Abstract
Background and Aims
The movement of water through mycorrhizal fungal tissues and between the fungus and roots is little understood. It has been demonstrated that arbuscular mycorrhizal (AM) symbiosis regulates root hydraulic properties, including root hydraulic conductivity. However, it is not clear whether this effect is due to a regulation of root aquaporins (cell-to-cell pathway) or to enhanced apoplastic water flow. Here we measured the relative contributions of the apoplastic versus the cell-to-cell pathway for water movement in roots of AM and non-AM plants.
Methods
We used a combination of two experiments using the apoplastic tracer dye light green SF yellowish and sodium azide as an inhibitor of aquaporin activity. Plant water and physiological status, root hydraulic conductivity and apoplastic water flow were measured.
Key Results
Roots of AM plants enhanced significantly relative apoplastic water flow as compared with non-AM plants and this increase was evident under both well-watered and drought stress conditions. The presence of the AM fungus in the roots of the host plants was able to modulate the switching between apoplastic and cell-to-cell water transport pathways.
Conclusions
The ability of AM plants to switch between water transport pathways could allow a higher flexibility in the response of these plants to water shortage according to the demand from the shoot.
Keywords: Apoplastic pathway, arbuscular mycorrhiza, cell-to-cell pathway, drought, root hydraulic conductivity
INTRODUCTION
As sessile organisms, plants are constantly challenged by a wide range of environmental stresses such as drought, which result in reduced productivity and significant crop losses (Bartels and Sunkar, 2005). Under conditions of water deficit, plants tend to control root water uptake and/or tissue water status by adjusting root hydraulic conductivity (Maurel et al., 2008; Parent et al., 2009). Studies have shown that, when water moves from soil to the root vascular tissues, the main hydraulic barrier is the radial transport path, between root epidermis and xylem, rather than the axial path along xylem conduits (Hachez et al., 2006).
The composite model of water transport across roots (Steudle and Peterson, 1998; Steudle, 2000) proposes that water flows along three major pathways in parallel between the soil medium and the root xylem/stele. In the absence of stress, water travels mainly by the apoplastic path, following a hydrostatic gradient created by transpiration. However, when stomata are closed, water goes mainly by the ‘cell-to-cell’ pathway thanks to the osmotic gradient between soil solution and xylem sap, and includes water circulating by the cytoplasm and plasmodesmata, and water crossing cell vacuoles. Thus, the cell-to-cell path involves transport of water across membranes, which is largely mediated by aquaporins (Maurel et al., 2008). It also involves transport of water through the symplast and plasmodesmata (Knipfer and Fricke, 2010). The relative contributions of the apoplastic, symplastic and transmembrane pathways to root hydraulic conductivity are not well characterized (Voicu et al., 2009). Moreover, depending on the environmental conditions, the relative contribution of these pathways to overall uptake or hydraulic conductivity may change substantially (Steudle, 2000; Martínez-Ballesta et al., 2003; Hachez et al., 2006). It is generally accepted that under conditions of no stress, the apoplastic path has much less resistance to water flow than the ‘cell-to-cell’ path (Steudle and Peterson, 1998). However, there is growing evidence that the contribution of aquaporin-mediated water transport to root water uptake is much larger than previously thought, even under conditions of transpiration (Knipfer and Fricke, 2010, 2011; Fritz and Ehwald, 2011).
Arbuscular mycorrhizal (AM) symbiosis is known to alter host plant–water relationships under both well-watered and drought stress conditions, and AM symbiosis is often beneficial in providing their hosts with water, enhancing the tolerance of the host plants to water deficit (reviewed by Augé, 2001; Ruiz-Lozano, 2003; Ruiz-Lozano and Aroca, 2010). In recent years, it has been demonstrated that AM symbiosis regulates root hydraulic properties, including root hydraulic conductivity. However, reports show both enhanced (Sánchez-Blanco et al., 2004; Aroca et al., 2008b) and reduced (Aroca et al., 2007; Ruiz-Lozano et al., 2009) root hydraulic conductivity due to mycorrhization, and these effects have been linked to regulation of plant aquaporins (Ruiz-Lozano and Aroca, 2010).
The movement of water and ions through mycorrhizal fungal tissue and between the fungus and root is currently little understood (Lehto and Zwiazek, 2011). For instance, in ectomycorrhizal symbiosis it has been proposed that structural modification, such as a looser network of mantle construction, could result in altered proportions of apoplastic and symplastic flux (Bogeat-Triboulot et al., 2004). Some studies have suggested that increases in root hydraulic conductivity in mycorrhizal roots could be the result of increased cell-to-cell water flux (Marjanović et al., 2005; Lee et al., 2010). Fungal mycelia contain their own aquaporins, although little is known about their contribution to the water transport of mycorrhizal plants (Aroca et al., 2009; Lehto and Zwiazek, 2011). On the other hand, it has also been suggested that water could be absorbed by the external AM mycelium and delivered to the cortical apoplast, at the symbiotic interfaces, where it would join water taken up via the root apoplastic pathway (Smith et al., 2010). Muhsin and Zwiazek (2002) suggested that ectomycorrhizal hyphae increase hydraulic conductance of roots by decreasing water flow resistance of the apoplast rather than by aquaporin-mediated transport. Thus, it is not clear whether the regulation of root hydraulic conductivity by AM symbiosis is due to an effect on root aquaporins (cell-to-cell pathway) or to an enhanced apoplastic water flow.
In this study we have addressed the regulation of root hydraulic conductivity by AM symbiosis via a combination of two experiments aimed at quantifying the effect of symbiosis on relative apoplastic water flow, as well as on the cell-to-cell pathway in the presence and absence of sodium azide as an inhibitor or aquaporin activity. Thus, the apoplastic tracer dye light green SF yellowish, which has the ability to move apoplastically but not symplastically (Martínez-Ballesta et al., 2003; López-Pérez et al., 2007), was used to test the relative contributions of the apoplastic versus the cell-to-cell pathway for water movement in roots. Therefore, if AM symbiosis increases water flow through the root apoplastic pathway, an increase in the sap dye concentration in AM plants would be expected.
The use of aquaporin inhibitors should also shed further light on the relative participation of apoplastic and cell-to-cell paths in whole-root water uptake. In the present study, we have used sodium azide as an aquaporin inhibitor (Tournaire-Roux et al., 2003; Fitzpatrick and Reid, 2009; Postaire et al., 2010) to examine possible changes in pathways for root water flow as a result of AM fungal inoculation.
