Abstract
Background and Aims
Grazing is a complex process involving the simultaneous occurrence of both trampling and defoliation. Clonal plants are a common feature of heavily grazed ecosystems where large herbivores inflict the simultaneous pressures of trampling and defoliation on the vegetation. We test the hypothesis that physiological integration (resource sharing between interconnected ramets) may help plants to deal with the interactive effects of trampling and defoliation.
Methods
In a field study, small and large ramets of the root-suckering clonal tree Populus simonii were subjected to two levels of trampling and defoliation, while connected or disconnected to other ramets. Plant responses were quantified via survival, growth, morphological and stem mechanical traits.
Key Results
Disconnection and trampling increased mortality, especially in small ramets. Trampling increased stem length, basal diameter, fibrous root mass, stem stiffness and resistance to deflection in connected ramets, but decreased them in disconnected ones. Trampling decreased vertical height more in disconnected than in connected ramets, and reduced stem mass in disconnected ramets but not in connected ramets. Defoliation reduced basal diameter, leaf mass, stem mass and leaf area ratio, but did not interact with trampling or disconnection.
Conclusions
Although clonal integration did not influence defoliation response, it did alleviate the effects of trampling. We suggest that by facilitating resource transport between ramets, clonal integration compensates for trampling-induced damage to fine roots.
Keywords: Biomechanics, clonal integration, defoliation, drylands, grazing, Populus simonii, resource sharing, root connections, root severing, trampling
INTRODUCTION
Grassland degradation and desertification is a major problem in drylands (Squires, 2003; Verón and Paruelo, 2010). Overgrazing is argued to be the major cause of desertification worldwide, although other factors also play a role (Yoav et al., 2006). Grazing is a complex process that has several effects on plants, the most important being biomass consumption and associated defoliation (Hanley and Sykes, 2009; Gruntman and Novoplansky, 2011), trampling and associated mechanical stress, and changes in soil properties such as compaction and nutrient addition (Sørensen et al., 2009).
Trampling can strongly affect plant performance, community structure and species composition (Cole, 1988; Sun and Liddle, 1993; Kobayashi et al., 1997; Striker et al., 2011), and in drylands it has been implicated as a cause of desertification (Ibáñez et al., 2007). Trampling has a direct mechanical effect on plants by causing physical damage through either excess flexural loading or crushing plant organs (Sun and Liddle, 1993). However, compared with other forms of mechanical stress such as wind loading and manual flexing and rubbing (known as ‘thigmorphogenesis’), trampling acts on one point instead of on the whole plant and entails a discontinuous overwhelming impact, especially for small individuals. Several mechanical traits, including leaf toughness, root strength and stem flexibility, have been suggested to play a role in plant tolerance to trampling (Kobayashi et al., 1999; Striker et al., 2011). Most studies on trampling, however, were conducted at the community level (Cole, 1988, 1995; Servane and Françoise, 2003; Andrés-Abellán et al., 2006), and few focused on the plastic responses of individual plants in terms of, for example, biomass allocation and mechanical traits. Sun and Liddle (1993) argued that short plants may be more resistant to trampling because they tend to be protected by taller plants. In moving or semi-moving dunes of drylands where vegetation is sparse, studying responses of individual plants to trampling is more practical. Although plastic responses of plants to other forms of mechanical stress have been well documented, little work has been done on trampling.
Biomass consumption and associated defoliation cause both direct loss of resources and reductions in photosynthetic surface. Yet plants exhibit compensatory traits that enable them to mitigate the negative effects of defoliation (Gruntman and Novoplansky, 2011; Muola et al., 2011; Zhang et al., 2011). The capacity for compensatory growth in turn may depend on the presence of other factors. Dry or nutrient-poor conditions favour compensatory growth, whereas shading tends to reduce it (Coughenour et al., 1990; Anten et al., 2003; Wise and Abrahams, 2007; Garbuzov et al., 2011). Although defoliation and trampling occur simultaneously during grazing, their potential interactive effects on plants have not been investigated. Plants respond to defoliation typically by increasing allocation to leaves at the expense of reduced allocation to roots and stems (Stevens et al., 2008), which could make them more vulnerable to mechanical damage through trampling.
Clonal growth has been considered as an important mechanism to allow plants to persist in adverse conditions (Beatty and Provan, 2011). Many plants in drylands exhibit clonal growth patterns whereby ramets are produced along horizontal runners (e.g. rhizomes, stolons or roots; Yu et al., 2008; Gruntman and Novoplansky, 2011; Sui et al., 2011). Connections between individuals (ramets) usually persist for several years, allowing the exchange of resources such as carbohydrates, water and nutrients (Yu et al., 2004, 2008; Roiloa et al., 2007; de Witte and Stöcklin, 2010). This physiological integration has been shown to enhance the performance of ramets suffering from different local stresses, including shading (Alpert, 1999; Alpert et al., 2003), water shortages (de Kroon et al., 1996; Dong and Alaten, 1999), nutrient depletion (Alpert, 1991), burial (Yu et al., 2004) and erosion (Yu et al., 2008). However, no study has examined the role of clonal integration in determining plant tolerance to trampling.
We conducted a field experiment, in which ramets of the common local tree species Populus simonii were subjected to two levels of root-severing (the horizontally growing roots connected to the ramets were disconnected or left connected), defoliation and trampling. Specifically, we address the following questions: (1) What are the effects of trampling and defoliation on growth, morphological and mechanical traits, and are these effects additive or interactive? (2) To what extent are trampling and defoliation effects changed by physiological integration, i.e. the presence of clonal connections between ramets?
