Abstract
Background and Aims
Ericales are a major group of extant asterid angiosperms that are well represented in the Late Cretaceous fossil record, mainly by flowers, fruits and seeds. Exceptionally well preserved fossil flowers, here described as Glandulocalyx upatoiensis gen. & sp. nov., from the Santonian of Georgia, USA, yield new detailed evidence of floral structure in one of these early members of Ericales and provide a secure basis for comparison with extant taxa.
Methods
The floral structure of several fossil specimens was studied by scanning electron microscopy (SEM), light microscopy of microtome thin sections and synchrotron-radiation X-ray tomographic microscopy (SRXTM). For direct comparisons with flowers of extant Ericales, selected floral features of Actinidiaceae and Clethraceae were studied with SEM.
Key Results
Flowers of G. upatoiensis have five sepals with quincuncial aestivation, five free petals with quincuncial aestivation, 20–28 stamens arranged in a single series, extrorse anther orientation in the bud, ventral anther attachment and a tricarpellate, syncarpous ovary with three free styles and numerous small ovules on axile, protruding-diffuse and pendant placentae. The calyx is characterized by a conspicuous indumentum of large, densely arranged, multicellular and possibly glandular trichomes.
Conclusions
Comparison with extant taxa provides clear evidence for a relationship with core Ericales comprised of the extant families Actinidiaceae, Roridulaceae, Sarraceniaceae, Clethraceae, Cyrillaceae and Ericaceae. Within this group, the most marked similarities are with extant Actinidiaceae and, to a lesser degree, with Clethraceae. More detailed analyses of the relationships of Glandulocalyx and other Ericales from the Late Cretaceous will require an improved understanding of the morphological features that diagnose particular extant groups defined on the basis of molecular data.
Keywords: Actinidiaceae, Clethraceae, Ericales, flowers, fossils, Glandulocalyx upatoiensis, Late Cretaceous, Santonian
INTRODUCTION
The rapid increase in knowledge of the fossil record of flowering plants in the past 30 years has provided important evidence for the antiquity of different lineages of angiosperms, which can be compared against the relative antiquity of those lineages predicted from phylogenetic analyses of extant taxa based on molecular data and age estimates extrapolated from rates of molecular evolution (Magallón et al., 1999; Magallón and Castillo, 2009; Bell et al., 2010; Magallón, 2010). A striking feature of the fossil record of angiosperms as it is currently known is the relatively late appearance of asterids [approx. 90 million years ago (Ma)] compared with early diverging angiosperm families such as Chloranthaceae and Nymphaeaceae (approx. 120 Ma), early monocots (Arales), several orders of magnoliids (Canellales, Laurales and Magnoliales) and early diverging groups of eudicots (Buxales and Proteales), all of which are recognized before the end of the Early Cretaceous (approx. 100 Ma; Friis et al., 2011). Among asterids there also appears to be a clear chronological pattern, with the two earliest diverging groups, Cornales and Ericales, both unequivocally present in the Late Cretaceous. In particular, there is clear evidence, from fruits, seeds and scattered records of fossil flowers, that Ericales were already diverse by this time.
In this paper we add to the fossil record of Late Cretaceous asterids with the description of an exceptionally well preserved ericalean fossil flower from the Santonian of Georgia, southeastern USA. The fossil is preserved as charcoal, and investigations by standard scanning electron microscopy (SEM) and synchrotron-radiation X-ray tomographic microscopy (SRXTM) have made it possible to describe the fossil in exceptional detail. Most notably, studies of almost mature floral buds with SRXTM have permitted the non-destructive investigation of the androecium and internal features of the ovary. Detailed characterization of the fossil indicates a relationship with extant core Ericales (Actinidiaceae, Roridulaceae, Sarraceniaceae, Clethraceae, Cyrillaceae and Ericaceae) and specifically with extant Actinidiaceae and Clethraceae. More detailed analyses of the phylogenetic position of the fossil and other Ericales from the Late Cretaceous will require improved knowledge of phylogenetic patterns among extant taxa and the morphological features that diagnose groups now recognized on the basis of molecular data.
MATERIALS AND METHODS
The fossil material described herein was collected along the banks of the Upatoi Creek from exposures of the lower Eutaw Formation on the Fort Benning Military Reservation in central-western Georgia, USA. Upatoi Creek forms the boundary between Chattahoochee County and Muscogee County, and the locality that yielded the fossils described here is on the south bank of the creek (Chattahoochee County), at 32°24'44''N and 84°50'01''W. The Eutaw Formation has been correlated stratigraphically with the Austin Group of Texas, which is of Coniacian–Santonian age (Christopher, 1982), and calcareous nanofossil evidence from the southern Atlantic Coastal Plain and eastern Gulf Coastal Plain suggests an early to middle Santonian age (Christopher, 1982).
The plant mesofossils occur in unconsolidated clays interspersed among fine to coarse sands and silts. Bulk matrix samples were dried, disaggregated in water and then washed through a series of sieves. Residual mesofossils were then treated with 40 % hydrofluoric acid (HF) and 10 % HCl to remove adhering mineral material, washed thoroughly in water, and allowed to dry before sorting. The mesofossil assemblage contains diverse well-preserved fossils including conifer shoots and cones, and diverse angiosperm flowers, fruits and seeds. Fossil taxa that have been formally described from the Upatoi Creek assemblage include Quadriplatanus georgianus (Magallón et al., 1997), Regnellidium upatoiensis (Lupia et al., 2000) and Upatoia barnardii (Leslie et al., 2009).
The fossil flower described here is known from 13 specimens. All specimens (catalogue numbers PP55153–PP55165) are housed in the paleobotanical collections of the Field Museum, Chicago, IL, USA. Specimens for SEM were mounted on aluminium stubs and sputter coated with gold or gold–palladium, and studied at the Field Museum using a Leo EVO 60 environmental SEM (5–10 kV), at the Swedish Museum of Natural History using a Hitachi S-4300 field emission SEM (1–5 kV), at the University of Vienna using a Jeol JSM-6390 (5–10 kV) or at the George Washington University using a Leo 1430VP SEM (5–10 kV). One specimen was embedded in 2-hydroxyethyl methacrylate (Kulzer's Technovit 7100; Heraeus Kulzer GmbH, Wehrheim/Ts., Germany) and cut at 6 µm with a rotary microtome. For a description of the embedding method and the products used, see Igersheim (1993) and, in particular, Igersheim and Cichocki (1996), who described the embedding and sectioning of charcoal specimens in detail.
Several specimens were also imaged using SRXTM at the BL20B2 beamline of the Super Proton ring–8 GeV (SPring-8) at the Japan Synchrotron Radiation Research Institute (PP55163; PP55165, Fig. 6A, B; Supplementary Data Videos S1 and S2) and at the beamline 2-BM of the Advanced Photon Source at the US Department of Energy Argonne National Laboratory (PP55153; PP55165, Fig. 6C–J, Supplementary Data Videos S3–S5).