MATERIALS AND METHODS
Experiment 1
Experimental design and statistical analysis
Experiment 1 consisted of a factorial design with: (1) non-inoculated control plants (C) and (2) plants inoculated with the AM fungus Glomus intraradices, strain EEZ 58 (Gi). In addition, half of the plants was grown under well-watered (WW) conditions throughout the entire experiment and the other half of the plants was subjected to drought stress (DS). Each treatment had six replicates, and Zea mays and Solanum lycopersicum were used in this experiment to test if the responses of AM symbiosis were mediated by the host plant, giving a total of 48 pots.
All experimental data were subjected to analysis of variance (ANOVA) with inoculation treatment and water regime as sources of variation. Post-hoc comparisons with the Duncan and least-significant difference (LSD) tests were used to investigate differences between groups.
Soil and biological materials
Loamy soil was collected from Granada province (Spain), sieved (5 mm), diluted with quartz-sand (<2 mm) (1 : 9, soil/sand, v/v) and sterilized by steaming (100 °C for 1 h on three consecutive days). The original soil had a pH of 8·2 [measured in water 1 : 5 (w/v)], 1·5 % organic matter and nutrient concentrations (g kg−1) were as follows: N, 1·9; P, 1; K, 6·9.
Seeds of maize (Zea mays L. cv. ‘Potro’) were sown in pots containing 1200 g of the 1 : 9 soil/sand mixture and thinned to one seedling per pot after emergence.
Tomato seeds were germinated on wet sand and seedlings were transplanted to pots containing 1200 g of the soil/sand mixture as described above for maize. The genotype used in this study was Solanum lycopersicum Mill. ‘Rheinlands Ruhm’ (accession LA0535; Aroca et al., 2008a).
Mycorrhizal inoculum was bulked in an open-pot culture of Zea mays L. and consisted of soil, spores, mycelia and infected root fragments. The AM species was Glomus intraradices (Schenck and Smith) strain EEZ 58. Ten grams of inoculum with about 60 infective propagules per gram (according to the most probable number test) was added to appropriate pots at sowing.
Uninoculated control plants received the same amount of autoclaved mycorrhizal inoculum together with a 3-mL aliquot of a filtrate (<20 µm) of the AM inoculum in order to provide a general microbial population free of AM propagules.
Growth conditions
The experiment was carried out under greenhouse conditions with temperatures ranging from 19 to 25 °C, 16/8-h light/dark period, a relative humidity of 50–70 % and an average photosynthetic photon flux density of 800 µmol m−2 s−1, as measured with a light meter (LI-188B; LICOR, Lincoln, NE, USA).
Plants were cultivated in 1·5-L pots filled with 1200 g of the soil/sand mixture (1 : 9). The substrate allows easy and fast recovery of the root system from the pot (Ruiz-Lozano and Azcón, 1997), with minimum disturbance of the fungal mycelium before measurement of relative apoplastic water flow. Plants were cultivated for 8 weeks, receiving 50 mL nutrient solution (Hoagland and Arnon, 1950) three times a week and 25 mL water on alternate days. The solution was modified to contain only 25 % phosphorus to avoid inhibition of AM symbiosis in the corresponding treatments.
As mentioned above, plants were grown almost entirely in sand, and thus this growing substrate had no water-holding capacity. Water treatments were imposed by modifying the volume of nutrient solution added to plants. Briefly, WW plants received 50 mL aqueous nutrient solution three times per week and 25 mL water on alternate days, as mentioned above. For the DS plants, the stock nutrient solution was diluted in only 25 mL water (keeping the same total amount of nutrients as for WW plants) and plants were irrigated with 25 mL nutrient solution three times per week and with 12·5 mL water on alternate days. Thus, during the period of drought stress, plants received 50 % of the water resources as compared with WW plants. This procedure was previously used by Ruiz-Sánchez et al. (2010) in rice, giving satisfactory results. Plants were maintained under such conditions for 12 d before harvesting.
Symbiotic development
At harvest, the percentage of mycorrhizal fungal colonization in maize or tomato plants was estimated by visual observation of root colonization after clearing washed roots in 10 % KOH and staining with 0·05 % trypan blue in lactic acid (v/v), according to Phillips and Hayman (1970). The extent of mycorrhizal colonization was calculated according to the gridline intersect method (Giovannetti and Mosse, 1980).
Leaf water potential
The mid-day leaf water potential (ψw) was determined 1 d before harvest with a C-52 thermocouple psychrometer chamber and an HR-33T dew point microvoltmeter (Wescor Inc., Logan, UT, USA). Leaf discs corresponding to the third full grown leaf were cut, placed inside the psychrometer chamber and allowed to reach temperature and water vapour equilibrium for 15 min before measurements were made by the dew-point method (Porcel and Ruiz-Lozano, 2004).
Relative water content
The relative water content (RWC) in plant leaves was determined at harvest as previously described by Ruiz-Lozano and Azcón (1997). Briefly, leaf samples (taken from the third full grown leaf) of around 1 cm2 were weighed (fresh weight, FW) immediately after harvesting, then placed in a water-saturated vial at 5 °C for 24 h and weighed (turgid weight, TW). Samples were dried in an oven at 75 °C for 48 h and their dry weights (DW) were obtained. RWC was calculated using the following equation: [(FW – DW)/(TW – DW)] × 100.
Stomatal conductance
Stomatal conductance was measured 2 h after the onset of the photoperiod with a porometer system (Porometer AP4; Delta-T Devices Ltd, Cambridge, UK) following the manufacturer's instructions. Stomatal conductance measurements were taken in the second fully-elongated leaf from four different plants from each treatment.
Photosynthetic efficiency
The efficiency of photosystem II was measured with FluorPen FP100 (Photon Systems Instruments, Brno, Czech Republic), which allows a non-invasive assessment of plant photosynthetic performance by measuring chlorophyll a fluorescence. FluorPen quantifies the quantum yield of photosystem II as the ratio between the actual fluorescence yield in the light-adapted state (FV′) and the maximum fluorescence yield in the light-adapted state (FM′), according to Oxborough and Baker (1997). Measurements were taken in the second full grown leaf of four different plants of each treatment.