MATERIAL AND METHODS
Study site and focal species
The experiment was conducted in the mobile dunes near Ordos Sandland Ecological Research Station (39°29′37·6″N, 110 °11′29·4″E, 1300 m a.s.l.) of the Institute of Botany, the Chinese Academy of Science, located in Mu Us Sandland in Inner Mongolia, China. The average summer temperature is 20–24 °C, and average annual precipitation is 260–450 mm (most during July–September) (Zhang, 1994). Due to severe desertification in recent decades, this area, which used to be typical grassland, is now dominated by different types of sandland, including fixed, semi-mobile and mobile dunes (Zhang, 1994). This desertification has resulted from persistent overgrazing in the area (Huang et al., 2000). The mobile dunes where the experiment was conducted are mainly occupied by Populus simonii. There are also a few plants of other species, including Artemisia ordosica, Caragana intermedia, Cynanchum komarovii and Hedysarum laeve.
Populus simonii Carrière (Salicaceae) is a tree that can reach a height of 20 m and a stem diameter of 0·5 m (Wang and Fang, 1984). As it is highly resistant to cold, drought and wind, it is widely distributed in many provinces in north and south-west China. Populus simonii is a root-suckering clonal species whose roots can extend horizontally; new ramets are produced on these roots. The horizontal roots in the study area mainly occur within the top 0·1 m of sand (L. Xu, pers. obs.). The small ramets from horizontal roots are often grazed by animals. The horizontal roots can generate small fibrous roots. In mobile dunes, the roots are easily damaged by exposure to the air when they are denuded, or by trampling. Populus simonii is an important local species used for the stabilization of mobile dunes and recovery of vegetation in many parts of northern China (Liu and Man, 2008).
Experimental design
On 5 June 2010, we selected 115 large ramets and 115 small ramets of P. simonii on three adjacent mobile dunes within an area of about 0·32 km2. At the start of the experiment, average ramet height and stem basal diameter were 26·65 ± 0·55 cm and 6·70 ± 0·23 mm, respectively, for the large ramets, and 11·77 ± 0·35 cm and 4·30 ± 0·14 mm for the small ramets. Of the 115 ramets in each size class, 15 were randomly selected and harvested to measure initial biomass. Immediately after harvesting, these plants were enclosed in polyethylene bags and taken back to the field station. The ramets were divided into leaves, stems, main roots and fibrous roots. Leaf images were obtained with a scanner (Uniscan e53, Qing Hua Ziguang, Beijing, China), and leaf area was measured with ImageJ (1·32j, National Institutes of Health, Bethesda, MD, USA). All plant parts were then dried at 70 °C for 48 h and dry mass was measured.
The remaining 100 ramets were randomly subjected to two levels of trampling treatments (trampling vs. no trampling), two levels of defoliation treatments (defoliation vs. no defoliation) and two levels of root-severing treatments (severing vs. no severing, i.e. the horizontal roots that connected the ramets were severed or left connected to prevent or allow clonal integration) in a factorial design. There were 15 replicate plants for the four treatments involving root-severing and ten for the other four treatments without root-severing because we expected that in the former the ramets would suffer higher mortality. After 1 week, a maximum of seven replicate ramets were added in the severed treatments due to high mortality.
Trampling was applied using a manually constructed load with a weight (6·22 kg) and surface area (diameter 3·07 cm) such that, when placed on a plant, it produced a stress level (0·84 kg cm−2) similar to that produced by the hoof of a sheep weighing 40 kg (Lin et al., 2008). Trampling was simulated by placing the load vertically for 3 s on the base of the stem. On each occasion plants were trampled three times in different directions. Trampling was performed twice a week from 8 June until the plants were harvested. For the defoliation treatment, 50 % of the leaves were removed by clipping one of every two leaves along the stems, top to bottom. For the severing treatments, we carefully removed the sand to the level at which the horizontally growing roots connecting the ramets were exposed. We cut off the horizontal roots 5 cm away from both sides of the ramet with sharp scissors and then put the sand back. For non-severing treatments, we also exposed the horizontally growing roots connected to the ramets and then reburied them to avoid the potential confounding effects of disturbance. During this process, care was taken not to disturb the adventitious roots of the ramets. During the experiment the defoliation and root-severing treatments were performed only once (6 June) and the trampling treatments were performed twice a week.
From 11 September 2010 to 14 September 2010, we harvested all the surviving plants. Length, deflection angle from vertical and basal diameter were measured in the field. Ramets were then excavated and transported to the field station laboratory. Leaves were scanned, leaf area was measured by ImageJ and leaf mass was obtained after drying at 70 °C for 48 h. Young's modulus, break stress and maximum load force of the main stem of each surviving ramet were measured with a universal electromechanical testing machine (Type 5540; Instron, Norwood, MA, USA), applying the three-point bending technique (for details see Anten et al., 2005). In short, stem samples were fixed at both ends in small clips and a vertical force was applied on the stem halfway between these clamps. We adjusted the distance between the clips to ensure it was at least ten times larger than the basal stem section. Dry mass of roots, stems and leaves was then measured using the methods described above.
Data processing
We calculated the above-ground leaf area ratio (m2 g−1; calculated according to Anten and Ackerly, 2001), which is the amount of leaf area per unit above-ground mass. We also calculated a number of mechanical stem traits, including the second moment of area (I, m4) describing the geometric contribution to stiffness of the stem (Jaffe et al., 1984; Niklas, 1996); Young's modulus (E) representing stiffness of an elastic material (Gere and Timoshenko, 1999; Anten et al., 2005); flexural stiffness (EI, N m−2) of the rigidity of a stem cross-section; and the stem break stress (σb), which quantifies the resistance of stem tissue to rupture. Details of calculations of these mechanical traits can be found in Anten et al. (2005).