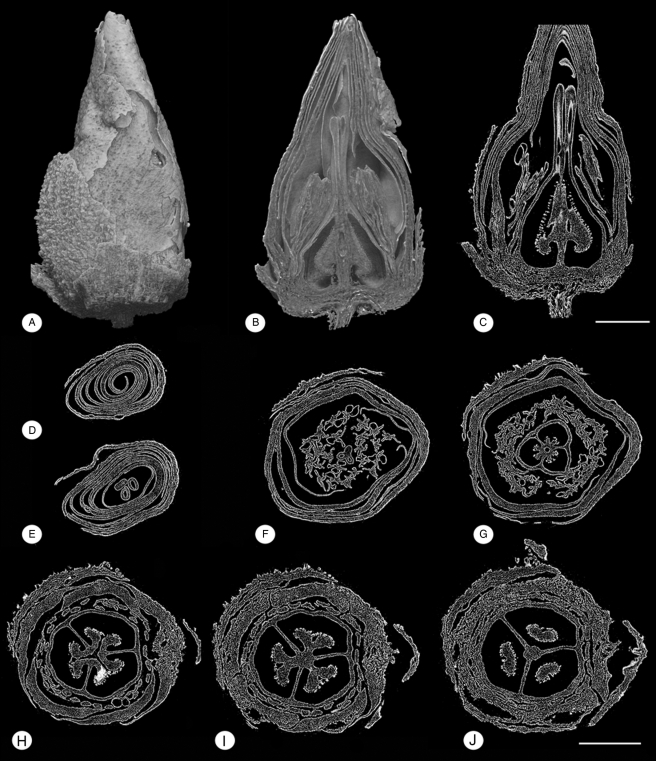
Fig. 6.
Glandulocalyx upatoiensis. Synchotron-radiation X-ray tomographic microscopy (SXRTM) reconstructions of a nearly mature floral bud. PP55165. (A) Lateral view showing fragmentary sepal with trichomes on the abaxial surface and smooth petals. (B) Three-dimensional reconstruction of half of floral bud in longitudinal section showing general organization of floral organs. (C) Longitudinal section through centre of floral bud showing hollow styles and pendant lower parts of placentae. (D–J) Series of transverse sections from apex (D) to base (J). (D) Level of corolla. (E) Level of tips of styles with stylar canals. (F) Level of anthers and distal part of symplicate ovary; note single sepal. (G) Level of symplicate ovary where the margins of each carpel are united; distal part of placentae. (H) Level of filaments and symplicate ovary with carpel margins free from each other and carpels not united in the centre. (I) Level of synascidiate (or possibly post-genitally united symplicate) part of ovary with protruding-diffuse placentae. (J) Level of pendant parts of placentae. Scale bars (A–C) = 600 µm; (D–J) = 700 µm.
At the Advanced Photon Source, specimens were examined at 15 keV, and 1500 projection images, each with an exposure time of 600 ms, were acquired equi-angularly over 180 °. Projection images were post-processed and rearranged into a flat- and dark-field corrected sinogram, and tomographic reconstruction was performed on a 64-nodescluster. Isotropic voxel dimensions were 1·54 µm. Three-dimensional images were reconstructed using the TRI/3DVOL software (64 bit) developed by Ratoc System Engineering (Tokyo).
At SPring-8, specimens were examined at 8 keV: 1800 projection images, each with an exposure time of 1·2 s, were acquired equi-angularly over 180 °. Projection images were post-processed and rearranged into a flat- and dark-field corrected sinogram, and tomographic reconstruction was performed on a windows or Linux PC by the convolution back projection method. Isotropic voxel dimensions were 2·74 µm.
Floral material of extant Clethraceae and Actinidiaceae
To compare androecial features in Glandulocalyx with those in potential extant relatives directly, we describe aspects of androecial development and structure of Clethra sp. [Jürg Schönenberger (JS) 552, Borneo, Malaysia], Actinidia kolomikta (JS 617, cult. Botanical Garden, University of Zürich, Switzerland) and Saurauia montana (JS 908, Monteverde, Costa Rica). Living material was fixed in FAA (formalin–acetic acid–alcohol) and subsequently stored in 70 % ethanol. For SEM, the material was dehydrated in an ethanol series (80, 95, 100 %) and acetone, critical point dried and sputter coated with gold.
To explore character evolution, we used the software package MESQUITE version 2·74 (Maddison and Maddison, 2010) using the parsimony option. Character evolution was traced on a tree topology for core Ericales mainly based on the phylogenetic analysis of Schönenberger et al. (2005). Relationships in Actinidiaceae are based on Keller et al. (1996). For an outgroup, we chose Theaceae, which form a polytomy with core Ericales and the styracoid clade (comprised of Styracaceae, Symplocaceae and Diapensiaceae) in the analysis of Schönenberger et al. (2005).
RESULTS
Order
Ericales (sensu APG III, 2009)
Family
Incertae sedis
Genus
Glandulocalyx Schönenberger, von Balthazar, Takahashi, Xiao, Crane & Herendeen, gen. nov.
Type
Glandulocalyx upatoiensis Schönenberger, von Balthazar, Takahashi, Xiao, Crane & Herendeen, sp. nov.
Etymology
The genus name Glandulocalyx refers to the conspicuous, multicellular, possibly glandular trichomes present on the sepals.
Generic diagnosis
Flower actinomorphic, pentamerous; calyx composed of five separate, imbricate sepals with numerous, prominent, more or less spherical to elongate, multicellular trichomes on the abaxial surface; corolla of five separate, imbricate petals; aestivation of calyx and corolla quincuncial. Androecium composed of 20–28 stamens. Each stamen with an elongate, ventrifixed and extrorse (in bud) anther. Pollen triaperturate. Gynoecium tricarpellate, syncarpous, superior, with three, long, free styles. Ovary trilocular, with pendant, protruding-diffuse, axile placentae bearing numerous ovules.
Species
Glandulocalyx upatoiensis Schönenberger, von Balthazar, Takahashi, Xiao, Crane & Herendeen, sp. nov.
Holotype
PP55153 (Figs 1 A–C and 2A, D). Deposited in the paleobotanical collection of the Field Museum of Natural History (Chicago).
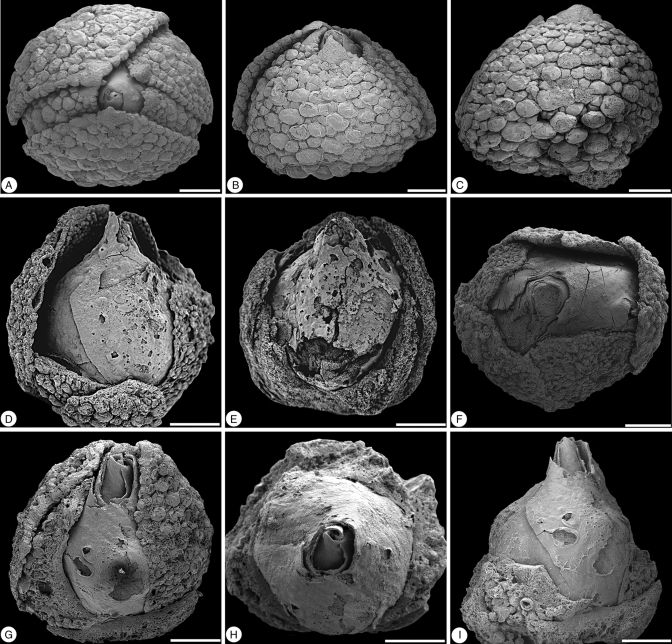
Fig. 1.