Hydrostatic root hydraulic conductivity
Hydrostatic root hydraulic conductivity (Lh) was measured with a Scholander pressure chamber, following the method described by López-Pérez et al. (2007). The aerial parts of the plants were removed, and the stems were placed in plastic tubes. Roots were placed in a pressure chamber in water and a gradual increase of pressure (0·2, 0·3 and 0·4 MPa) was applied to the detached roots. Each pressure interval was maintained for 2 min and the sap exuded during each pressure interval was collected in Eppendorf tubes and weighed. The roots and the tubes were weighed in a precision balance. Sap flow (Jv) was expressed in mg H2O (g root DW)−1 h−1 and plotted against pressure (MPa), with the slope being the L value in mg H2O (g root DW)−1 MPa−1 h−1.
Relative apoplastic water flow
Relative changes in apoplastic water flux were estimated using a light green dye (light green SF yellowish; Sigma-Aldrich Chemical, Gillingham, UK; colour index 42095, molecular weight 792·85 g mol−1), which has the ability to move apoplastically but not symplastically (López-Pérez et al., 2007). Detopped root systems were immersed in 250 µmol L−1 light green solution inside the pressure chamber 5 min before pressure application and kept in this solution during measurement. Sap was collected after 2 min at 0·2, 0·3 and 0·4 MPa in a Scholander pressure chamber. At the end of the experiment, the concentration of the dye in the whole collected sap was determined immediately with a spectrophotometer (Hitachi U-1900) at 630 nm (López-Pérez et al., 2007). The average baseline fluorescence value in the nutrient solution before addition of the dye was subtracted from the values obtained after adding the dye and in the collected sap. The percentage of the apoplastic pathway was calculated from the ratio between dye concentration in the sap flow and in the nutrient solution. The concentration of dye in the nutrient solution of each treatment was considered to be 100 %.
Apoplastic transport was estimated based on the assumption that, due to its large molecular weight (792·85 g mol−1), light green SF yellowish is excluded from the symplastic and transcellular pathways (Zimmermann and Steudle, 1998). Although fluorescent dyes may not precisely measure the total apoplastic water flux (Zimmermann and Steudle, 1998), they can be used to detect relative changes in the apoplastic transport of plants from different treatments (Kamaluddin and Zwiazek, 2001; Voicu and Zwiazek, 2004).
Experiment 2
Experimental design and statistical analysis
Experiment 2 consisted of a factorial design with two inoculation treatments: (1) non-inoculated control plants (C) and (2) plants inoculated with the AM fungus Glomus intraradices, strain EEZ 58 (Gi). Half of the plants were cultivated under WW conditions throughout the entire experiment and the other were subjected to DS. Thus, there were four microbial per water treatments with 24 replicates each, totalling 96 pots (one plant per pot), so that half of the plants (12 for each treatment, totalling 48 plants) were treated with sodium azide, while the other half (12 for each treatment, totalling 48 plants) remained untreated. After the treatment with sodium azide (or with water, for untreated plants) six replicates of each treatment were used for the measurement of osmotic root hydraulic conductivity and the other six replicates for measurement of hydrostatic root hydraulic conductivity.
Data were subjected to ANOVA with inoculation treatment, water regime and presence or absence of sodium azide as sources of variation. Post-hoc comparisons with the Duncan and LSD tests were used to determine differences between groups.
Soil and biological materials
The soil used was the same as described for expt 1, but diluted with sand in a proportion of 1 : 1 (v/v). Two seeds of maize (Zea mays L. cv. ‘Potro’) were sown in pots containing 1200 g of the soil/sand mixture and thinned to one seedling per pot after emergence.
Mycorrhizal inoculum and inoculation procedures were the same as described for expt 1.
Growth conditions
The experiment was carried out under greenhouse conditions with temperatures, humidity and photoperiod as described for expt 1.
Plants were cultivated for 8 weeks and, 4 weeks after sowing, non-AM plants started receiving Hoagland nutrient solution (Hoagland and Arnon, 1950; 10 mL per pot once a week) with complete formulation, to obtain AM and non-AM plants of comparable size before starting the drought treatment.
Soil moisture was measured with an ML2 ThetaProbe (AT Delta-T Devices Ltd, Cambridge, UK), which measures volumetric soil moisture content by responding to changes in the apparent dielectric constant of moist soil, as previously described (Porcel and Ruiz-Lozano, 2004). Water was supplied daily to maintain soil at 100 % of field capacity during the first 6 weeks after sowing. The 100 % soil water-holding capacity corresponds to 20 % volumetric soil moisture measured with the ThetaProbe, as determined experimentally in a previous experiment using a pressure plate apparatus. Then, half of the plants were allowed to dry until soil water content reached 70 % of field capacity (2 d were needed), while the other half were maintained at field capacity. A soil water-holding capacity of 70 % corresponds to 11 % volumetric soil moisture measured with the ThetaProbe (also determined experimentally with a pressure plate apparatus in a previous assay). The soil water content was measured daily with the ML2 ThetaProbe before rewatering (at the end of the afternoon), reaching a minimum soil water content of approx. 65% field capacity. The amount of water lost was added to each pot to keep the soil water content at the desired level of 70 % field capacity (Porcel and Ruiz-Lozano, 2004). As the pot volume is small (<1·5 L), the water added daily is rapidly redistributed into the entire pot, so that the drought stress imposed is perceived by the entire root system. Plants were maintained under such conditions for an additional 12 d before harvesting.
Treatment with sodium azide
Before measuring root hydraulic conductivity and harvest, half of the plants from each treatment were treated with sodium azide at a final concentration of 7 mm (Fitzpatrick and Read, 2009). Whole pots were immersed in an aerated aqueous solution of sodium azide 7 mm, so that the root system was kept undisturbed during the treatment. The pots were maintained under submersion for 50 min to allow the compound to diffuse along the pot substrate until roots and subsequent absorption by roots. The concentration of sodium azide and the time of exposure were decided according to preliminary tests with sodium azide concentrations ranging from 1 to 30 mm and exposure times ranging from 15 min to 2 h. The selected concentration (7 mm) and exposure time (50 min) are in the range of use by Postaire et al. (2010), who treated Arabidopsis plants under hydroponic conditions with 2 mm sodium azide for 60 min. Untreated plants were immersed in water also for 50 min and were kept as a control for sodium azide treatment.
Symbiotic development
The percentage of mycorrhizal root infection in maize roots was determined as described for expt 1.