The mechanical traits mentioned above were scaled up to calculate resistance to bending and breaking at the whole-stem level. We note that these are qualitative measures of stem mechanical behaviour solely used for comparative purposes. Resistance to bending force (Fbend), referring to the force needed to bend the stem by a small angle (θ), was calculated via (Gere and Timoshenko, 1999):
| (1) |
where Hv is the vertical height of the main stem from the force acting point to the ground. Here we assume θ = 1° because larger deflections require a different bending model (Gere and Timoshenko, 1999).
Vertical height (Hv) is the height between the sand and the highest tip of the main stem given by:
| (2) |
where β is the angle between the main stem and ground.
The minimum lateral break force (Fbreak) exerting on the top of the stem, required to rupture the stem at its base, was calculated as (Gere and Timoshenko, 1999):
| (3) |
where d is the basal diameter.
As noted in the Introduction, trampling tends to entail an overwhelmingly large force and trampling tolerance could thus be expected to be associated with a favourable balance between strength and flexibility. Here we use the ratio of Fbreak to Fbend as a simple proxy for this balance:
| (4) |
As mentioned above, we assume θ = 1°. Equation (4) shows that the balance between strength and flexibility increases with the break stress (σb) and shoot vertical height (Hv) and decreases with Young's modulus (E) and basal diameter (d).
Statistical analysis
We used a generalized linear model to analyse the effect of size, trampling, defoliation and severing on ramet survival, with logit as the link function. A three-way ANOVA was used to examine the effects of trampling, defoliation, severing and their interactions on growth, morphological and mechanical traits of the surviving, large ramets. All the data were ln-transformed and examined by Levene's test for equality of variance and the Shapiro–Wilk test for normality. This analysis was not applied to the small ramets because 93 % of the ramets in the root-severing treatment died. All analyses were conducted with SPSS 16·0 (SPSS Inc., Chicago, IL, USA). The effects are considered significant at P < 0·05.
RESULTS
Survival rate
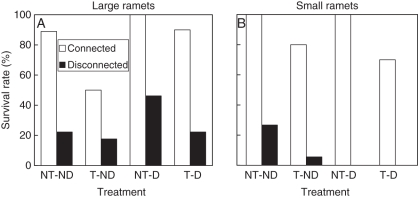
Survival rate of the ramets was significantly affected by ramet size (Likelihood ratio χ2 = 4·05, P = 0·042), root-severing (χ2 = 108·64, P < 0·001) and trampling (χ2 = 11·63, P = 0·001), but not by defoliation (χ2 = 0·37, P = 0·559). The presence of root connections between ramets had a strong positive effect on survival, and this effect was larger for smaller than for larger plants (Fig. 1). At the end of the experiment, survival rates of large connected ramets ranged from 50 to 100 % and those of large disconnected ones from 18 to 46 %. For the small ramets, survival rates were 30–100 % for connected treatments and 0–27 % for the disconnected treatments.
Fig. 1.
Survival rate of large (A) and small (B) Populus simonii ramets subjected to root severing (disconnected) or not (connected), defoliation (D) or not (ND), and trampling (T) or not (NT).
Morphology and growth responses
Trampling increased the length and basal diameter of the main stem in connected ramets, but decreased them in disconnected ramets, as reflected by the significant trampling × severing effect on these traits (Table 1, Fig. 2A, C). Moreover, trampling significantly increased the deflection angle of the stems, which was reflected in a smaller height above the sand, i.e. vertical height. This effect was stronger in the disconnected than in the connected ramets (Fig. 2B, Table 1: significant trampling × severing effect). Both severing and defoliation significantly decreased basal diameter of the stems (Table 1, Fig. 2C).
Table 1.
Statistiacl effects of trampling, defoliation and severing clonal connections on the growth, morphological and mechanical properties of large ramets of Populus simonii
| Trampling (T) | Defoliation (D) | Severing (S) | T × S | D × S | D × T | D × T × S | |
|---|---|---|---|---|---|---|---|
| Growth and morphological properties | |||||||
| Main stem length | 0·00n.s. | 1·17n.s. | 0·23n.s. | 5·77* | 0·09n.s. | 0·07n.s. | 0·34n.s. |
| Vertical height | 21·00*** | 0·02n.s. | 0·04n.s. | 6·02* | 1·86n.s. | 1·56n.s. | 3·09n.s. |
| Basal diameter | 0·22n.s. | 8·66** | 7·01* | 7·82** | 0·05n.s. | 0·78n.s. | 0·38n.s. |
| Leaf mass | 4·49** | 5·49** | 32·75*** | 0·72n.s. | 2·07n.s. | 1·32n.s. | 0·99n.s. |
| Stem mass | 1·88n.s. | 5·08* | 10·82** | 4·19* | 0·16n.s. | 0·16n.s. | 1·33n.s. |
| Fibrous root mass | 0·33n.s. | 0·07n.s. | 5·02* | 6·09* | 0·09n.s. | 1·92n.s. | 0·00n.s. |
| Leaf area ratio | 0·01n.s. | 22·69*** | 2·94n.s. | 0·54n.s. | 0·01n.s. | 0·05n.s. | 0·14n.s. |
| Mechanical properties | |||||||
| Young's modulus | 0·84n.s. | 0·88n.s. | 4·44* | 4·30* | 0·22n.s. | 0·01n.s. | 0·08n.s. |
| Second moment of area | 0·22n.s. | 8·66** | 7·01* | 7·82* | 0·05n.s. | 0·78n.s. | 0·38n.s. |
| Flexural stiffness | 0·98n.s. | 9·55** | 12·51** | 12·53** | 0·00n.s. | 0·58n.s. | 0·54n.s. |
| Break stress | 0·15n.s. | 0·01n.s. | 1·67n.s. | 5·06* | 0·16n.s. | 0·01n.s. | 1·68n.s. |
| Break force (Fbreak) | 1·25n.s. | 4·19* | 12·48** | 12·95** | 0·02n.s. | 0·76n.s. | 2·51n.s. |
| Bend force (Fbend) | 7·17* | 7·42* | 16·51*** | 10·38** | 0·02n.s. | 1·05n.s. | 0·27n.s. |
| Fbreak/Fbend | 9·06** | 3·20n.s. | 3·79n.s. | 0·00n.s. | 0·11n.s. | 0·27n.s. | 2·03n.s. |
F values and significance levels (***P < 0·001, **P < 0·01, *P < 0·05, n.s.P ≥ 0·05) are given; degrees of freedom of dry biomass of fibrous roots are (1,34); degrees of freedom of the others for all the effects are (1,40). Data were ln-transformed before analyses.