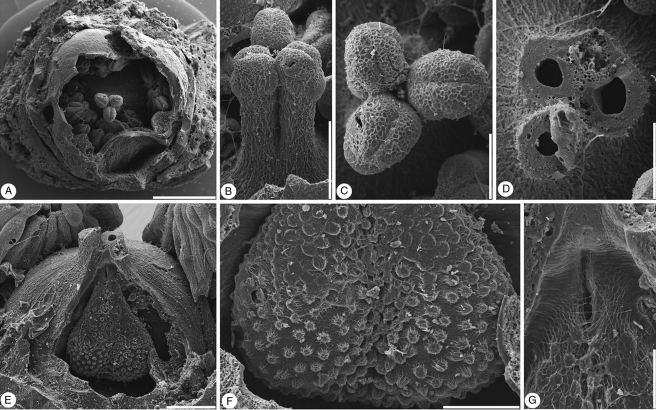
Glandulocalyx upatoiensis. Floral buds; five different specimens shown in different views and partly dissected. (A–C) PP55153 (holotype). (A) Apical view showing five sepals with quincuncial aestivation and well-developed multicellular trichomes on abaxial sepal surfaces and sepal margins. (B) Lateral view of the same bud as in (A). (C) Lateral view from the other side; note larger multicellular trichomes at the base of the sepal. (D) Lateral view of bud with sepals partly abraded and revealing petals; see Fig. 5 for microtome sections of this specimen; PP55160. (E) Lateral view of abraded bud showing thick sepals and fragmentary petals; PP55161. (F) Apical view of bud showing five sepals in a quincuncial arrangement and corolla partly protruding from calyx; PP55154; this specimen was dissected in several steps to reveal internal floral organs; see Fig. 3. (G–I) PP55155; this specimen was dissected to reveal internal floral organs; see Figs 2–4. (G) Lateral view of a bud with sepals partly abraded revealing the corolla. (H) Apical view of the specimen with calyx removed to reveal the petals fully. (I) Lateral view of the specimen in (G) with calyx removed to reveal the petals. Scale bars = 500 µm.
Fig. 2.
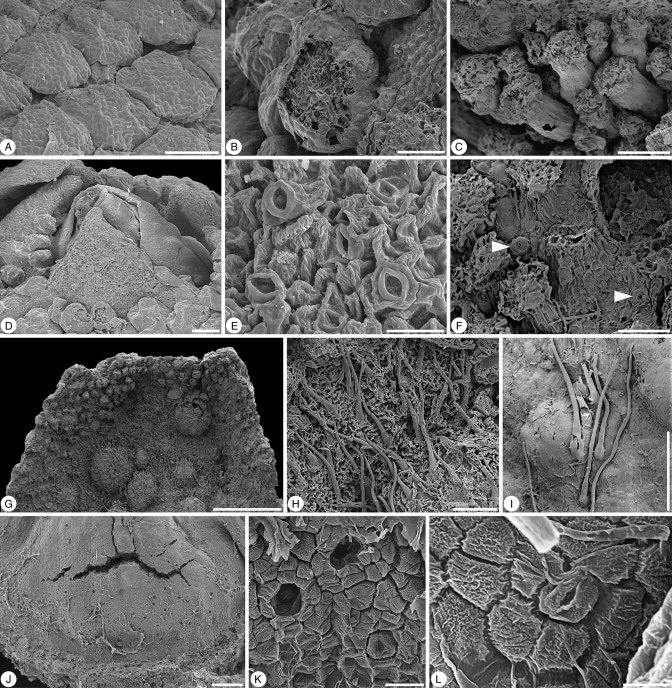
Glandulocalyx upatoiensis. Surface details of sepals, petals and gynoecium. (A) Well-developed, densely spaced multicellular trichomes on the abaxial surface of the sepals; PP55153 (holotype). (B) Abraded trichome from abaxial surface of sepal showing multicellular internal structure; PP55155. (C) Stalked multicellular trichomes at the base of a sepal; PP55158. (D) Close-up of apex of a floral bud showing sepal tips that are free of multicellular trichomes surrounding the tip of the corolla with its smooth petals; PP55153 (holotype). (E) Detail of stomata from the tip of a sepal; PP55153 (holotype). (F) Stomata (arrows) scattered among multicellular trichomes on abaxial surface of sepals; PP55158. (G) Adaxial surface in the distal half of a sepal showing multicellular trichomes at the periphery and simple, unicellular trichomes in the centre; note blisters formed during charcoalification; PP55155. (H) Detail of simple, unicellular trichomes on adaxial surface of sepal; PP55158. (I) Simple, unicellular trichomes on abaxial surface of petal; PP55158. (J) Base of ovary with stomata (nectary?); PP55155. (K) Detail of partly collapsed stomata at base of ovary; PP55155. (L) Detail of individual stoma at base of ovary, PP55155. Scale bars (A, C, D, I, J) = 100 µm; (B, F, H) = 50 µm; (E) = 20 µm; (G) = 500 µm; (L) = 5 µm; (K) = 10 µm.
Additional material
PP55154–PP55165 (dissections of PP55155 on associated SEM stubs 136, 137, 141, 142).
Locality
32°24′44′′N and 84°50'01''W on the south bank of Upatoi Creek on the Fort Benning Military Reservation, Chattahoochee County, Georgia, USA.
Stratigraphy and age
Lower part of the Eutaw Formation (early to middle Santonian) Late Cretaceous.
Etymology
The specific epithet refers to the Upatoi Creek in central-western Georgia where the fossil material was collected.
Specific diagnosis
As for genus with the following additions: floral buds close to anthesis (with corolla protruding) approx. 2 mm in diameter and 5 mm in length (specimens PP55157 and PP55165). Sepals broadly triangular and acuminate; multicellular trichomes present on the entire abaxial surface of sepals except for the sepal tips, and also on the distal third of the adaxial surface; sepal tips with stomata; simple, unicellular trichomes present on adaxial sepal surface. Petal shape (most probably) broadly ovate with a rounded apex; simple, unicellular trichomes present on abaxial petal surface. Stamen filaments inserted individually on floral base; anthers sagittate and with short connective tip; joint between anther and filament narrow and connective tissue between thecae weakly developed. Pollen with smooth surface. Ovary conical close to anthesis; styles slender, weakly capitate distally, with stylar canal; ovary glabrous with numerous stomata around the base.
Detailed description and remarks
The description is based entirely on dispersed floral buds preserved at slightly different developmental stages. The preservation of the material is not sufficient to study floral vasculature in detail. Anthetic flowers and mature fruits and seeds are not known. There is also no information about inflorescence structure or other parts of the plant.
Floral buds are small, ranging from 1·7 to 2·0 mm in diameter. In the earliest developmental stages preserved, in which the sepals completely enclose the petals (Figs 1 and 3A, J), the buds are more or less spherical. Closer to anthesis, the corolla elongates, extending beyond the calyx to give the bud a pointed apex. At this stage the bud is narrowly ovoid and approx. 5 mm long (Fig. 6A–C; Supplementary Data Video S1). The size of anthetic flowers is uncertain. However, the largest bud (approx. 2 mm in diameter; Fig. 3J) contains clearly distinguishable in situ pollen grains (Fig. 3L). The pollen grains were apparently almost mature when the bud was charcoalified, suggesting that the flower was probably close to anthesis. It is therefore likely that anthetic flowers were not much larger than this bud. Flowers are bisexual, polysymmetric and hypogynous, and the perianth is pentamerous (Figs 1, 5 and 6).