Osmotic root hydraulic conductivity
After incubation of roots in sodium azide solution or water, osmotic hydraulic conductivity (Los) was measured on detached roots exuding under atmospheric pressure (Aroca et al., 2007). Under these conditions, water is moving due only to an osmotic gradient between the soil solution and the root tissue. Therefore, according to Steudle (2000), the water would be moving exclusively by the cell-to-cell path. Pots were immersed in aerated nutrient solution. The aerial part was separated from the root and a pipette tip or an Eppendorf tube (as stem diameter) with cotton inside was attached to the stem. We discarded the liquid exuded in the first 15 min to avoid phloem contamination. The exudate of the following 2 h was collected in the cotton, then removed by two centrifugations of 2 min at 13 000 r.p.m. The osmolarities of the exuded sap and the nutrient solution were determined using a cryoscopic osmometer (Osmomat 030; Gonotec Gmbh, Berlin, Germany).
Osmotic root hydraulic conductance (L) was calculated as L = Jv/ΔΨ, where Jv is the exuded sap flow rate and ΔΨ the osmotic potential difference between the exuded sap and nutrient solution. These measurements were carried out 3 h after lights were turned on.
Hydrostatic root hydraulic conductivity
Hydrostatic root hydraulic conductivity (Lh) was measured with a high-pressure flow meter (HPFM) (Tyree et al., 1995), as previously described by Voicu et al. (2009), with slight modifications. Measurements of root Lh were carried out during the transient mode, where the pressure increases over a range and Lh is calculated from the slope of flow versus pressure. De-topped roots were connected to the HPFM using compression couplings, and were perfused with water at increasing pressure ranging from 350 to 450 kPa. A computer recorded the water flow and the applied pressure calculating the hydrostatic root hydraulic conductivity as mg H2O (g root DW)−1 h−1 MPa−1.
RESULTS
First experiment with maize plants
Plant growth and water status
Maize plants grew well under the new growing conditions and produced an average of 18 g shoot fresh weigh (data not shown) during the 8-week growing period. The proportion of AM root colonization was over 73%, with no effect of water treatment (Table 1).
Table 1.
Percentage of arbuscular mycorrhizal colonization in roots of maize and tomato plants from experiments 1 and 2
| Treatment | Experiment 1 |
Experiment 2* | |
|---|---|---|---|
| Maize | Tomato | Maize | |
| Control WW | 0b | 0b | 0b |
| G. intraradices WW | 74a | 68a | 63a |
| Control DS | 0b | 0b | 0b |
| G. intraradices DS | 73a | 70a | 60a |
Within each column, means followed by different letters are significantly different (P < 0·05) as determined by Duncan's multiple range test. WW, well watered; DS, drought stressed.
* The treatment with sodium azide was applied for 50 min just before measurement of root hydraulic conductivities. Thus, no effect of sodium azide on the percentage of AM root colonization was observed.
The shoot water potential of maize plants decreased significantly only in non-AM plants when subjected to drought, while in AM plants the decrease was not significant (Fig. 1A). The relative water content in shoots decreased under DS conditions, the decrease being more pronounced in non-AM plants (Fig. 1B). Stomatal conductance also decreased with DS imposed, reaching similar values in AM and non-AM plants (Fig. 1C). Finally, the efficiency of photosystem II was maximum in AM plants under WW conditions. The drought stress imposed reduced the efficiency of photosystem II in AM plants, but remained higher than in non-AM plants subjected to drought (Fig. 1D).
Fig. 1.
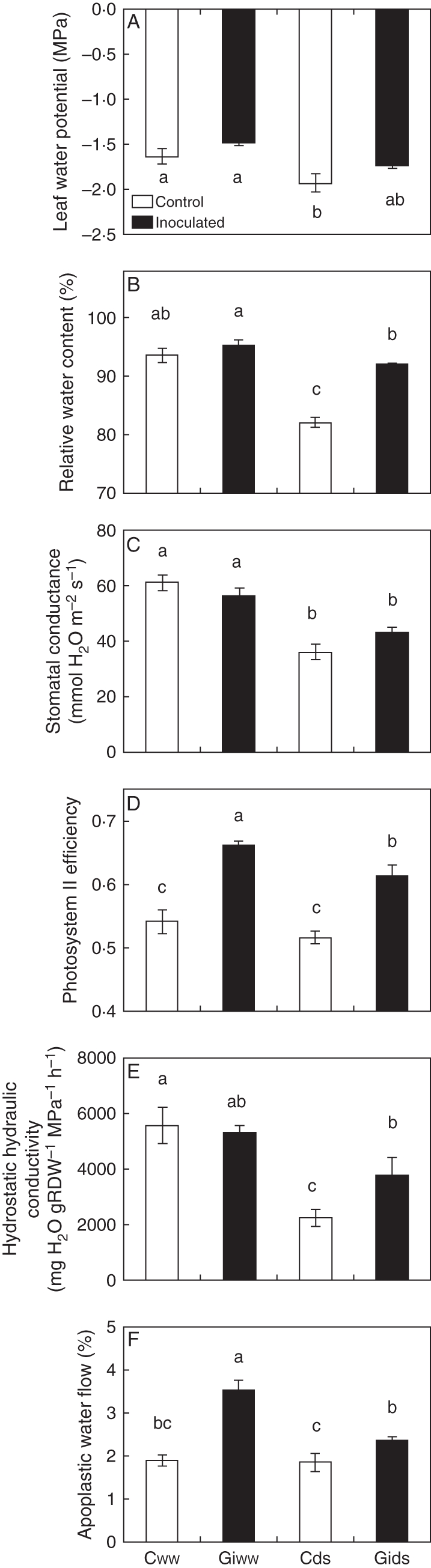
Effects of arbuscular mycorrhizal (AM) symbiosis and drought stress on (A) leaf water potential, (B) relative water content, (C) stomatal conductance, (D) efficiency of photosystem II, (E) hydrostatic root hydraulic conductivity (RDW = root dry weight) and (F) relative apoplastic water flow in maize plants grown under conditions of experiment 1. Treatments are designed as uninoculated controls (C, open bars) or G. intrarradices-inoculated plants (Gi, black bars). Plants were cultivated under well-watered conditions (ww) or subjected to drought stress (ds). Bars represent means plus s.e. (n = 6). Means followed by different letters are significantly different (P < 0·05) as determined by Duncan's multiple range and LSD tests.
