides in the parenchyma. (H) A styloid with numerous raphides in the parenchyma. (I) A styloid druse in a parenchyma cell. (J) A bundle of crystals which are intermediate forms of styloid and raphide in the mesophyll. (K) A platy aggregation cluster which occupied almost a whole mesophyll cell. (L) A platy aggregation cluster in a mesophyll cell. (M) A tabular crystal druse in a parenchyma cell. (N) A blocky and tabular crystal druse in a mesophyll cell. (O) A platy crystal druse in a parenchyma cell. (P) Crystal sand in a mesophyll cell. (Q) A tetrahedral crystal druse in a parenchyma cell. (R) A spherical crystal in a parenchyma cell. Scale bars: (A, D, F, G) = 20 µm; (B, I, K, P, Q) = 10 µm; (C, E) = 50 µm; (H) = 25 µm; (J, L–O) = 5 µm; (R) = 2 µm.
Fig. 3.
SEM images of various crystals in a branchlet of Acacia robeorum. (A) A prismatic crystal (arrow). (B) A pseudo-prismatic block. (C) Raphides (arrows). (D) A few solitary styloids (arrows). (E) A cluster of raphides and styloids. (F) A bundle of crystals which are intermediate forms of styloid and raphide. (G) Bladed aggregation clusters. (H) A bladed aggregation cluster. Scale bars: (A, D, E, F) = 20 µm; (B) = 5 µm; (C) = 50 µm; (G, H) = 2 µm.
Table 1.
Elemental compositions, shapes, sizes, locations and abundance of crystals formed in phyllodes and branchlets of Acacia robeorum
| Elemental composition (type) | Shape | Size | Location | Abundance |
|---|---|---|---|---|
| Ca C O (1) | Prisms (Figs 2A, B, 3A, B) | 5–10 µm side length | Phloem fibre cells and cells associated with phloem fibre cells in phyllodes; pith, pith ray cells, xylem fibre cells and cortical cells associated with phloem fibre cells in branchlets | Present in >50 % of the cells, only one per cell |
| Ca C O (1) | Raphide bundles (Fig. 2C, D) | 25–29·5 µm wide, 64·5–181·5 µm long | Mesophyll and parenchyma cells in phyllodes | Varied greatly in different parts of a phyllode, ≥1 per cell |
| Ca C O (1) | Raphide clusters on styloids (Fig. 2E, G, H) | Not measured | Parenchyma cells in phyllodes | Varied greatly in different parts of a phyllode, only one per cell |
| Ca C O (1) | Solitary raphides (Fig. 3C) | Not measured | Pith, pith ray cells, xylem fibre cells and cortical cells in branchlets | Present in <50 % of the cells, ≥1 per cell |
| Ca C O (1) | Solitary styloids (Figs 2F, 3D) | 6–112·5 µm long | Mesophyll and parenchyma cells in phyllodes; pith, pith ray cells, xylem fibre cells and cortical cells in branchlets | Present in <50 % of the cells, only one per cell |
| Ca C O (1) | Styloid druses (Fig. 2I) | 12–25 µm diameter | Mesophyll and parenchyma cells in phyllodes; cortical cells in branchlets | Present in <50 % of the cells, ≥1 per cell |
| Ca C O (1) | Bundles or clusters of crystals which are intermediate forms of styloids and raphides (Figs 2J, 3E, F) | Not measured | Mesophyll and parenchyma cells in phyllodes; pith, pith ray cells, xylem fibre cells and cortical cells in branchlets | Sporadic |
| Ca S C O (2) | Platy aggregation clusters (Fig. 2K, L) | 10–25 µm wide, 20–100 µm long | Mesophyll cells in phyllodes | Present in >50 % of the cells, only one per cell |
| Ca S C O (2) | Tabular crystal druses (Fig. 2M) | 20 µm diameter | Parenchyma cells in phyllodes | Sporadic, only one per cell |