Fig. 3.
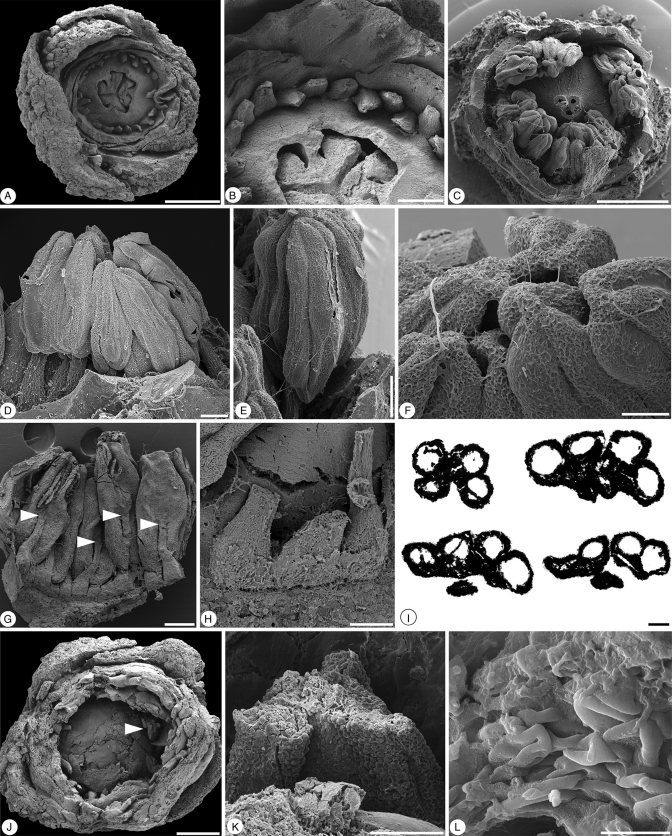
Glandulocalyx upatoiensis. Morphological details of stamens and pollen grains. (A) Apical view of damaged flower with part of perianth, anthers and top of ovary broken off, showing bases of stamen filaments arranged in a single series around the trilocular ovary with protruding-diffuse placentae; PP55156. (B) Detail of filament bases and protruding-diffuse placentae from the specimen in A; PP55156. (C) Apical view of the specimen in Fig. 1G–I dissected to reveal androecium and apex of gynoecium; PP55155. (D) Lateral view of the specimen in (C) showing stamens with filament and extrorse anther; PP55155. (E) Lateral view of stamen from specimen in (C); PP55155. (F) Apices of anthers from the specimen in (C) showing weakly developed connective tips; PP55155. (G) Adaxial view of group of stamens dissected from the specimen in Fig. 1F showing ventral (adaxial) anther attachment (arrows); PP55154. (H) Detail of broad filament bases; same specimen as in (G); PP55154. (I) Series of transverse sections through an anther of the specimen in Fig. 1D; ventral (adaxial) side facing downward; first section (upper left) showing the tetrasporangiate structure of the anther; second section (upper right) at the level of the joint between anther and filament; third section (bottom left) at the level below the joint of anther and filament with two theca united by a weakly developed connective; fourth section (bottom right) at the lower-most level where the theca are free from each other (sagittate anther); PP55160. (J) Apical view of broken, almost mature, floral bud; the arrow indicates partly preserved anther with in situ pollen shown in (K) and (L); PP55157. (K) Detail of broken anther with in situ pollen from specimen in J; PP55157. (L) In situ triaperturate pollen from the specimen in (J); PP55157. Scale bars (A, C, J) = 500 µm; (B, G) = 200 µm; (D, E, H, K) = 100 µm; (F, I, L) = 50 µm.
Fig. 5.
Glandulocalyx upatoiensis. Series of transverse microtome sections (left) and interpretative line drawings (right) of a relatively young floral bud, from apex (A) to base (I) of specimen PP55160 in Fig. 1D showing sepals (green), petals (red), stamens (yellow), gynoecium (blue) and floral base (grey); bud is laterally damaged with part of the calyx missing. (A) Distal part of bud with sepals, corolla and tips of styles. (B, C) Level of styles and anthers. (D) Level of distal part of ovary with three locules. (E) Level of stamen filaments and symplicate region of ovary. (F) Level of synascidiate region of ovary with protruding axile placentae. (G) Level of pendant parts of placentae. (H, I) Level of floral base. (J) Reconstruction of floral diagram showing five quincuncial sepals, five quincuncial petals, 24 extrorse stamens and the trilocular gynoecium. Scale bar (A–I) = 500 µm.
Calyx
The five sepals are nearly equal in size, separate from each other and inserted at approximately the same level on the receptacle (Fig. 5I). Aestivation is quincuncial, indicating a spiral sequence of organ initiation (Figs 1A and 5). Individual sepals are broadly triangular and acuminate (Fig. 1A, B), with the area of attachment narrower than their breadth (Fig. 5). The bases of at least the two outermost sepals are cordate. Sepals are several cell layers thick and the sepal bases are well developed. Partially broken sepals indicate that there are several vascular bundles in each sepal.
Over their entire abaxial surface the sepals bear numerous, prominent, closely spaced multicellular trichomes (Fig. 1A–C). The trichomes are largest near the median plane and base of each sepal and decrease in size distally and toward the sepal margins (Fig. 1B, C). Trichomes are only lacking from the tips of the sepals, and in this region there are numerous, densely spaced stomata (Fig. 2D, E). Individual stomata are also scattered on the abaxial sepal surface among the multicellular trichomes (Fig. 2F). In some buds (Fig. 1C) the trichomes are more or less spherical and almost sessile. In larger flower buds, especially toward the base of the sepals, the trichomes are more elongated with a large multicellular head (Fig. 2A–C) and a generally short multicellular stalk. Multicellular trichomes are also present along the sepal margins (Fig. 1A) and in the distal third of the adaxial sepal surface (Fig. 2G). Most of the adaxial epidermis of the sepals is densely covered by simple, unicellular trichomes (Fig. 2G, H).
Corolla
Petals are free (Figs 5 and 6), alternate with the sepals and also have quincuncial aestivation. Petal shape is difficult to reconstruct based on the material, but is most probably broadly obovate with a rounded apex. Petals are thin, only a few cell layers thick distally (Figs 5A, B and 6D–G), but almost as thick as the sepals proximally (Figs 5G–I and 6H–J). No vascular structure was observed in the petals. Simple, unicellular trichomes are present, scattered on the abaxial surface of the petals (Fig. 2I). In buds, the distal parts of the petals are erect, twisted with each other, and taper together to a point at the apex (Figs 1D, I, 2G, 6A, D, E, and Supplementary Data Video S1, S2). The length of the corolla in the larger, more mature, flower buds is 2·8–3·4 mm.