Root hydraulic properties
The highest Lh of maize plants was found under WW conditions, with no significant differences between AM and non-AM plants (Fig. 1E). It decreased under drought, especially in non-AM plants, while the decrease in AM plants was not statistically significant.
Under the growing conditions of this experiment, the relative apoplastic water flow (Fig. 1F) was significantly enhanced in AM plants as compared with non-AM plants, under both WW conditions (87 % enhancement) and DS conditions (27% enhancement). However, non-AM plants did not reduce their apoplastic water flow as a consequence of drought, given that they showed similar values under WW and DS conditions.
First experiment with tomato plants
Plant growth and water status
Under the conditions of this experiment, tomato plants also grew well and produced and average of 15 g shoot FW (data not shown) during the 8-week growing period. The proportion of AM root colonization was over 68 %, with no effect of water treatment (Table 1).
Data obtained with tomato plants showed that DS decreased leaf water potential in AM and non-AM plants, but the decrease was more pronounced in non-AM plants (Fig. 2A). The relative water content also decreased as a consequence of drought, with similar values in AM and non-AM plants (Fig. 2B). Stomatal conductance was highest in AM tomato plants cultivated under WW conditions and decreased to similar values both in AM and in non-AM plants as a consequence of drought (Fig. 2C). The efficiency of photosystem II was similar in AM and non-AM plants when cultivated under WW conditions (Fig. 2D). It decreased in both treatments after DS, reaching lowest values in non-AM plants.
Fig. 2.
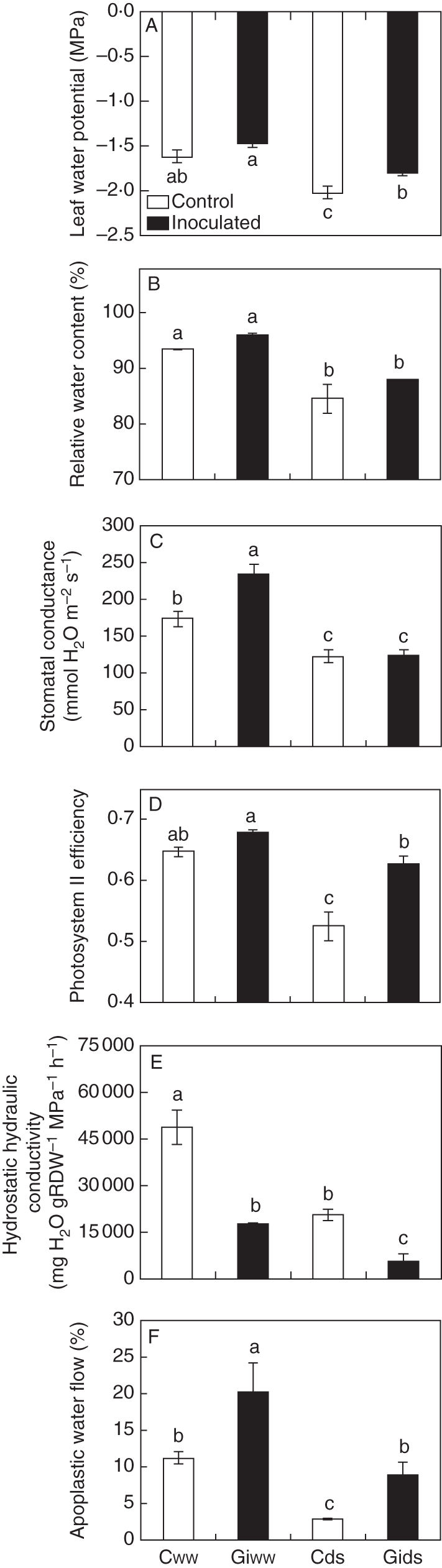
Effects of arbuscular mycorrhizal (AM) symbiosis and drought stress on (A) leaf water potential, (B) relative water content, (C) stomatal conductance, (D) efficiency of photosystem II, (E) hydrostatic root hydraulic conductivity and (F) relative apoplastic water flow in tomato plants grown under conditions of experiment 1. See legend to Fig. 1 for other details.
Root hydraulic properties
AM tomato plants exhibited a significantly lower Lh value under WW conditions than non-AM plants (Fig. 2E). Drought decreased this parameter in both treatments, with AM plants always exhibiting a lower Lh than non-AM plants.
Regardless of the effects of AM symbiosis on Lh, the relative apoplastic water flow (Fig. 2F) was significantly higher in AM tomato plants under both WW conditions (81 % increase) and DS conditions (195 % increase).
Second experiment with maize plants treated with sodium azide
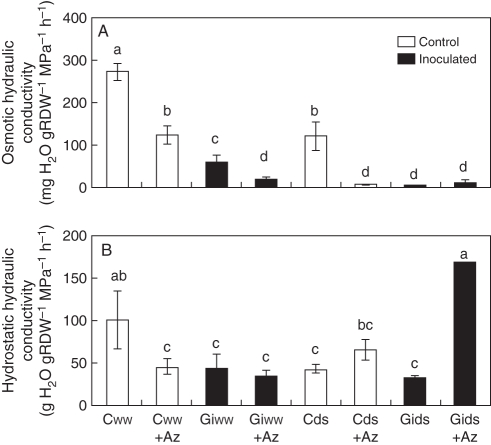
Data from expt 2 show that maize plants reached over 60 % of AM root colonization (Table 1). The osmotic root hydraulic conductivity (Los) of maize plants cultivated under WW conditions was 355 % higher in non-AM plants than in AM plants (Fig. 3A). The application of sodium azide decreased considerably this parameter in both AM and non-AM plants, 54 % in non-AM plants and 68 % in AM plants. When plants were subjected to drought, Los was lower in all treatments as compared with WW plants. However, non-AM plants exhibited the highest Los values, which were almost suppressed by treatment with sodium azide. AM plants subjected to drought exhibited a very low Los value that was not further affected by sodium azide treatment.
Fig. 3.
Effects of arbuscular mycorrhizal (AM) symbiosis, drought stress and sodium azide treatment on (A) osmotic root hydraulic conductivity and (B) hydrostatic root hydraulic conductivity, in maize plants grown under conditions of experiment 2 (RDW = root dry weight). Treatments are designed as uninoculated controls (C, open bars) or G. intrarradices-inoculated plants (Gi, black bars). Plants were cultivated under well-watered conditions (ww) or subjected to drought stress (ds). A group of plants within each treatment was treated with 7 mm sodium azide (+Az) before measurement of hydraulic conductivity. Bars represent means plus standard error (n = 6). Means followed by different letters are significantly different (P < 0·05) as determined by Duncan's multiple range and LSD tests.