Fig. 2.
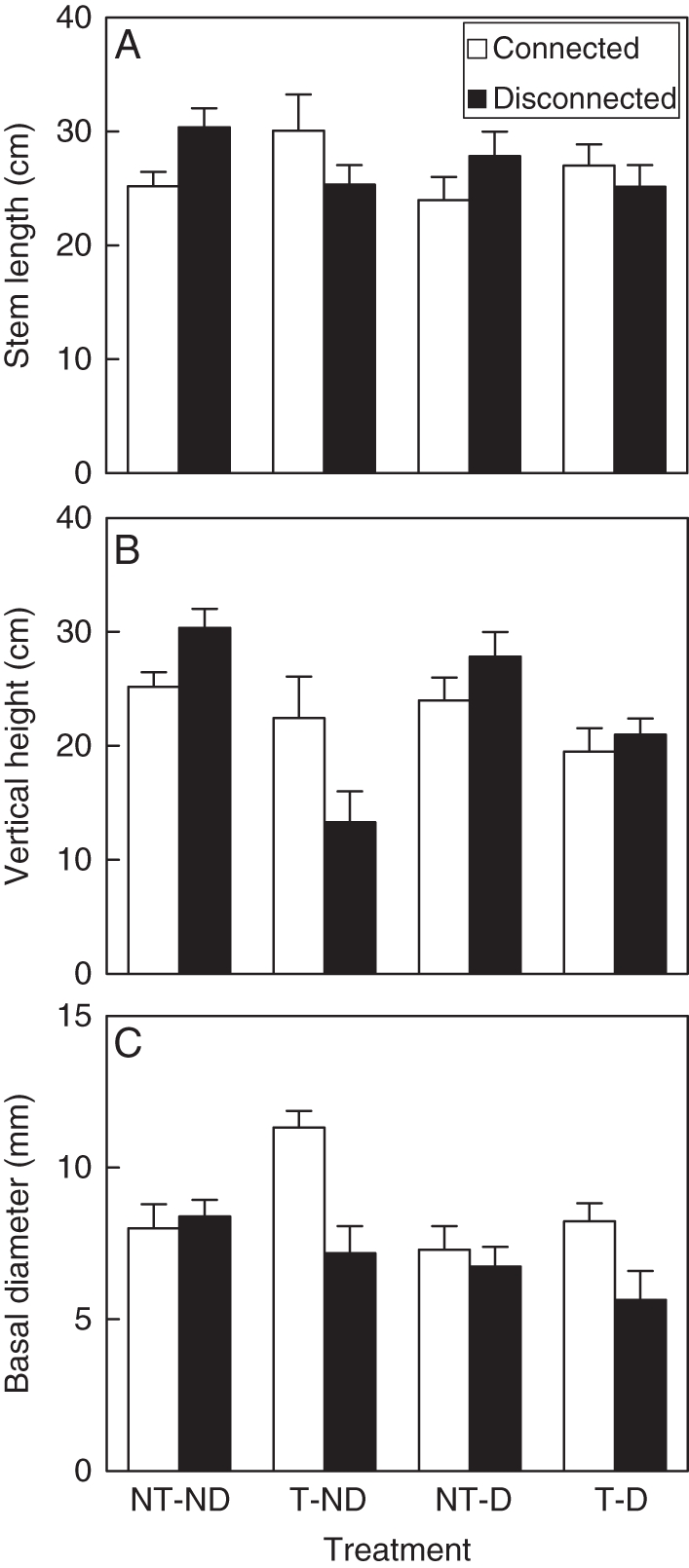
Length of main stem (A), vertical height (B) and basal diameter (C) of the large Populus simonii ramets subjected to root severing (disconnected) or not (connected), defoliation (D) or not (ND), and trampling (T) or not (NT). Data are means + s.e.
Trampling significantly decreased stem mass in disconnected ramets, but had no significant effect in connected ramets (Fig. 3A, Table 1: significant trampling × severing effect). Trampling increased fibrous root mass in connected ramets, but significantly decreased it in disconnected ramets (Fig. 3C, Table 1: significant trampling × severing effect). Trampling decreased leaf mass significantly in both connected and disconnected ramets (Table 1, Fig. 3B). Defoliation reduced leaf mass, stem mass and leaf area ratio (Table 1, Fig 3A, B, D), but did not interact with severing or trampling (Table 1). Severing significantly decreased leaf mass and stem mass of the surviving ramets (Table 1, Fig. 3A, B).
Fig. 3.