Androecium
Stamen number varies between 20 and 28, and the stamens are arranged in a single series (Figs 3A, B and 5, and Supplementary Data Video S3, S4). Specimen PP55160 (Fig. 5) has 20, specimen PP55156 (Fig. 3A, B) has 27 and specimen PP55165 has 28 stamens (Fig. 6). Filaments are inserted individually on the floral base (Figs 3B, G–H, 6H, I and 5H–I) and are not fused to each other or the corolla. Filament bases are dorsiventrally flattened and broader proximally (Fig. 3B, G–H). Broken filaments show a single vascular strand. Anthers are dithecal, tetrasporangiate, elongate and, at least in pre-anthetic flowers, longer than the filaments (Fig. 3D–F, I). Anthers are sagittate at the base and have a short connective tip. Anther attachment is ventral (adaxial) and the anthers are extrorse, i.e. stomia are directed towards the periphery of the flower (Fig. 3D, G, I). Whether anther dehiscence extends along the full length of the anther cannot be established; there are no anthetic anthers available. The joint between anther and filament is narrow (Fig. 3G). The connective tissue between the thecae is only weakly developed (Fig. 3I). Pollen grains preserved in situ in the anthers are triaperturate, but perhaps not quite mature. The pollen surface appears smooth with no supratectal elements. Other details of pollen structure are not preserved (Fig. 3L).
Gynoecium
The gynoecium is syncarpous, superior and consists of three carpels. Syncarpy extends to the top of the ovary; the three styles are free from each other (Figs 4B, 6E, and Supplementary Data Video S5). The ovary is semi-globose in younger developmental stages (Fig. 4A, E) and becomes cone shaped closer to anthesis (Fig. 6 B, C, and Supplementary Data Video S1, S5). The gynoecium is trilocular and apparently synascidiate below (Figs 5F–I and 6J) and symplicate above (Figs. 4G, 5D–E and 6H). The boundary between these two zones cannot be determined accurately based on the material available. Styles are slender and weakly capitate distally (Fig. 4B, and Supplementary Data Video S5). Internally, they have a distinct stylar canal that extends for their whole length (Figs 4D, 6E). A distinct ventral groove is clearly visible on the stigma in younger developmental stages (Fig. 4B, C).
Fig. 4.
Glandulocalyx upatoiensis. Details of gynoecium from the specimen in Fig. 1G–I. PP55155. (A) Apical view of dissected specimen showing tips of three styles in the centre of the flower and remains of calyx, corolla and stamens. (B) Oblique lateral view of three styles showing expanded stigmatic surfaces with ventral groove. (C) Apical view of the tips of three styles with ventral grooves. (D) Apical view of ovary showing three broken styles, each with a central canal. (E) Lateral view of dissected ovary showing one of the three locules with protruding placenta and diffuse insertion of numerous ovules. (F) Detail of protruding-diffuse placenta showing developing ovules. (G) Detail of distal part of locule showing symplicate region where carpel margins are not united. Scale bars (A) = 500 µm; (B, E) = 200 µm; (C, D, F) = 100 µm; (G) = 50 µm.
The gynoecium is glabrous with numerous stomata around its base (Fig. 2J). Most of these stomata are collapsed, leaving small, circular holes in the epidermis of the ovary (Fig. 2K, L), but whether these stomata and the underlying tissues were nectariferous cannot be established. No vascular structure has been observed in the gynoecium.
Ovules are numerous and inserted on axile, protruding-diffuse placentae, which reach deeply into the locules (Figs 4E, F, 5E–G and 6H, I). Placentae are also pendant and, below their level of attachment, extend downwards into the lowermost parts of the locules. At this level, the placentae appear free in transverse sections (Figs 5G–I, 6B, C, J, and Supplementary Data Video S1). Details of the structure of the ovules, mature seeds and fruits are not known.
DISCUSSION
Comparison with other fossil flowers of Ericales
The pentamerous, polysymmetric and hypogynous flowers of Glandulocalyx, with their polystemonous androecium and trimerous, syncarpous gynoecium, suggest a possible relationship with extant Ericales. All of these floral features are widespread in the order. There are also similarities between Glandulocalyx and previously described fossil flowers from the Late Cretaceous that have been assigned to Ericales.
Most similar to Glandulocalyx is the fossil flower Parasaurauia allonensis described from the Allon locality (Santonian) in Georgia, USA, which was assigned to Actinidiaceae based on a preliminary cladistic analysis (Keller et al., 1996). Parasaurauia has prominent multicellular trichomes on the calyx similar to those of Glandulocalyx and is also pentamerous and hypogynous and has a trimerous gynoecium with protruding-diffuse axile placentae. However, Glandulocalyx differs from P. allonensis in its polystemonous androecium (ten stamens in two whorls of five in Parasaurauia), the absence of a depression at the apex of the ovary and the more prominently pendant placentae with numerous small ovules. The anthers in Parasaurauia are also more bulky, much more strongly sagittate at the base and basifixed.
Also similar to Glandulocalyx is Pentapetalum trifasciculandricus described from the Old Crossman locality (Turonian) in New Jersey, USA, which may be related to Pentaphylacaceae (Martínez-Millán et al., 2009). Pentapetalum is pentamerous and hypogynous with a polystemonous androecium and a trimerous, trilocular gynoecium. However, Pentapetalum is interpreted as having the stamens arranged in three groups, and the stamens have long filaments and short anthers in bud. Also, the placentae, which bear fewer and larger ovules arranged in two rows, are not protruding nor do they seem to be pendant. In addition, whereas the calyx of Pentapetalum appears to be covered with trichomes, these are different from the prominent multicellular trichomes on the calyx of Glandulocalyx.
Similarities between Glandulocalyx and other fossil flowers assigned to Ericales are much less pronounced. Glandulocalyx differs from Paleoenkianthus sayrevillensis, also from the Old Crossman locality (Turonian) (Nixon and Crepet, 1993), in the number of stamens (eight in Paleoenkianthus), which have long slender filaments and anthers that are described as U-shaped and awned. The gynoecium of Paleoenkianthus is also tetramerous. Paleoenkianthus is regarded as an early diverging lineage of Ericaceae, although it was not formally assigned to any suprageneric taxon (Nixon and Crepet, 1993).
Glandulocalyx differs from Paradinandra suecica from the Åsen locality (Campanian–Santonian) in southern Sweden (Schönenberger and Friis, 2001) in the number and arrangement of stamens in the polystemonous androecium (15 in two whorls in Paradinandra vs. 20–28 in a single series in Glandulocalyx), the hollow rather than solid styles and the trilocular rather than unilocular ovary. The stamens in Paradinandra also have long filaments with short anthers. Paradinandra suecica appears to be related to Pentaphylacaceae (sensu APG, III) (Schönenberger and Friis, 2001). Actinocalyx bohrii from the Åsen locality is also distinct in having an androecium of only five stamens, with long slender filaments and short anthers, and a sympetalous corolla. Actinocalyx is thought to be closely related to Diapensiaceae (Friis, 1985).