Hydrostatic root hydraulic conductivity (Lh) was also higher in non-AM plants cultivated under WW conditions than in the corresponding AM plants (Fig. 3B). Indeed, non-AM plants had 126 % greater Lh than AM plants. Treatment with sodium azide reduced this parameter by 55 % in non-AM plants but did not affect it significantly in AM plants. Under DS conditions Lh of non-AM plants was 57 % lower than under WW conditions and treatment with sodium azide enhanced this parameter slightly (but not significantly). In contrast, AM plants did not reduce Lh significantly either as a consequence of drought or as a consequence of sodium azide treatment. Indeed, under DS conditions, AM plants treated with sodium azide exhibited higher values of Lh than under WW conditions or than for untreated AM plants.
DISCUSSION
In the two experiments considered in this study, we obtained AM and non-AM plants of similar size. To achieve this, from week 4 of cultivation to the beginning of the drought period, non-AM plants received an extra application of nutrient solution once a week. This is important because it is well known that AM symbiosis generally increases host plant growth due to improved plant nutrition (Smith and Read, 1997). In studies on water relationships of plants confined to containers, it is often difficult to compare treatments if plants are not of similar size as unequal size causes different degrees of soil water depletion, plant transpiration and, consequently, unequal stress intensity (Goicoechea et al., 1997; Ruiz-Sánchez et al., 2011). On the other hand, it has been reported that the effects of AM fungi on plant physiology are not directly linked to the extent of AM root colonization (Smith et al., 2010). Regardless, in this study, levels of AM root colonization are comparable in maize and tomato and not affected by the water stress treatments (Table 1). Thus, the results obtained here are not mediated by differences in plant size or in the extent of AM root colonization.
The results obtained in tomato and maize for leaf water potential, relative water content, stomatal conductance and efficiency of photosystem II demonstrated that the drought stress imposed significantly decreased these parameters. These results also suggest that AM plants exhibited enhanced water and physiological status as compared with non-AM plants. One of the most common explanations for the improved water status and physiology in mycorrhizal plants is the strongly increased absorbing surface caused by soil-growing hyphae combined with the fungal ability to take up water from soils with low water potential (Augé, 2001; Ruiz-Lozano, 2003; Lehto and Zwiazek, 2011). Hyphal length density associated with AM root has been estimated to vary from 1 to more than 100 m per gram of soil (Smith et al., 2010). Average diameters of hyphae of AM fungi are in the range 2–20 µm, one or two orders of magnitude narrower than roots. This size difference has important implications for access to water-filled pores, because hyphae will be able to penetrate a much higher proportion of pores than roots (Smith et al., 2010). Indeed, hyphal water uptake and transfer to the host plants has been demonstrated in several studies (Ruiz-Lozano and Azcón, 1995; Marulanda et al., 2003; Khalvati et al., 2005). The increased water uptake by hyphae may be less important when the soil is near saturation and large pores are filled with water as the root surfaces are also in contact with water. However, as the soil dries and water is retained only in smaller pores where fungal hyphae can grow, but roots cannot, the water uptake function of hyphae becomes more significant for survival (Allen, 2007; Lehto and Zwiazek, 2011).
The pathway of water in mycelial strands was considered to be apoplastic by Duddridge et al. (1980), and Muhsin and Zwiazek (2002) concluded that the increased root hydraulic conductance in ectomycorrhizal Ulmus americanus was due to apoplastic rather than cell-to-cell transport. Moreover, also in ectomycorrhizas, it has been proposed that infected fine roots were covered by hyphae and the fungal symbiont was integrated into transpiration-driven bulk water movement in soil (Plamboeck et al., 2007). Results from the present study suggest that water moving through the AM fungal hyphae could contribute to the apoplastic water flow in roots of maize and tomato plants, as was also suggested for ectomycorrhizas (Muhsin and Zwiazek, 2002; Plamboeck et al., 2007; Lehto and Zwiazek, 2011). Indeed, the cell-to-cell pathway of water across the cortex should be more arduous in mycorrhizas because in addition to the membranes of the host cells, the water would have to pass through the hyphal membrane as there is no symplastic connection between fungal cells and host root cells (Lehto and Zwiazek, 2011). However, symplastic transport in hyphae cannot be excluded, and symplastic movement within the hyphae themselves may provide an additional important pathway for water movement in the root cortex of the colonized roots (Reid, 1979). Moreover, mycorrhizas can affect the cell-to-cell pathway at least through effects on plant aquaporin expression and/or activity (Ruiz-Lozano and Aroca, 2010).
The previous results prompted the realization of a new experiment to measure both osmotic and hydrostatic hydraulic conductivities in the presence and absence of an inhibitor of aquaporin activity. Mercurials are the most commonly used aquaporin inhibitors in the literature. They act through covalent modification of cysteine residues within the water pore and in other regions of the protein, causing either block or conformational changes leading to inhibition of water transport (Niemietz and Tyerman, 2002). However, Hg has been reported to have a larger array of side effects depending on the dose and the duration of the treatments (Niemietz and Tyerman, 2002; Maurel et al., 2008). For instance, it also alters membrane permeability and indirectly inhibits cellular metabolism (Kamaluddin and Zwiazek, 2001). Thus, the use of alternative compounds is recommended. Fitzpatrick and Reid (2009) used sodium azide (0·5 mm) and butyric acid (10 mm) to inhibit the activity of aquaporins in barley plants grown hydroponically and they observed that sodium azide inhibited more efficiently the activity of aquaporins than butyric acid. Such an effect may be due to the fact that azide inhibits the phosphorylation and causes acidification of cytoplasm, both effects contributing to close the aquaporin channel (Tournaire-Roux et al., 2003).
The high values of Lh in AM plants subjected to drought and treated with sodium azide are opposite to the very low values of Los observed in these treatments. This suggests that when drought or sodium azide inhibited Los in AM plants they must have compensated for the lack of water flow through the cell-to-cell pathway by increasing apoplastic water flow. Under DS conditions, non-AM plants treated with sodium azide also exhibited this compensatory mechanism, but to a lesser extent than AM plants, in spite of the fact that sodium azide inhibited much more Los than in AM plants. In contrast, under WW conditions the compensatory mechanism was only observed in AM plants because sodium azide inhibited considerably Los, but Lh remained unaffected. In non-AM plants cultivated under WW conditions sodium azide decreased Los significantly and Lh also, indicating no compensation by the apoplastic pathway. Thus, an ability of AM plants to switch between water transport pathways is evidenced by the results obtained here.