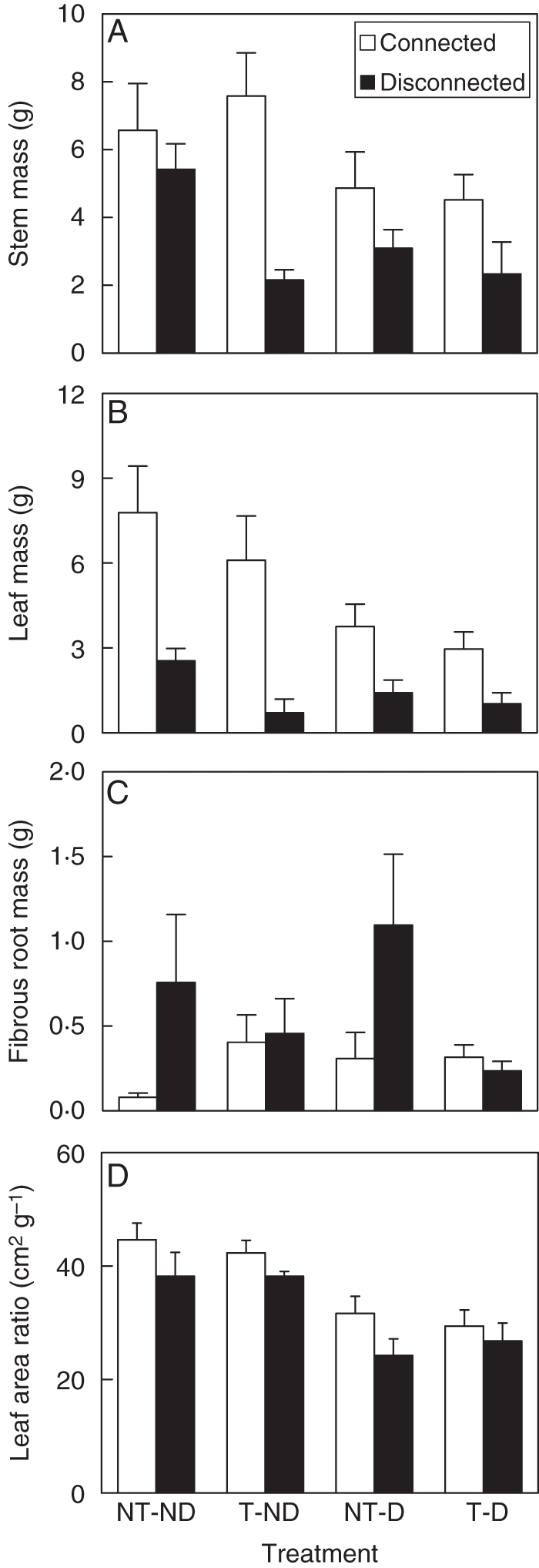
Dry mass of stems (A), leaves (B), fibrous roots (C) and leaf area ratio (D) of the large Populus simoni ramets subjected to root severing (disconnected) or not (connected), defoliation (D) or not (ND), and trampling (T) or not (NT). Data are means + s.e.
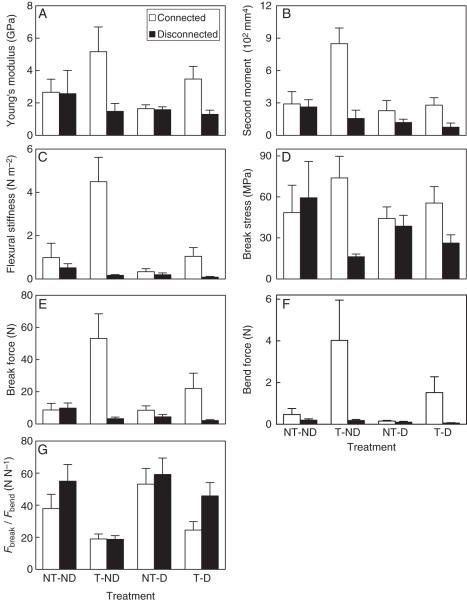
Mechanical properties
In connected ramets, trampling significantly increased Young's modulus (E), second moment of area (I), flexural stiffness (EI), break stress (σb) and the minimum force needed to break (Fbreak) and bend (Fbend) stems, whereas in disconnected ramets it decreased them (Fig. 4A–E, Table 1: significant trampling × severing effect). Trampling markedly decreased Fbreak/Fbend in both connected and disconnected ramets (Table 1, Fig. 4F). Defoliation significantly reduced I, EI, Fbreak and Fbend (Table 1, Fig. 4A, C, E), which are all a function of stem diameter (eqns 1 and 3), but it did not interact with severing or trampling (Table 1).
Fig. 4.
Young's modulus (A), second moment of area (B), flexural stiffness (C), break stress (D), minimum lateral break force (E), bend force (F) and Fbreak/Fbend (G) of the large Populus simonii ramets subjected to root severing (disconnected) or not (connected), defoliation (D) or not (ND), and trampling (T) or not (NT). Data are means + s.e.
DISCUSSION
Effects of trampling and severing on survival
Successful colonization in dry sandland has often been associated with a clonal growth form, the argument being that the exchange of resources through clonal connections facilitates the establishment of vegetative offspring (Guàrdia et al., 2000; Yu et al., 2004, 2008; Li, 2010). Demographic studies have further documented that population growth of clonal species in drylands is mainly governed by successful establishment of vegetative offspring (Li, 2010). However, experimental evidence for the role of clonal integration in determining growth and survival of vegetative offspring in drylands is limited (Yu et al., 2004, 2008). Both severing and trampling strongly decreased survival rates of P. simonii, particularly in small plants, but defoliation did not have a significant effect. These results suggest that clonal integration and the associated exchanges of resources between ramets strongly determined survival of new P. simonii ramets. They also indicate that the negative effects of grazing on survival of vegetative offspring are mediated by trampling rather than defoliation associated with grazing.