Comparison with extant taxa
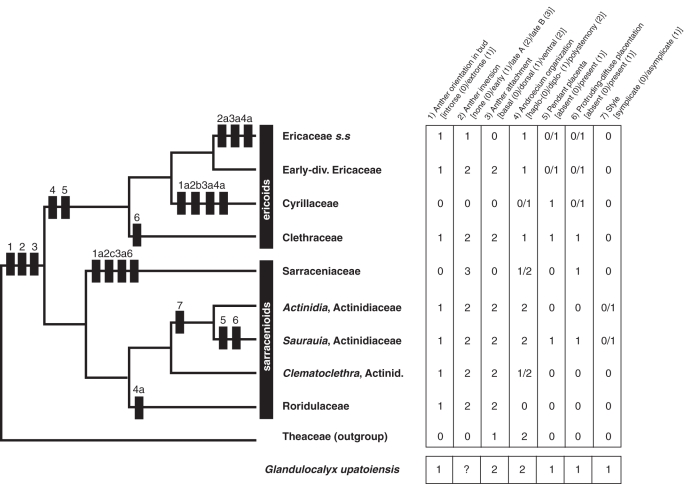
Ericales are a large and morphologically diverse order of asterids (Stevens, 2001 onwards) for which the composition has been circumscribed and monophyly established only relatively recently, largely based on phylogenetic analyses of molecular data (Soltis et al., 2000; Albach et al., 2001; Barkman et al., 2004). In earlier classifications the groups that now comprise Ericales were often assigned to 10–12 different angiosperm orders (e.g. Cronquist, 1981; Dahlgren, 1983), many of which were thought to be only distantly related. Current phylogenetic concepts place Ericales as the second lineage to diverge from the main line of asterid evolution after Cornales. In Ericales, results from molecular phylogenetics (Anderberg et al., 2002; Schönenberger et al., 2005) suggest that the balsaminoids (Balsaminaceae, Marcgraviaceae and Tetrameristaceae) are sister to the other 19 families of the order, with the polemonioids (Polemoniaceae and Fouquieriaceae) as the next diverging lineage. The remaining families fall into three broad groups, the primuloids plus Ebenaceae, the styracoids and ‘core Ericales’. The exact relationships among these groups and several additional families are still not resolved (Stevens, 2001 onwards; Schönenberger et al., 2005). ‘Core Ericales’ comprise a strongly supported clade of six families (Actinidiaceae, Roridulaceae, Sarraceniaceae, Clethraceae, Cyrillaceae and Ericaceae), in which the sarracenioid clade (Actinidiaceae, Roridulaceae and Sarraceniaceae) and the ericoid clade (Clethraceae, Cyrillaceae and Ericaceae) are sister taxa (Fig. 8). Interfamilial relationships in the sarracenioids and ericoids are well established (Fig. 8; Schönenberger et al., 2005).
Fig. 8.
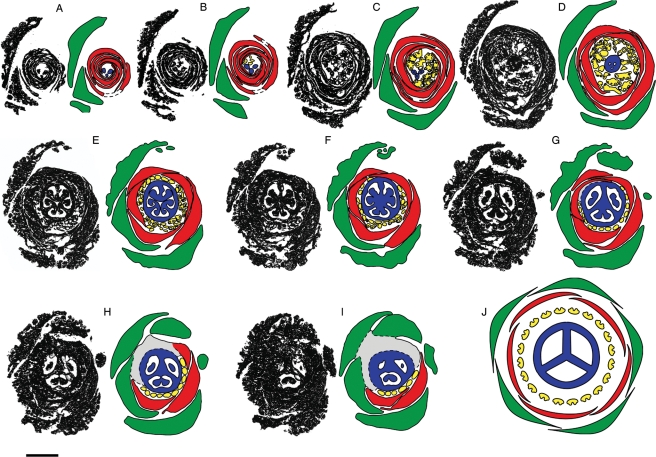
Distribution and likely evolution of selected floral characters in core Ericales. Phylogenetic relationships largely based on Schönenberger et al. (2005); sister group relationship of Actinidia and Saurauia based on Keller et al. (1996); early diverging lineages of Ericaceae include Enkianthoideae and Monotropoideae (sensu Stevens et al., 2004). Character coding of extant taxa based on primary literature and family descriptions in Kubitzki (2004b) mentioned in the Discussion. Black rectangles indicate character state changes: (1) from introrse to extrorse anther orientation in bud; (1a) reversal to introrse anther orientation; (2) from non-inverting anthers to ‘late anther inversion type A’; (2a) from ‘late anther inversion type A’ to early anther inversion; (2b) reversal to non-inverting anthers; (2c) from ‘late anther inversion type A’ to ‘late anther inversion type B’; (3) from dorsal to ventral anther attachment; (3a) from ventral to basal anther attachment; (4) from polystemony to diplostemony; (4a) from polystemony to haplostemony in Roridulaceae and from diplostemony to haplostemony in Cyrillaceae; (5) from non-pendant to pendant placentae; (6) from non-protruding-diffuse to protruding-diffuse placentae; (7) from symplicate to asymplicate styles.
Nevertheless, despite recent progress in molecular phylogenetics, conflicting patterns in the distributions of morphological characters make the determination of phylogenetic relationships among the extant taxa based on morphology, and comparisons with molecular results, challenging. Except for the balsaminoids (Schönenberger et al., 2010) and the polemonioids (Schönenberger, 2009), there are also no recent comparative studies of floral structure. It is therefore not surprising that there are currently no clear-cut non-molecular synapomorphies for the order as a whole, or for most of its larger sub-clades. Poor knowledge of the structure and diversity of extant Ericales is a major impediment to assessing the phylogenetic relationships of fossil taxa.
Keller et al. (1996) used the early cladistic analyses of Anderberg (1992, 1993) based on morphological features to evaluate the relationships of the fossil taxon P. allonensis. That analysis placed P. allonensis sister to Actinidia and Saurauia in Actinidiaceae. However, since then there have been numerous changes in family delimitations and an increase in the number of families assigned to Ericales as the clade is now circumscribed (Stevens, 2001 onwards). All attempts so far to use morphological characters for phylogenetic analyses of Ericales (Anderberg, 1992, 1993; Keller et al., 1996; Luna and Ochoterena, 2004; Martínez-Millán et al., 2009) have either suffered from an absence of appropriate structural data or have been based on an outdated concept of the taxonomic circumscription of the order. As a result, their explanatory power for interpreting interfamilial or higher level relationships in Ericales has been limited and often at odds with hypotheses of relationships based on molecular analyses.
A series of comparative studies of floral structure covering all the major clades of Ericales (Schönenberger, 2009; Schönenberger et al., 2010; J. Schönenberger et al., unpubl. res.) is currently underway to compile a comprehensive structural data set for morphology-based phylogenetic analyses of extant and fossil taxa. Until this is complete, a formal cladistic analysis of the relationships of Glandulocalyx would be premature and potentially misleading. Instead, we focus here on similarities and differences with the most relevant extant and fossil taxa, placing particular emphasis on the organization of the androecium and the gynoecium.
Androecium
One important feature is the presence of so-called inverted anthers in the six families of core Ericales (Actinidiaceae, Roridulaceae, Sarraceniaceae, Clethraceae, Cyrillaceae and Ericaceae). There seems to be confusion in the literature as to what comprises anther inversion in the different groups, but initial studies by one of us (J.S.) suggest that there are three different patterns that probably have different ontogenetic bases.
So-called ‘early anther inversion’ is restricted to Ericaceae sensu stricto (s.s.; all lineages of Ericaceae except for Enkianthoideae and Monotropoideae sensu Stevens et al., 2004). This type of anther inversion is characterized by a complete inversion of the anther that begins during early stages of stamen development so that at maturity the morphological apex appears at the base and the morphologically dorsal (abaxial) side of the anther is brought into an adaxial position (Artopoeus, 1903; Matthews and Knox, 1926; Matthews and Taylor, 1926; Paterson, 1961).