| Ca S C O (2) | Blocky and tabular crystal druses (Fig. 2N) | 2·5–50 µm diameter | Mesophyll and parenchyma cells in phyllodes | Present in >50 % of the cells, ≥1 per cell |
| Ca S C O (2) | Platy crystal druses (Fig. 2O) | 12–16·5 µm diameter | Parenchyma cells in phyllodes | Sporadic, >1 per cell |
| Ca S C O (2) | Crystal sand (Fig. 2P) | Not measured | Mesophyll and parenchyma cells in phyllodes; pith in branchlets | Present in >50 % of the cells, numerous per cell |
| Ca S C O (2) | Bladed aggregation clusters (Fig. 3G, H) | 3–4 µm wide, 4–5 µm long | Xylem fibre cells | Present in >50 % of the cells, ≥1 per cell |
| Ca S C O (2) | Bladed aggregation druses (not shown in figures) | 2–5 µm diameter | Pith and cortical cells in branchlets | Sporadic, >1 per cell |
| Ca K S C O (2) | Tetrahedral crystal druses (Fig. 2Q) | 16–20 µm diameter | Mesophyll and parenchyma cells in phyllodes | Sporadic, only one per cell |
| Ca Mg K Si S C O (3) | Spheres (Fig. 2R) | 3·5–16 µm diameter | Mesophyll and parenchyma cells in phyllodes | Present in almost every cell, ≥ 1 per cell |
Data are summarized from SEM images of five cross-sections of a phyllode and three cross-sections of a branchlet, at least three EDS spectra for each type of crystal, together with optical microscope images of six cross-sections of a phyllode.
In branchlets, the crystals observed included: prismatic crystals (Fig. 3A, B), raphides (Fig. 3C), solitary styloids (Fig. 3D), clusters of raphides and styloids (Fig. 3E), bundles of crystals which are intermediate forms of styloids and raphides (Fig. 3F) and bladed aggregation clusters (Fig. 3G, H). Styloid druses, bladed aggregation druses and crystal sand were also observed in branchlets.
Elemental compositions of crystals
Results of qualitative X-ray microanalyses showed that crystals formed in phyllodes and branchlets of A. robeorum were of various elemental compositions (Fig. 4, Table 1). Based on the elemental compositions, the crystals were classified into three major groups.
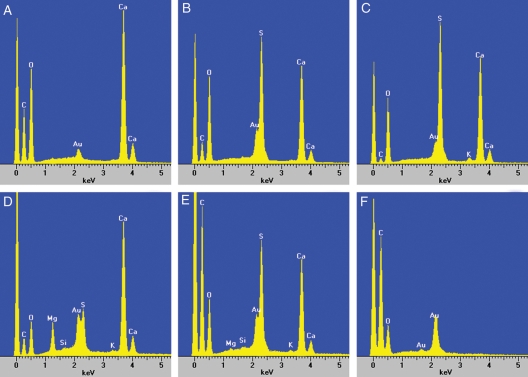
Fig. 4.
EDS spectra of crystals and different areas of the cross-sections of Acacia robeorum phyllodes. The large peak to the left of the carbon (C) peak in each spectrum is background noise, and the peak of gold (Au) is from the gold used to coat the samples. (A) Typical spectrum showing large calcium (Ca), carbon and oxygen (O) peaks from EDS of crystals in Fig. 2A–J and Fig. 3A–F. (B) Typical spectrum showing large calcium, sulfur (S) and oxygen peaks and a small carbon peak from EDS of crystals in Fig. 2K–P and Fig. 3G–H. (C) Typical spectrum showing large calcium, sulfur and oxygen peaks and small carbon and potassium (K) peaks from EDS of crystals in Fig. 2Q. (D) Typical spectrum showing large calcium, magnesium (Mg), sulfur and oxygen peaks and small carbon, silicon (Si) and potassium peaks from EDS of spherical crystal in Fig. 2R. (E) Typical spectrum showing large calcium, carbon, sulfur and oxygen peaks and small magnesium, silicon and potassium peaks from EDS of the whole or part of the cross-sections of a phyllode. (F) Typical spectrum showing only a large carbon peak and a small oxygen peak from EDS of cells without crystals.