Growth and morphological responses to trampling, defoliation and severing
For connected ramets of P. simonii, repeated trampling not only increased stem length and basal diameter, but also increased declination of stems from the vertical (plants became less vertically inclined). As a result, vertical plant heights above the soil surface were significantly reduced. These findings are consistent with studies on other woody plants (Sun and Liddle, 1993; Andrés-Abellán et al., 2006). For disconnected ramets, however, trampling decreased stem length, basal diameter, vertical height and stem mass. These results indicate that clonal integration can strongly modify plant responses to trampling. Clonal integration has been associated with tolerance to many stress factors, but its role in modifying responses to mechanical stress in general and trampling in particular has received little attention.
The interaction between clonal integration and trampling could have important functional significance in the study system. First, the study area has been under heavy grazing, and P. simonii has been planted to restore overgrazed lands. Second, the probability of being trampled as well as its associated impact strongly declines with increasing size (as also shown in our study); beyond a certain height plants are unlikely to be trampled. Thus, larger ramets that suffer little or no trampling can mitigate the damage imposed by trampling on their small vegetative offspring.
Defoliation negatively affected growth, and the final biomass was reduced by about 50 %, which is proportional to the fraction of leaves that was removed (also 50 %). This indicates that compensatory growth in P. simonii was very limited (see Anten et al., 2003). Also, we did not find any interactive effect of trampling and defoliation. These results suggest that effects of trampling and defoliation are additive. The magnitude of the defoliation effect was similar among connected and disconnected plants, suggesting that clonal integration did not modify effects of defoliation on P. simonii. Thus, the role of clonal integration in increasing grazing tolerance may act through its effects in mitigating trampling effects rather than defoliation effects, which has not previously been reported.
We suggest that the effects of clonal integration, trampling and defoliation are at least partly mediated by water supply. In the study area, precipitation is low (260–450 mm annually) and ramets of P. simonii grew in almost pure sand. Thus, the young ramets most likely experienced water limitations. In theory, a given ramet can obtain water from its own fibrous roots, from its mother ramet or other ramets through the main connecting root. Due to the shallow root system and sand movement, these roots can easily become exposed, making them vulnerable to drying or damage. Interestingly, 16 % of the ramets that died during the experiment had not yet produced fibrous roots, while all the surviving ramets had. For the surviving ramets, those that were disconnected tended to have more fibrous roots (0·70 ± 0·19 g) than those that were connected (0·27 ± 0·06 g), suggesting that the amount of fibrous roots is important in determining survival of disconnected ramets. Disconnected, trampled plants had considerably smaller rooting systems than non-trampled plants (0·33 ± 0·09 vs. 0·96 ± 0·29 g), suggesting that trampling caused either reduced root growth or damage to existing roots. This effect could be more severe in woody species such as P. simonii than in herbaceous species because woody stems are relatively rigid. As a result, trampling induced a large bending moment on the root system. Fibrous roots in sandy soils tend to have low shear strengths (Berry et al., 2004). Together these factors contributed to the low root anchorage strength (Crook and Ennos, 1994; van Delden et al., 2010). Thus we suggest that trampling exacerbated drought stress by causing root damage, and that this effect could be mitigated by water transported through clonal connections.
Effects of trampling and severing on mechanical traits
Plants can prevent mechanical damage either by increasing their resistance to breakage or by enhancing their flexibility to minimize the forces encountered (Puijalon et al., 2008, 2011; Paul-Victor and Rowe, 2011). At the stem level, the former can be represented by the minimum break force (Fbreak) and the latter is the inverse of the force needed to bend the stem (1/Fbend). Across species there appears to be a tight negative correlation between Fbreak and Fbend, indicating that plants cannot maximize both flexibility and strength (Puijalon et al., 2011). Because trampling entails an overwhelming force, flexibility rather than strength should be expected to increase trampling tolerance (Sun and Liddle, 1993). This is true for disconnected ramets of P. simonii: trampling reduced stem diameter and break stress, which resulted in a reduction in both stem strength (Fbreak) and resistance to bending (Fbend).
For connected ramets, however, we observed clear opposite responses. Trampling increased stem diameter and associated flexural stiffness and as a result increased both Fbreak and Fbend. These results support the previously noted point that clonal integration can strongly modify plant responses to trampling. Moreover, trampling increased bending resistance more than strength, as reflected in the reduced value of Fbreak/Fbend. These results are consistent with the observed responses of plants to other forms of mechanical stress (e.g. wind, rubbing and flexing; Biro et al., 1980; Telewski, 1990; Anten et al., 2005). It is possible that in P. simonii thigmomorphogenic responses induced by trampling are similar to those induced by other forms of mechanical stress such as wind, and such responses may reduce the chance of mechanical damage and thus enhance trampling resistance (Anten et al., 2005).
Conclusions
Although a high relative frequency of rhizomatous species is known to be associated with intense grazing pressure (van Staalduinen et al., 2007), our study is the first to document that clonal integration may contribute to grazing tolerance by mitigating the effects of trampling. This interactive effect may be mediated by the capacity for the transport of water or other key resources through clonal connections, which partly compensates for the trampling-induced damage to the root system. This observation adds to our understanding of plant adaptation to grazing by suggesting that anti-herbivore tolerance in clonal plants is at least partly related to the ability to withstand trampling rather than defoliation.