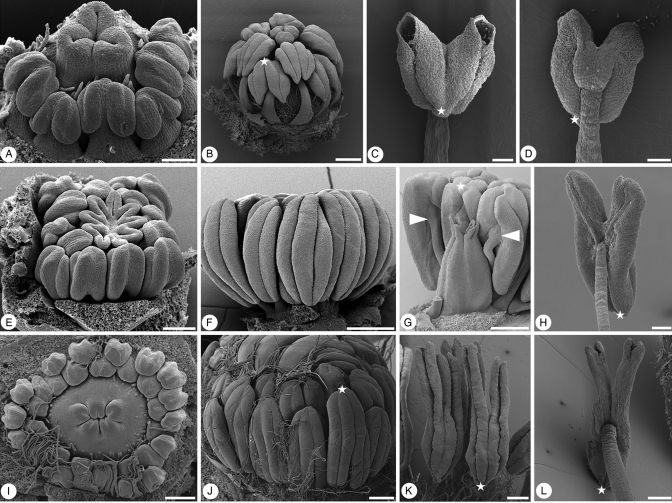
The second type of anther inversion, here called ‘late anther inversion type A’, is characterized by ‘normal’ development of the stamens (Fig. 7). Only at the beginning of anthesis do the anthers invert more or less strongly through growth processes in the distal part of the filament (Fig. 7D, H, L). ‘Late anther inversion type A’ is generally associated with ventral (adaxial) anther attachment and extrorse anther orientation in the bud stage as shown here for extant representatives of Clethra, Actinidia and Saurauia (Fig. 7B, F, G, J).
Fig. 7.
Androecium organization in extant representatives of core Ericales. (A–D) Clethra sp. (Clethraceae). (A) Oblique lateral view of young floral bud showing extrorse orientation of anthers; perianth removed. (B) Lateral view of floral bud with extrorse and ventrifixed anthers in a developmental stage just before anthesis; star indicates morphological apex of one of the anthers. (C) Ventral view (as seen in intact flower) of anthetic stamen with inverted (now introrse) anther; star indicates morphological apex of anther. (D) Dorsal view (as seen in intact flower) of anthetic stamen showing anther attachment; star indicates morphological apex of anther. (E–H) Actinidia kolomikta (Actinidiaceae). (E) Oblique lateral view of young floral bud showing extrorse orientation of anthers; perianth removed. (F) Lateral view of floral bud with extrorse and ventrifixed anthers in a developmental stage close to anthesis. (G) Lateral view of floral bud in a developmental stage close to anthesis; perianth and part of stamens removed; arrows indicate points of ventral anther attachment; star indicates morphological apex of one of the anthers. (H) Dorsal view (as seen in intact flower) of anthetic stamen showing anther attachment; star indicates morphological apex of anther. (I–L) Saurauia montana (Actinidiaceae). (I) Apical view of young floral bud showing developing stamens with extrorse orientation of anthers; perianth removed. (J) Lateral view of floral bud with extrorse and ventrifixed anthers in a developmental stage close to anthesis; star indicates morphological apex of one of the anthers; perianth removed. (K) Group of anthetic stamens with inverted anthers; star indicates morphological apex of one of the anthers. (L) Dorsal view (as seen in intact flower) of anthetic stamen showing anther attachment; star indicates morphological apex of anther. Scale bars (A, K) = 100 µm; (B, F, G, J) = 500 µm; (C–E, H, I, L) = 200 µm.
In Clethra sp., the androecium is diplostemonous; anther attachment is ventral and the orientation is extrorse in pre-anthetic flowers (Fig. 7A, B). At the beginning of anthesis, the anthers invert through growth processes in the distal part of the filament, turning the anther upside down, resulting in an introrse orientation of the anthetic anther (Fig. 7C, D). Anther dehiscence is by pores, which are located at the morphological base of the thecae. Due to the inversion of the anthers, the pores come to lie at the distal end of the anthetic stamen (Fig. 7C).
In A. kolomikta, the androecium is polystemonous with about 15 stamens. Again, anther orientation is extrorse in pre-anthetic stages (Fig. 7E, F) and anther attachment is ventral (Fig. 7G). Anther dehiscence is by longitudinal slits (not shown). At the start of anthesis, anthers invert more or less strongly, again resulting in an upside down orientation of the anthetic anther (Fig. 7H).
This type of anther inversion, ‘late anther inversion type A’, in which inversion of the anther at anthesis brings the dorsal side (abaxial) of the anther into an adaxial position making the anthers functionally introrse at anthesis, occurs in early diverging lineages of Ericaceae (Copeland, 1941, 1947; Hermann and Palser, 2000), in Clethraceae (Thomas, 1961; Schneider and Bayer, 2004), in Roridulaceae (Conran, 2004), and in Actinidiaceae (Hunter, 1966; Soejarto, 1969; Dickison, 1972).
A third type of anther inversion, here called ‘late anther inversion type B’, occurs in Sarraceniaceae. In this family, anther attachment is basal and anthers are introrse in bud. At the beginning of anthesis, anthers also invert in this family, but in the other direction compared with the families mentioned above, resulting in functionally extrorse anthers (see figures in Renner, 1989 and Kubitzki, 2004c; J.S., pers. obs.). No anther inversion is present in Cyrillaceae (Kubitzki, 2004a).
Based on the phylogenetic relationships of the above families as assessed from molecular data (Schönenberger et al., 2005), it is likely that ‘late anther inversion type A’ and possibly also the associated features of ventral anther attachment and extrorse orientation of the anthers in bud, are synapomorphies of the large clade including all six families (Fig. 8). These features have apparently been lost in Cyrillaceae, which have basally attached introrse anthers that do not invert. Early anther inversion as present in Ericaceae s.s. is most probably a derived feature and a synapomorphy for that particular clade. Likewise, ‘late anther inversion type B’ in Sarraceniaceae is also most probably derived from ‘early anther inversion type A’.
In the flowers of Glandulocalyx, the anthers are clearly ventrally attached and anther orientation is extrorse in the bud stage (Fig. 3D, G, I). Since all specimens found so far are in a pre-anthetic stage, it is not possible to say whether the anthers of the fossils did in fact invert at anthesis. However, the presence of ventral attachment and extrorse anther orientation strongly suggests that they did. The presence of these features also allows Glandulocalyx to be referred to the six-family clade and at the same time to be excluded from the Ericaceae s.s., Cyrillaceae and Sarraceniaceae. Outside this larger clade, ventrally attached anthers are rare and are to our knowledge only present in one other ericalean family, Polemoniaceae (Schönenberger, 2009). Polemoniaceae, however, differ markedly from Glandulocalyx in their strongly sympetalous corolla, exclusive haplostemony and introrse anthers (despite ventral attachment). In Polemoniaceae the gynoecium is trimerous, as in Glandulocalyx, but the style is symplicate over most of its length. The number of ovules is much smaller than in Glandulocalyx and the ovules are arranged in two collateral rows.
A further important androecial feature relates to stamen number, which in Ericales is highly variable among and within families (see Schönenberger et al., 2005 for discussion of androecium organization in Ericales). The same is also true for core Ericales: Ericaceae and Clethraceae are diplostemonous throughout; Cyrillaceae are haplo- or diplostemonous; Roridulaceae are haplostemonous; Sarraceniaceae are diplo- or polystemonous; and most Actinidiaceae are polystemonous, with diplostemony occurring only in Clematoclethra. The most parsimonious explanation for androecium evolution in core Ericales is that the most recent common ancestor of this clade was polystemonous (Fig. 8). Whereas polystemony is still dominant in the sarracenioid clade, diplostemony has probably evolved along the stem lineage leading to the ericoid clade. With 20 or more stamens, the androecium of Glandulocalyx is plesiomorphic in this respect, leaving open the possibility of a probable phylogenetic relationship to the sarracenioid clade.