The first group (1) of crystals showed large calcium, carbon and oxygen peaks (Fig. 4A), suggesting these crystals were calcium oxalate; this group included prismatic crystals, raphides (solitary, bundles and clusters) and styloids (solitary, bundles, druses and clusters) (Figs 2A–J, 3A–F).
The second group (2) of crystals showed obvious calcium, sulfur and oxygen peaks and a small carbon peak (Fig. 4B), and sometimes there was a small potassium peak (Fig. 4C), indicating these crystals were possible complex compounds of which calcium sulfate was the major component and calcium oxalate was the minor component (crystals of this group are referred to hereafter as calcium sulfate). Crystals of this group included platy aggregation clusters, tabular crystal druses, blocky and tabular crystal druses, platy crystal druses, bladed aggregation clusters, bladed aggregation druses, crystal sand and tetrahedral crystal druses (Figs 2K–Q, 3G, H).
The third group (3) of crystals showed large calcium, magnesium, sulfur and oxygen peaks and small potassium, carbon and silicon peaks (Fig. 4D), indicating these crystals were compounds that were more complex than the second group. These crystals were possibly mixtures of calcium sulfate, calcium oxalate, magnesium oxalate and silica; crystals belonging to this group are referred to as calcium sulfate · magnesium oxalate hereafter, and they were all spherical (Fig. 2R).
EDS spectra from the whole or part of the cross-sections of a phyllode rather than from a single crystal showed large calcium, carbon, sulfur and oxygen peaks and small magnesium, potassium and silicon peaks (Fig. 4E), whereas cells without crystals showed only a large carbon peak and a small oxygen peak (Fig. 4F), confirming calcium, magnesium, potassium, sulfur and silicon peaks were from the crystals, rather than from tissues that did not form crystals.
Locations and abundance of crystals
As listed in Table 1, crystals of all three major groups were of great abundance in phyllodes, while calcium oxalate and calcium sulfate were also found in great abundance in branchlets. Both calcium oxalate and calcium sulfate were observed in almost all tissues in phyllodes and branchlets, including mesophyll, parenchyma, sclerenchyma (fibre cells), pith, pith ray and cortex (Figs 2, 3, 5). Locations of some types of crystals were tissue-specific: for example, prismatic crystals were found only in phloem fibre cells or in cells associated with phloem fibre cells in phyllodes (Fig. 5B, C, F), and they were found in pith, pith ray cells and cortical cells associated with phloem fibre cells in branchlets; calcium sulfate · magnesium oxalate crystals were present only in mesophyll and parenchyma cells in phyllodes; platy aggregation clusters were found only in mesophyll cells; and tabular crystal druses and platy crystal druses occurred only in parenchyma cells in phyllodes.
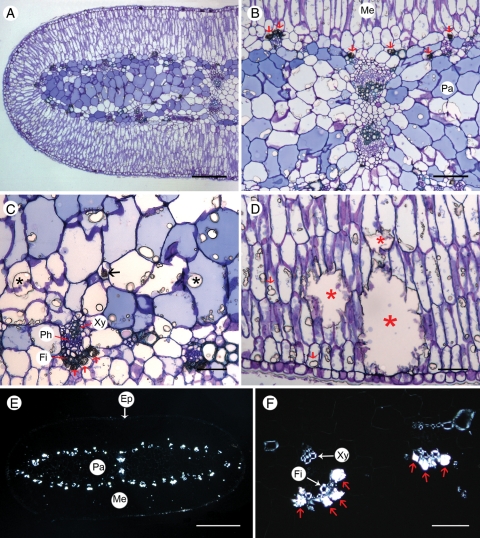
Fig. 5.