ACKNOWLEDGEMENTS
We thank Yu-Kun Hu and Baoyin for assistance with fieldwork, Xue-Hua Ye, Qing-Guo Cui and Zeng-Juan Fu for help with measurements, Dr Heinjo During for comments on the experimental design, and Dr Mick Hanley and two reviewers for helpful comments on an early version of the manuscript. This work was supported by the Royal Netherlands Academy of Arts and Sciences (02CDC015 and 99CDC027) and National Natural Science Foundation of China (31070371 and 30821062).
LITERATURE CITED
- Alpert P. Nitrogen sharing among ramets increases clonal growth in Fragaria chiloensis. Ecology. 1991;72:69–80. [Google Scholar]
- Alpert P. Effects of clonal integration on plant plasticity in Fragaria chiloensis. Plant Ecology. 1999;141:99–106. [Google Scholar]
- Alpert P, Holzapfel C, Slominski C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. Journal of Ecology. 2003;91:27–35. [Google Scholar]
- Andrés-Abellán M, López-Serrano FR, Morote FAG, Cerro-Barja AD. Assessment of trampling simulation impacts on native vegetation in Mediterranean sclerophyllous forest. Environmental Monitoring and Assessment. 2006;120:93–107. doi: 10.1007/s10661-005-9051-2. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Ackerly DD. A new method of growth analysis for plants that experience periodic losses of leaf mass. Functional Ecology. 2001;15:804–811. [Google Scholar]
- Anten NPR, Martínez-Ramos M, Ackerly DD. Defoliation and growth in an understory palm: quantifying the contributions of compensatory responses. Ecology. 2003;84:2905–2918. [Google Scholar]
- Anten NPR, Casado-Garcia R, Nagashima H. Effects of mechanical stress and plant density on mechanical characteristics, growth, and lifetime reproduction of tobacco plants. The American Naturalist. 2005;166:650–660. doi: 10.1086/497442. [DOI] [PubMed] [Google Scholar]
- Beatty GE, Provan J. High clonal diversity in threatened peripheral populations of the yellow bird's nest (Hypopitys monotropa; syn. Monotropa hypopitys). Annals of Botany. 2011;107:663–670. doi: 10.1093/aob/mcr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry P, Sterling M, Spink J, et al. Understanding and reducing lodging in cereals. Advances in Agronomy. 2004;84:215–269. [Google Scholar]
- Biro RL, Hunt E, Erner Y, Jaffe MJ. Thigmomorphogenesis: changes in cell division and elongation in internodes of mechanically perturbed or ethrel-treated bean plants. Annals of Botany. 1980;45:655–664. [Google Scholar]
- Cole DN. Research paper INT-389. 1988. Disturbance and recovery of trampled montane grassland and forests in Montana. US Department of Agriculture, Forest Service, Intermountain Research Station. [Google Scholar]
- Cole DN. Experimental trampling of vegetation. I. Relationship between trampling intensity and vegetation response. Journal of Applied Ecology. 1995;32:203–214. [Google Scholar]
- Coughenour MB, Detling JK, Bamberg IE, Mugambi MM. Production and nitrogen responses of the African dwarf shrub Indigofera spinosa to defoliation and water limitation. Oecologia. 1990;83:546–552. doi: 10.1007/BF00317208. [DOI] [PubMed] [Google Scholar]
- Crook MJ, Ennos AR. Stem and root characteristics associated with lodging resistance in four winter wheat cultivars. Journal of Agricultural Science. 1994;123:167–174. [Google Scholar]
- van Delden SH, Vos J, Ennos AR, Stomph TJ. Analysing lodging of the panicle bearing cereal teff (Eragrostis tef) New Phytologist. 2010;186:696–707. doi: 10.1111/j.1469-8137.2010.03224.x. [DOI] [PubMed] [Google Scholar]
- Dong M, Alaten B. Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolia dune, China. Plant Ecology. 1999;141:53–58. [Google Scholar]
- Garbuzov M, Reidinger S, Hartley SE. Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Annals of Botany. 2011;2011:1355–1363. doi: 10.1093/aob/mcr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gere J, Timoshenko S. Mechanics of materials. Cheltenham, UK: Stanley Thornton; 1999. [Google Scholar]
- Gruntman M, Novoplansky A. Ontogenetic contingency of tolerance mechanisms in response to apical damage. Annals of Botany. 2011;108:965–973. doi: 10.1093/aob/mcr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guàrdia R, Raventós J, Caswell H. Spatial growth and population dynamics of a perennial tussock grass (Achnatherum calamagrostis) in a badland area. Journal of Ecology. 2000;88:950–963. [Google Scholar]
- Hanley ME, Sykes RJ. Impact of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F-X, Gao Q, Zhao S-Y. The concept of carrying capacity on ecological angle of view. Acta Partaculturae Sinica. 2000;9:48–57. [Google Scholar]
- Ibáñez J, Martínez J, Schnabel S. Desertification due to overgrazing in a dynamic commercial livestock-grass-soil system. Ecological Modelling. 2007;205:227–288. [Google Scholar]
- Jaffe MJ, Telewski FW, Cooke PW. Thigmomorphogenesis: on the mechanical properties of mechanically perturbed bean plants. Physiologia Plantarum. 1984;62:73–78. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hori Y, Nomoto N. Effects of trampling and vegetation removal on species diversity and micro-environment under different shade conditions. Journal of Vegetation Science. 1997;8:873–880. [Google Scholar]
- Kobayashi T, Ikeda H, Hon Y. Growth analysis and reproductive allocation of Japanese forbs and grasses in relation to organ toughness under trampling. Plant Biology. 1999;1:445–452. [Google Scholar]