In expt 1 we also observed that the apoplastic water flow was consistently enhanced by AM symbiosis, but Lh was reduced significantly in tomato plants but not in maize plants. Thus, the effect of AM symbiosis on Lh can be mediated by the plant species considered, as suggested by results in different plants showing both enhanced (Sánchez-Blanco et al., 2004; Aroca et al., 2008b) and reduced (Aroca et al., 2007; Ruiz-Lozano et al., 2009) root hydraulic conductivity due to mycorrhization. Because Lh is the sum of both apoplastic and cell-to-cell water flow, the above results also mean that the cell-to-cell water flow of AM tomato plants should have been reduced considerably, outweighing the increase of apoplastic water flow. In fact, it has been shown that, under drought conditions, root hydraulic conductivity decreases but the proportion of water circulating by the apoplastic path increases with respect to that circulating by the ‘cell-to-cell’ pathway (Siemens and Zwiazek, 2003), and this may explain the results observed in this study. However, what makes the composite model so appealing is that it provides a low-resistance path of water flow, driven by hydrostatic forces, when demand for water by the shoot is highest and when the main driving force is hydrostatic, and that it provides a higher resistance path of water flow, driven by osmotic forces, when demand for water is lower and osmotic forces predominate (Knipfer and Fricke, 2010). In the short term, root hydraulics is adjusted by switching between the cell-to-cell and apoplastic pathways, depending on the driving forces (Ranathunge et al., 2004). According to the model, a two-stage type of limitation or regulation of root water flow has been proposed. A coarse regulation is achieved by the fact that the contribution of apoplastic water flow around protoplasts increases with increasing tension set up during transpiration. A fine regulation would be maintained by the action of aquaporins or water channels in the parallel cell-to-cell path, which has a relatively high hydraulic resistance. Coarse regulation would be important in normal situations of unstressed plants, allowing for an adjustment of root water flow in response to the demands from the shoot. Fine regulation, by contrast, would dominate in stressed roots, when the apoplast is largely blocked by apoplastic barriers. In addition, fine regulation by water channels would either allow water uptake in the presence of transpiration (water channels open) or would prevent water losses to a dry soil in its absence (channels closed). Thus, the presence of AM fungi in the roots of host plants could modulate the switching between water transport pathways depending on both the driving forces and the water permeability of components of the pathway (Steudle, 2000). However, two questions remain unanswered: What is the physiological meaning of this switching between water transport pathways? What is its relevance for plant water status? Given the amount of experimental data supporting improved water relationships in AM plants (Augé, 2001; Ruiz-Lozano, 2003; Ruiz-Lozano and Aroca, 2010), it could be hypothesized that the ability of AM plants to switch between water transport pathways could allow a higher flexibility in the response of these plants to water shortage according to demands from the shoot.
CONCLUSIONS
Data from this study show that roots of AM plants enhanced significantly the relative apoplastic water flow as compared with non-AM plants and that this increase is evident under both WW and DS conditions. The data also show that the presence of AM fungi in the roots of host plants could modulate the switching between the apoplastic and cell-to-cell water transport pathways, which may allow AM plants to respond better to water demands from the shoot, especially under water-deficit conditions.
ACKNOWLEDGEMENTS
This work was financed by MICINN-FEDER (Project AGL2008-00898). G.B. was financed by a JAE pre-doctoral programme (CSIC).
LITERATURE CITED
- Allen MF. Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone Journal. 2007;6:291–297. [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporin in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytologist. 2007;173:808–816. doi: 10.1111/j.1469-8137.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- Aroca R, Alguacil M, Vernieri P, Ruiz-Lozano JM. Plant responses to drought stress and exogenous ABA application are differently modulated by mycorrhization in tomato and an ABA-deficient mutant (sitiens) Microbial Ecology. 2008a;56:704–719. doi: 10.1007/s00248-008-9390-y. [DOI] [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Ruiz-Lozano JM. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. Journal of Experimental Botany. 2008b;59:2029–2041. doi: 10.1093/jxb/ern057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Bago A, Sutka M, et al. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Molecular Plant–Microbe Interactions. 2009;22:1169–1178. doi: 10.1094/MPMI-22-9-1169. [DOI] [PubMed] [Google Scholar]
- Augé RM. Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11:3–42. [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Science. 2005;21:1–36. [Google Scholar]
- Bogeat-Triboulot MB, Bartoli F, Garbaye J, Marmeisse R, Tagu D. Fungal ectomycorrhizal community and drought affect root hydraulic properties and soil adherence to roots of Pinus pinaster seedlings. Plant and Soil. 2004;267:213–223. [Google Scholar]
- Duddridge JA, Malibari A, Read DJ. Structure and function of mycorrhizal rhizomorph with special reference to their role in water transport. Nature. 1980;287:834–836. [Google Scholar]
- Fitzpatrick KL, Reid RJ. The involvement of aquaglyceroporins in transport of boron in barley roots. Plant, Cell and Environment. 2009;32:1357–1365. doi: 10.1111/j.1365-3040.2009.02003.x. [DOI] [PubMed] [Google Scholar]
- Fritz M, Ehwald R. Mannitol permeation and radial flow of water in maize roots. New Phytologist. 2011;189:210–217. doi: 10.1111/j.1469-8137.2010.03452.x. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytologist. 1980;84:489–500. [Google Scholar]
- Goicoechea N, Antolin MC, Sánchez-Díaz M. Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiologia Plantarum. 1997;100:989–997. [Google Scholar]
- Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Molecular Biology. 2006;62:305–323. doi: 10.1007/s11103-006-9022-1. [DOI] [PubMed] [Google Scholar]
- Hoagland D, Arnon D. The water-culture method for growing plants without soil. California Agricultural Experimental Station Circular. 1950;347:1–32. [Google Scholar]