Optical microscope images of cross-sections of an Acacia robeorum phyllode. (A–D) Transmitted light; (E–F) polarized light. (A) A view of half of a cross-section stained with toluidine blue. (B) Dark-stained prismatic calcium oxalate crystals (red arrows) in cells associated with phloem fibre cells and unstained calcium sulfate crystals in mesophyll and parenchyma cells. (C) A few prismatic calcium oxalate crystals (red arrows) associated with phloem fibre cells, a calcium oxalate druse (black arrow) and many calcium sulfate crystals (black asterisks) in parenchyma cells. (D) Calcium sulfate crystals in mesophyll cells (red arrows) and occupying more than one cell (red asterisks). (E) A polarized view of a whole unstained cross-section showing birefringent calcium oxalate crystals along vascular tissues and non-birefringent cells in the epidermis, parenchyma and mesophyll. (F) A polarized view showing birefringent xylem, phloem fibre cells and calcium oxalate crystals (red arrows) associated with phloem fibre cells. Abbreviations: Ep, epidermis; Fi, fibre; Me, mesophyll; Pa, parenchyma; Ph, phloem; Xy, xylem. Scale bars: (A) = 200 µm; (B) = 100 µm; (C, D, F) = 50 µm; (E) = 500 µm.
Phyllode elemental concentrations
As listed in Table 2, calcium, magnesium and sulfur concentrations of A. robeorum were considerably higher than the average values of three other Acacia species which grow at nearby sites in the Great Sandy Desert (H. He et al., unpubl. res.). The sulfur concentration was more than 40 times higher than that sufficient for plant growth, while the calcium concentration was about 15 times as high and the magnesium concentration was four times higher than that for sufficient growth (Kirkby, 2011).
Table 2.
Phyllode element concentrations
| Element | Acacia robeorum* | Other Acacia spp. average† | Level sufficient for growth‡ |
|---|---|---|---|
| C (mg g−1) | 313 ± 3 | 494 | Not applicable |
| N (mg g−1) | 11·7 ± 0·3 | 15·9 | 15 |
| P (mg g−1) | 0·44 ± 0·02 | 0·33 | 2 |
| K (mg g−1) | 4·3 ± 0·3 | 4·4 | 10 |
| S (mg g−1) | 42·2 ± 1·2 | 1·6 | 1 |
| Ca (mg g−1) | 72·0 ± 3·3 | 10·4 | 5 |
| Mg (mg g−1) | 10·3 ± 0·4 | 3·3 | 2 |
| Na (mg g−1) | 0·39 ± 0·08 | 1·04 | 0·01 |
| Al (μg g−1) | 67·1 ± 16·3 | 145·5 | Not applicable |
| Fe (μg g−1) | 178 ± 22 | 136 | 100 |
| Mn (μg g−1) | 160 ± 19 | 107 | 50 |
| Cu (μg g−1) | 3·7 ± 1·0 | 5·7 | 6 |
| Zn (μg g−1) | 63·0 ± 14·4 | 18·5 | 20 |
| Mo (μg g−1) | 0·77 ± 0·09 | 0·56 | 0·1 |
* All values for A. robeorum are presented as means ± s.e. (n = 6).
† Average values of three other Acacia species which grow at nearby sites in the Great Sandy Desert (He et al., 2011; H. He et al., unpubl. res.).
‡ Average concentrations of mineral elements in plant shoot dry matter sufficient for adequate growth (Kirkby, 2011).
DISCUSSION
The presence of calcium oxalate in plants is relatively common (Franceschi and Nakata, 2005); however, biogenic formation of calcium sulfate in plants is rare (Pritchard et al., 2000). The present microscopic techniques showed that both calcium oxalate and calcium sulfate crystals were present in great abundance in phyllodes and branchlets of A. robeorum, and elemental analyses revealed very high calcium, magnesium and sulfur concentrations in phyllodes. Possible causes and potential functions are proposed for the formation of the crystals.