- de Kroon H, Fransen B, van Rheenen JWA. High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia. 1996;106:73–84. doi: 10.1007/BF00334409. [DOI] [PubMed] [Google Scholar]
- Li S-L. Woody plants in dry sands: life history traits and population dynamics. 2010 PhD Thesis, Utrecht University, The Netherlands. [Google Scholar]
- Lin H-L, Hou F-J, Ren J-Z. Evaluation of indicators of grazing trampling intensity. Acta Pratacul Turae Sinica. 2008;17:85–92. [Google Scholar]
- Liu X, Man X-L. Distribution patterns of root systems of Populus simonii carr in highland of Mu Us Sandland. Science of Soil and Water Conservation. 2008;6:48–53. [Google Scholar]
- Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R. The role of inbreeding and outbreeding in herbivore resistance and tolerance in Vincetoxicum hirundinaria. Annals of Botany. 2011;108:547–555. doi: 10.1093/aob/mcr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. Differences between Acer saccharum leaves from open and wind-protected sites. Annals of Botany. 1996;78:61–66. [Google Scholar]
- Paul-Victor C, Rowe N. Effect of mechanical perturbation on the biomechanics, primary growth and secondary tissue development of inflorescence stems of Arabidopsis thaliana. Annals of Botany. 2011;107:209–218. doi: 10.1093/aob/mcq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puijalon S, Léna J-P, Rivière N, Champagne J-Y, Rostan J-C, Bornette G. Phenotypic plasticity in response to mechanical stress: hydrodynamic performance and fitness of four aquatic plant species. New Phytologist. 2008;177:907–917. doi: 10.1111/j.1469-8137.2007.02314.x. [DOI] [PubMed] [Google Scholar]
- Puijalon S, Bouma TJ, Douady CJ, et al. Plant resistance to mechanical stress: evidence of an avoidance-tolerance trade-off. New Phytologist. 2011;191:1141–1149. doi: 10.1111/j.1469-8137.2011.03763.x. [DOI] [PubMed] [Google Scholar]
- Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- Servane L, Françoise R. Response of three plant communities to trampling in a sand dune system in Brittany (France) Environmental Management. 2003;31:227–235. doi: 10.1007/s00267-002-2813-5. [DOI] [PubMed] [Google Scholar]
- Sørensen LI, Mikola J, Kytöviita M-M, Olofsson J. Trampling and spatial heterogeneity explain decomposer abundances in a sub-Arctic grassland subjected to simulated reindeer grazing. Ecosystems. 2009;12:830–842. [Google Scholar]
- Squires VR. Desertification, climate change and the world's drylands. In: Alsharhan AS, Wood WW, Goudie AS, Fowler A, Abdellatif EM, editors. Desertification in third millennium. 2003. pp. 21–26. Rotterdam: AA Balkema. [Google Scholar]
- van Staalduinen MA, During H, Werger MJA. Impact of grazing regime on a Mongolian forest steppe. Applied Vegetation Science. 2007;10:299–306. [Google Scholar]
- Stevens MT, Kruger EL, Lindroth RL. Variation in tolerance to herbivory is mediated by differences in biomass allocation in aspen. Functional Ecology. 2008;22:40–47. [Google Scholar]
- Striker GG, Mollard FPO, Grimoldi AA, León RJC, Insausti P. Trampling enhances the dominance of graminoids over forbs in flooded grassland mesocosms. Applied Vegetation Science. 2011;14:95–106. [Google Scholar]
- Sui Y, He W, Pan X, Dong M. Partial mechanical stimulation facilitates the growth of the rhizomatous plant Leymus secalinus: modulation by clonal integration. Annals of Botany. 2011;107:693–697. doi: 10.1093/aob/mcr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Liddle MJ. Plant morphological characteristics and resistance to simulated trampling. Environmental Management. 1993;17:511–521. [Google Scholar]
- Telewski FW. Growth, wood density, and ethylene production in response to mechanical perturbation in Pinus taeda. Canadian Journal of Forest Research. 1990;20:1277–1282. [Google Scholar]
- Verón SR, Paruelo JM. Desertification alters the response of vegetation to changes in precipitation. Journal of Applied Ecology. 2010;47:1233–1241. [Google Scholar]
- Wang Z, Fang Z-F. Flora of China. Beijing: Science Press; 1984. [Google Scholar]
- Wise MJ, Abrahamson WG. Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. American Naturalist. 2007;169:443–454. doi: 10.1086/512044. [DOI] [PubMed] [Google Scholar]
- de Witte LC, Stöcklin J. Longevity of clonal plants: why it matters and how to measure it. Annals of Botany. 2010;106:859–870. doi: 10.1093/aob/mcq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoav A, Naomi P, Joseph P, Gideon A. Geomorphic changes leading to natural desertification versus anthropogenic land conservation in an arid environment, the Negev Highlands, Israel. Geomorphology. 2006;82:177–200. [Google Scholar]
- Yu F-H, Dong M, Krüsi B. Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist. 2004;162:697–704. doi: 10.1111/j.1469-8137.2004.01073.x. [DOI] [PubMed] [Google Scholar]
- Yu F-H, Wang N, He W-M, Chu Y, Dong M. Adaptation of rhizome connections in drylands: increasing tolerance of clones to wind erosion. Annals of Botany. 2008;102:571–577. doi: 10.1093/aob/mcn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhao C, Lamb EG. Cotyledon damage affects seed number through final plant size in the annual grassland species Medicago lupulina. Annals of Botany. 2011;107:437–442. doi: 10.1093/aob/mcq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Ecological background, principles and optimised models for rangeland management of the Maowusu sandland. Acta Phytoecologica Sinica. 1994;18:1–16. [Google Scholar]