- Kamaluddin M, Zwiazek JJ. Metabolic inhibition of root water flow in red-osier dogwood (Cornus stolonifera) seedlings. Journal of Experimental Botany. 2001;52:739–745. doi: 10.1093/jexbot/52.357.739. [DOI] [PubMed] [Google Scholar]
- Khalvati MA, Hu Y, Mozafar A, Schmidhalter U. Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biology. 2005;7:706–712. doi: 10.1055/s-2005-872893. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare) New Phytologist. 2010;187:159–170. doi: 10.1111/j.1469-8137.2010.03240.x. [DOI] [PubMed] [Google Scholar]
- Knipfer T, Fricke W. Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.) Journal of Experimental Botany. 2011;62:717–733. doi: 10.1093/jxb/erq312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Calvo-Polanco M, Chung GC, Zwiazek JJ. Cell water flow properties in root cortex of ectomycorrhizal (Pinus banksiana) seedlings. Plant, Cell and Environment. 2010;33:769–780. doi: 10.1111/j.1365-3040.2009.02103.x. [DOI] [PubMed] [Google Scholar]
- Lehto T, Zwiazek JJ. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza. 2011;21:71–90. doi: 10.1007/s00572-010-0348-9. [DOI] [PubMed] [Google Scholar]
- López-Pérez L, Fernández-García N, Olmos E, Carvajal M. The phi thickening in roots of broccoli plants: an acclimation mechanisms to salinity? International Journal of Plant Sciences. 2007;168:1141–1149. [Google Scholar]
- Marjanović Ž, Uehlein N, Kaldenhoff R, et al. Aquaporins in poplar: what a difference a symbiont makes! Planta. 2005;222:258–268. doi: 10.1007/s00425-005-1539-z. [DOI] [PubMed] [Google Scholar]
- Martínez-Ballesta MC, Aparicio F, Pallás V, Martínez V, Carvajal M. Influence of saline stress on root hydraulic conductance and PIP expression in Arabidopsis. Journal of Plant Physiology. 2003;160:689–697. doi: 10.1078/0176-1617-00861. [DOI] [PubMed] [Google Scholar]
- Marulanda A, Azcón R, Ruiz-Lozano JM. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa L. plants under drought stress. Physiologia Plantarum. 2003;119:526–533. [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Muhsin TM, Zwiazek JJ. Ectomycorhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytologist. 2002;153:153–158. [Google Scholar]
- Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Letters. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- Oxborough K, Baker NR. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynthesis Research. 1997;54:135–142. [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology. 2009;149:2000–2012. doi: 10.1104/pp.108.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JM, Hayman DS. Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970;55:159–161. [Google Scholar]
- Plamboeck AH, Dawson TE, Egerton-Warburton LM, North M, Bruns TD, Querejeta JI. Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza. 2007;17:439–447. doi: 10.1007/s00572-007-0119-4. [DOI] [PubMed] [Google Scholar]
- Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, et al. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiology. 2010;152:1418–1430. doi: 10.1104/pp.109.145326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany. 2004;55:433–447. doi: 10.1093/jxb/erh041. [DOI] [PubMed] [Google Scholar]
- Reid CPP. Mycorrhizae and water stress. In: Riedacker A, Gagnaire-Michard J, editors. Root physiology and symbiosis. Nancy, France: Proceedings of the IUFRO Symposium; 1979. pp. 392–409. [Google Scholar]
- Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza. 2003;13:309–317. doi: 10.1007/s00572-003-0237-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Aroca R. Modulation of aquaporin genes by the arbuscular mycorrhizal symbiosis in relation to osmotic stress tolerance. In: Seckbach J, Grube M, editors. Symbioses and stress: joint ventures in biology, cellular origin, life in extreme habitats and astrobiology. Dordrecht: Springer Science+Business Media; 2010. pp. 359–374. [Google Scholar]
- Ruiz-Lozano JM, Azcón R. Hyphal contribution to water uptake in mycorrhizal plants as affected by fungal species and water status. Physiologia Plantarum. 1995;95:472–478. [Google Scholar]
- Ruiz-Lozano JM, Azcón R. Effect of calcium application on the tolerance of mycorrhizal lettuce plants to polyethylene glycol-induced water stress. Symbiosis. 1997;23:9–22. [Google Scholar]
- Ruiz-Lozano JM, Alguacil MM, Bárzana G, Vernieri P, Aroca R. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Molecular Biology. 2009;70:565–579. doi: 10.1007/s11103-009-9492-z. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. Journal of Plant Physiology. 2010;167:862–869. doi: 10.1016/j.jplph.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sánchez M, Armada E, Munoz Y, et al. Azospirillum and arbuscular mycorrhizal colonization enhanced rice growth and physiological traits under well-watered and drought conditions. Journal of Plant Physiology. 2011;168:1031–1037. doi: 10.1016/j.jplph.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blanco MJ, Ferrández T, Morales MA, Morte A, Alarcón JJ. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. Journal of Plant Physiology. 2004;161:675–682. doi: 10.1078/0176-1617-01191. [DOI] [PubMed] [Google Scholar]
- Siemens JA, Zwiazek JJ. Effects of water deficit stress and recovery on the root water relations of trembling aspen (Populus tremuloides) seedlings. Plant Sciences. 2003;165:113–120. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego: Academic Press; 1997. [Google Scholar]
- Smith SE, Facelli E, Pope S, Smith FA. Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil. 2010;326:3–20. [Google Scholar]
- Steudle E. Water uptake by roots: effects of water deficit. Journal of Experimental Botany. 2000;51:1532–1542. doi: 10.1093/jexbot/51.350.1531. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. How does water get through roots? Journal of Experimental Botany. 1998;49:775–788. [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, et al. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Patiño S, Bennink J, Alexander J. Dynamic measurements of roots hydraulic conductance using a high-pressure flow meter in the laboratory and field. Journal of Experimental Botany. 1995;46:83–94. [Google Scholar]
- Voicu MC, Zwiazek JJ. Cycloheximide inhibits root water flow and stomatal conductance in aspen (Populus tremuloides) seedlings. Plant, Cell and Environment. 2004;27:199–208. [Google Scholar]
- Voicu MC, Cooke JEK, Zwiazek JJ. Aquaporin gene expression and apoplastic water flow in bur oak (Quercus macrocarpa) leaves in relation to the light response of leaf hydraulic conductance. Journal of Experimental Botany. 2009;60:4063–4075. doi: 10.1093/jxb/erp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Steudle E. Apoplastic transport across young maize roots: effects of the exodermis. Planta. 1998;206:7–19. [Google Scholar]