Elemental compositions of the crystals and phyllode elemental concentrations
Crystals in plants have been identified as calcium oxalate using EDS in many studies (Horner and Wagner, 1992; Berg, 1994; Braissant et al., 2004; Lersten and Horner, 2011). In contrast, only a few studies have reported calcium sulfate (Huttunen et al., 1990; Storey and Thomson, 1994; Pritchard et al., 2000) and magnesium oxalate (Rao and Tewari, 1989). In the present study, elements such as calcium, magnesium, potassium, carbon, silicon, sulfur and oxygen were detected in crystals formed in phyllodes and branchlets of A. robeorum, and the crystals were classified into three major groups according to their elemental compositions: (1) calcium oxalate, (2) calcium sulfate and (3) calcium sulfate · magnesium oxalate. Determination of atomic percentages to determine the stoichiometry of the crystals was not possible as the nature of the samples does not allow quantitative X-ray analysis. Due to the birefringent character of crystals, polarizing microscopes are widely used for studying the presence and distributional patterns of crystals in plants (Lersten and Horner, 2011). However, in the present study, only calcium oxalate crystals were birefringent, whereas the other two groups of crystals were not (Fig. 5), and we speculate that the orientations of these crystals may affect the degree of their birefringence.
The results of the present study provide strong support that more than one crystal habit, based on chemical composition, may occur in the same plant, and that crystals may not necessarily be calcium oxalate or calcium carbonate (Horner and Wagner, 1992). It is clear that the high calcium, magnesium and sulfur concentrations were due to the presence of abundant crystals with these elements in phyllodes. The phyllode carbon concentration of A. robeorum (313 mg g−1 DM) was significantly lower than the average of three other Acacia species (494 mg g−1 DM, H. He et al., unpubl. res.) which grow at nearby sites in the Great Sandy Desert. The latter three species formed much fewer calcium oxalate and calcium sulfate crystals than A. robeorum, and no magnesium oxalate crystals were observed in the three species (H. He et al., unpubl. res.). Compared with A. robeorum, the three species have significantly lower LMA and higher phyllode nitrogen concentration (He et al., 2011). Concentrations of some elements, for example, nitrogen, phosphorus and potassium, might be low in phyllodes of A. robeorum, in part because of the presence of the crystals.
Possible causes of the formation of the crystals
Crystals in higher plant families may occur in a single tissue or in multiple tissues of an individual species. Distribution of crystals within the plant is highly variable among species and there are no generalities about the locations where crystals can be formed (Franceschi and Nakata, 2005). Crystals are commonly found in members of all three subfamilies of the family Leguminosae, and certain members of the family contain leaf crystals which have distinct shapes and very specific patterns of distribution (Horner and Zindler-Frank, 1982; Zindler-Frank, 1987). It is generally accepted that the morphologies and precise locations of crystals are under strict genetic control, and that a particular species will form only a certain crystal type or subset of crystal morphologies (Franceschi and Nakata, 2005).
A. robeorum is a striking example of a species that forms numerous crystals with different habits in cells within close proximity to each other (Bharadwaj, 1988; Horner and Wagner, 1992). Indeed, calcium oxalate and calcium sulfate of different morphologies coexist in phyllodes and branchlets of A. robeorum. For example, calcium sulfate (platy aggregation clusters and druses) and calcium sulfate · magnesium oxalate crystals were found in close contact with each other within the same mesophyll and parenchyma cells in phyllodes. Similarly, calcium oxalate raphides and calcium sulfate crystal sand were also observed within the same mesophyll and parenchyma cells. Locations of certain types of crystals were tissue-specific, while others were not. In addition, the abundance of certain types of crystals, e.g. raphides, from different parts of a phyllode varied considerably. Sizes of some crystals in the mesophyll and parenchyma were much larger than in other surrounding cells, possibly because of considerable expansion of the idioblasts during crystal deposition (Franceschi, 1989). Some of the crystals (Figs 2E, H, I, K, L, 3E–H) could be extreme variations of a druse with many crystals developing from a common nucleation site (Horner et al., 1981). Based on SEM images, some crystals were speculated to occur extracellularly in phyllodes; however, this could not be confirmed by optical microscopy. Transmission electron microscopy was not performed.
When A. robeorum seedlings were grown in soils with different levels of nutrients in a glasshouse, only calcium oxalate prismatic crystals and spherical crystals occurred abundantly in phyllodes; the number of calcium sulfate crystals in phyllodes of these plants was much less than that found in the phyllodes collected from the plants' natural habitat, styloids occurred sporadically and no raphides were observed (H. He et al., unpubl. res.). This indicates that the formation of most of the calcium oxalate and calcium sulfate crystals was not constitutive in A. robeorum, but was biologically induced; that is, the secondary precipitation of minerals occurs as a result of interactions between biological activity and the environment (Weiner and Dove, 2003). The subsoil in the plants' natural habitat is relatively rich in calcium, magnesium and sulfur, in contrast to those of three other Acacia species (Table 3), and it is likely that A. robeorum takes up these elements in excess of its requirement, rather than reducing their uptake by roots.
Table 3.
Plant-available nutrient concentrations of the soil*
| Element (μg g−1) | Acacia robeorum† | Other Acacia spp. average‡ |
|---|---|---|
| P | 5·0 | 2·5 |
| K | 70·5 | 50·5 |
| S | 13·5 | 2·6 |
| Ca | 385·0 | 172·3 |
| Mg | 79·5 | 50·8 |
| Na | 1·0 | 3·0 |
| Al | 280·0 | 193·3 |
| Fe | 23·0 | 17·0 |
| Mn | 170·0 | 63·5 |
| Cu | 2·9 | 0·3 |
| Zn | 0·2 | 0·2 |
| Mo | 0·02 | 0·01 |
* Plant-available nutrient concentrations of the first 10 cm topsoil were obtained using a Mehlich No. 3 extraction.
† All values for the soil in the natural habitat of A. robeorum are presented as means (n = 2, and each sample was a bulk sample of soil collected under the crowns of three shrubs).
‡ Average values of the soil in the natural habitats of three other Acacia species which grow at nearby sites in the Great Sandy Desert.
Possible functions of the crystals
Calcium oxalate production is considered necessary for regulating bulk calcium levels in plants that grow in environments where soluble calcium is relatively abundant and exclusion of entry in the root zone is limited (Franceschi, 1989; Volk et al., 2002). For A. robeorum, the crystals probably play an important role in removing excess calcium, magnesium and sulfur. The shape, size, placement and sheer number of crystals may also prevent herbivory by large animals as well as insects (Berg, 1994; Finley, 1999; Hudgins et al., 2003). No aluminium or heavy metals were incorporated into any of the three major groups of crystals formed in phyllodes and branchlets of A. robeorum, but aluminium, iron, mangnese and titanium were incorporated into some calcium oxalate crystals formed on the phyllode surface (data not shown). In addition, we found that aluminium, iron, manganese, copper, zinc, titanium and vanadium were incorporated into calcium oxalate and calcium sulfate crystals in phyllodes of A. robeorum seedlings grown in a glasshouse (H. He et al., unpubl. res.). This indicates a potential role of the crystals in detoxification of aluminium and heavy metals, as reported previously (Franceschi and Schueren, 1986; Mazen, 2004).
Conclusions
Both calcium oxalate and calcium sulfate crystals were found in almost all studied tissues in phyllodes and branchlets collected from the natural habitat of A. robeorum, including mesophyll, parenchyma, sclerenchyma (fibre cells), pith, pith ray and cortex, and they occurred in great abundance. The crystals were of various morphologies, including prisms, raphides, styloids, druses, crystal sand and clusters. Calcium sulfate · magnesium oxalate crystals, which are possibly mixtures of calcium sulfate, calcium oxalate, magnesium oxalate and silica, were also abundant in mesophyll and parenchyma cells in phyllodes. Due to presence of the crystals, concentrations of calcium, magnesium and sulfur were very high in A. robeorum phyllodes. Formation of most of the crystals was biologically induced, but the exact factors that induce formation of the crystals require further investigation.
ACKNOWLEDGEMENTS
This work was supported by the Australian Research Council, Mineral and Energy Research Institute of Western Australia, Newcrest Mining Ltd (Telfer Gold Mine), and the School of Plant Biology. The University of Western Australia, China Scholarship Council and The University of Western Australia are acknowledged for providing a scholarship to Honghua He. We acknowledge the facilities, scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by The University, and State and Commonwealth Governments. We thank Chris Bray who provided the Olympus BX43 microscope and Eleanor Hoy who provided enormous help in sample collection and transport.
LITERATURE CITED
- Arnott H, Pautard F. Calcification in plants. In: Schraer H, editor. Biological calcification: cellular and molecular aspects. Amsterdam: North-Holland Publishing Co; 1970. pp. 375–446. [Google Scholar]
- Berg RH. A calcium oxalate-secreting tissue in branchlets of the Casuarinaceae. Protoplasma. 1994;183:29–36. [Google Scholar]
- Bharadwaj K. SEM studies and chemical nature of crystals of 4 plant species. Biological Memoirs. 1988;14:117–128. [Google Scholar]
- Boke NH. Histogenesis and morphology of the phyllode in certain species of Acacia. American Journal of Botany. 1940;27:73–90. [Google Scholar]
- Bosca S, Barton CJ, Taylor NG, et al. Interactions between MUR10/CesA7-dependent secondary cellulose biosynthesis and primary cell wall structure. Plant Physiology. 2006;142:1353–1363. doi: 10.1104/pp.106.087700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouropoulos N, Weiner S, Addadi L. Calcium oxalate crystals in tomato and tobacco plants: morphology and in vitro interactions of crystal-associated macromolecules. Chemistry – a European Journal. 2001;7:1881–1888. doi: 10.1002/1521-3765(20010504)7:9<1881::aid-chem1881>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Braissant O, Cailleau G, Aragno M, Verrecchia EP. Biologically induced mineralization in the tree Milicia excelsa (Moraceae): its causes and consequences to the environment. Geobiology. 2004;2:59–66. [Google Scholar]
- Fink S. Unusual patterns in the distribution of calcium oxalate in spruce needles and their possible relationships to the impact of pollutants. New Phytologist. 1991;119:41–51. doi: 10.1111/j.1469-8137.1991.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Finley DS. Patterns of calcium oxalate crystals in young tropical leaves: a possible role as an anti-herbivory defense. Revista De Biologia Tropical. 1999;47:27–31. [Google Scholar]
- Franceschi VR. Calcium oxalate formation is a rapid and reversible process in Lemna minor L. Protoplasma. 1989;148:130–137. [Google Scholar]
- Franceschi VR, Horner HT., Jr Calcium oxalate crystals in plants. Botanical Review. 1980;46:361–427. [Google Scholar]
- Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annual Review of Plant Biology. 2005;56:41–71. doi: 10.1146/annurev.arplant.56.032604.144106. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Schueren AM. Incorporation of strontium into plant calcium oxalate crystals. Protoplasma. 1986;130:199–205. [Google Scholar]
- Hawkesford M, Horst W, Kichey T, et al. Functions of macronutrients. In: Marschner P, editor. Marschner's Mineral nutrition of higher plants. 3rd edn. London: Academic Press; 2011. pp. 135–178. [Google Scholar]
- He H, Bleby TM, Veneklaas EJ, Lambers H. Dinitrogen-fixing Acacia species from phosphorus-impoverished soils resorb leaf phosphorus efficiently. Plant, Cell and Environment. 2011;34:2060–2070. doi: 10.1111/j.1365-3040.2011.02403.x. [DOI] [PubMed] [Google Scholar]
- Horner HT, Wagner BL. Association of four different calcium crystals in the anther connective tissue and hypodermal stomium of Capsicum annuum (Solanaceae) during microsporogenesis. American Journal of Botany. 1992;79:531–541. [Google Scholar]
- Horner HT, Zindler-Frank E. Calcium oxalate crystals and crystal cells in the leaves of Rhynchosia caribaea (Leguminosae: Papilionoideae) Protoplasma. 1982;111:11–18. [Google Scholar]
- Horner HT, Kausch AP, Wagner BL. Growth and change in shape of raphide and druse calcium oxalate crystals as a function of intracellular development in Typha angustifolia L. (Typhaceae) and Capsicum annum L. (Solanaceae) Scanning Electron Microscopy. 1981;Part 3:251–262. [Google Scholar]
- Hudgins JW, Krekling T, Franceschi VR. Distribution of calcium oxalate crystals in the secondary phloem of conifers: a constitutive defense mechanism? New Phytologist. 2003;159:677–690. doi: 10.1046/j.1469-8137.2003.00839.x. [DOI] [PubMed] [Google Scholar]
- Huttunen S, Turunen M, Reinikainen J. Scattered CaSO4-crystallites on needle surfaces after simulated acid rain as an indicator of nutrient leaching. Water, Air, & Soil Pollution. 1990;54:169–173. [Google Scholar]
- Ilarslan H, Palmer RG, Horner HT. Calcium oxalate crystals in developing seeds of soybean. Annals of Botany. 2001;88:243–257. [Google Scholar]
- Kirkby E. Introduction, definition and classification of nutrients. In: Marschner P, editor. Marschner's Mineral nutrition of higher plants. 3rd edn. London: Academic Press; 2011. pp. 3–5. [Google Scholar]
- Lersten NR, Horner HT. Unique calcium oxalate “duplex” and “concretion” idioblasts in leaves of tribe Naucleeae (Rubiaceae) American Journal of Botany. 2011;98:1–11. doi: 10.3732/ajb.1000247. [DOI] [PubMed] [Google Scholar]
- Marschner H, editor. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Maslin BR, van Leeuwen S. New taxa of Acacia (Leguminosae: Mimosoideae) and notes on other species from the Pilbara and adjacent desert regions of Western Australia. Nuytsia. 2008;18:139–188. [Google Scholar]
- Mazen A. Calcium oxalate deposits in leaves of Corchorus olitotius as related to accumulation of toxic metals. Russian Journal of Plant Physiology. 2004;51:281–285. [Google Scholar]
- Miller R. Potassium calcium sulfate crystals in the secondary xylem of Capparis. International Association of Wood Anatomists Bulletin. 1978;2–3:50. [Google Scholar]
- Nakata PA. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science. 2003;164:901–909. [Google Scholar]
- O'Brien TP, McCully ME. The study of plant structure: principles and selected methods. Melbourne: Termarcarphi Pty. Ltd; 1981. [Google Scholar]
- Pritchard SG, Prior SA, Rogers HH, Peterson CM. Calcium sulfate deposits associated with needle substomatal cavities of container-grown longleaf pine (Pinus palustris) seedlings. International Journal of Plant Sciences. 2000;161:917–923. [Google Scholar]
- Prychid CJ, Rudall PJ. Calcium oxalate crystals in monocotyledons: a review of their structure and systematics. Annals of Botany. 1999;84:725–739. [Google Scholar]
- Rao DV, Tewari JP. Occurrence of magnesium oxalate crystals on lesions incited by Mycena citricolor on coffee. Phytopathology. 1989;79:783–787. [Google Scholar]
- Storey R, Thomson WW. An X-ray microanalysis study of the salt glands and intracellular calcium crystals of Tamarix. Annals of Botany. 1994;73:307–313. [Google Scholar]
- Volk GM, Lynch-Holm VJ, Kostman TA, Goss LJ, Franceschi VR. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biology. 2002;4:34–45. [Google Scholar]
- Weiner S, Dove P. An overview of biomineralization processes and the problem of the vital effect. Reviews in Mineralogy and Geochemistry. 2003;54:1–29. [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Annals of Botany. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric-acid digestion and multielement analysis of plant-material by inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis. 1987;18:131–146. [Google Scholar]
- Zindler-Frank E. Oxalate biosynthesis in relation to photosynthetic pathway and plant productivity – a survey. Zeitschrift für Pflanzenphysiologie. 1976;80:1–13. [Google Scholar]
- Zindler-Frank E. Calcium oxalate crystals in legumes. In: Stirton CH, editor. Advances in legume systematics, Part 3. Kew: Royal Botanic Gardens; 1987. pp. 279–316. [Google Scholar]