Pollen structure has been comparatively studied among all families of core Ericales by Zhang and Anderberg (2002) and was found to be rather uniform. Pollen in this clade is oblate, speroidal or sub-prolate, tricolporate with three long, tapering furrows. The general pollen structure of Glandulocalyx (Fig. 3L) fits well with these features. The pollen surface has been described as rather unspecialized for Actinidiaceae (Dressler and Bayer, 2004), either with no discernible ornamentation (Saurauia; Hunter, 1966) or with a rugulate tectum (Actinidia; Zhang and Anderberg, 2002). In Clethraceae, the pollen surface varies from almost smooth to rugulate (Zhang and Anderberg, 2002). Surface ornamentations of Actinidiaceae and Clethraceae (and Sarraceniaceae) are similar to each other, and Zhang and Anderberg (2002) hypothesized that this pollen type may be symplesiomorphic for core Ericales. Pollen of Glandulocalyx with its smooth surface most probably also reflects this symplesiomorphic type and therefore does not help to place the fossil among lineages of extant core Ericales.
In summary, androecial features strongly support a relationship of Glandulocalyx with core Ericales. Taking into account stamen numbers, a potential relationship with the sarracenioids, in particular with Actinidia and Saurauia (Actinidiaceae), is possible and, based on other features, seems likely (see below).
Gynoecium
While all Ericales apparently have pluricarpellate, syncarpous gynoecia and superior ovaries, there is considerable diversity in carpel number, degree of carpel fusion, type of placentation and number of ovules. Three carpels per flower are particularly common in the order, but whether this is plesiomorphic for the order as a whole remains to be established. In core Ericales, three carpels are particularly common in the sarracenoid clade, but also occur in the other families. Higher numbers are particularly common in Actinidia. Most Cretaceous ericalean fossils including Glandulocalyx are apparently tricarpellate (Paleoenkianthus is tetracarpellate; Nixon and Crepet, 1993). However, since carpel number is variable and since three carpels are also present in all core ericalean families, this feature is uninformative for establishing the phylogenetic relationships of Glandulocalyx.
Style structure is more relevant to determining the relationships of Glandulocalyx. Most extant Ericales have a single united (symplicate) style, with variation in the degree to which the stigmatic lobes are free from each other (asymplicate region). Among core Ericales, style structure varies considerably, but styles that are free to the base (asymplicate) as in Glandulocalyx and most other ericalean fossils described so far from the Late Cretaceous occur only in Saurauia and Actinidia (Actinidiaceae; Dressler and Bayer, 2004) among extant lineages. Given that a symplicate style is apparently plesiomorphic for Ericales as a whole and for core Ericales, the free styles in these taxa are most parsimoniously interpreted as a synapomorphy for the clade comprising Saurauia, Actinidia and Parasaurauia (see also Keller et al., 1996). The free styles seen in Glandulocalyx may be evidence of a close relationship with this clade.
An apical depression of the top of the ovary, in which the styles are inserted, is sometimes present in Actinidiaceae (Dressler and Bayer, 2004) and was also found in Parasaurauia (Keller et al. 1996). However, this feature is not seen in the ovary of Glandulocalyx. The systematic utility of this feature is limited by the fact that it is not only variable in Actinidiaceae, but also occurs in many other representatives of core Ericales including Sarraceniaceae and most lineages of the ericoid clade (see also Keller et al., 1996).
Placentation is generally axile in core Ericales, and axile placentae are also present in Glandulocalyx. Two less common placental features of Glandulocalyx among Ericales as a whole are the presence of distinct protruding-diffuse placentae that are pendant in their lower part. This combination of placental features is apparently common among core Ericales (Fig. 8). Among ericoids, it is present in Clethra (Aghard, 1858; Schnarf, 1924) and various Ericaceae (e.g. Aghard, 1858; Paterson, 1961), particularly among early diverging lineages of Ericaceae such as Pyroloideae and Monotropoideae (Pyykkö, 1969). In Cyrillaceae, the placentae are also pendant but are rather small, bearing only 1–3 ovules each (Thomas, 1961; Kubitzki, 2004a). In the sarracenioid clade, protruding-diffuse, pendant placentae are restricted to Saurauia (Schnarf, 1924; Dickison, 1972). In Actinidia, placentae do not protrude and placentation is simply axile, whereas in Clematoclethra the axile placentae project upward into the carpel locules (Dickison, 1972). In Roridulaceae, placentation is simple and axile, with only 1–4 ovules per locule (Diels, 1930; Conran, 2004). In Sarraceniaceae placentation is axile, protruding-diffuse but apparently not pendant (Shreve, 1906; Uphof, 1936).
In summary, the gynoecial features of Glandulocalyx, especially the free styles, support a possible relationship with the sarracenioids, particularly Saurauia. However, according to the literature, placentation in Saurauia and Clethra is remarkably similar (Schnarf, 1924) and a possible relationship of Glandulocalyx with Clethraceae cannot be excluded. Current knowledge of the diversity of ovary structure in core Ericales suggests that the similarities in placentation of Saurauia and Clethra are the result of convergent evolution. However, details of placenta structure are not yet well known among extant core Ericales, and an alternative explanation might be that protruding-diffuse pendant placentae are plesiomorphic for the group as a whole.
Floral indumentum
Ericales are diverse in specialized epidermal features (see Kubitzki, 2004b, and citations therein). Several families exude oils or other substances, and these are sometimes associated with multicellular epidermal structures. For example, perianth parts of both Actinidiaceae (Hunter, 1966; Keller et al., 1996; Dressler and Bayer, 2004) and Clethraceae (Kavaljian, 1952) exhibit a variety of different multicellular trichomes. Nevertheless, we are not aware of epidermal structures in any extant taxa that are comparable with the distinctive multicellular trichomes that cover the calyx of Glandulocalyx. However, other Late Cretaceous ericalean flowers are also characterized by complex multicellular and/or potentially glandular trichomes on the sepals [e.g. Parasaurauia (Keller et al., 1996); Palaeoenkianthus (Nixon and Crepet, 1993); and other, so far unnamed, Cretaceous taxa with ericalean affinities (Crepet, 1996, 2008)]. These kinds of floral indumentum composed of sclerified or glandular trichomes are thought to have a protective function, e.g. against phytophagous insects (Strauss and Zangerl, 2002), but may also be linked to other ecological factors.
Floral biology
A clearly differentiated perianth with well-developed petals suggests that Glandulocalyx was insect pollinated. It is likely that the polystemonous flowers produced relatively large amounts of pollen, which may have been offered as a reward. In general, pollen-flowers are mainly visited by beetles and bees (Endress, 1994). Only pre-anthetic stages are known of Glandulocalyx, but the general floral organization with free petals indicates that the flowers were simple, open bowl shaped at anthesis. Such an open floral construction is also consistent with a pollen-flower syndrome as is found for instance in many Theaceae, Ranunculaceae or Papaveraceae (Papaver-type pollen-flowers; Vogel, 1978). In these flowers the pollen is often powdery and is either shed on the perianth or collected directly from the (sometimes poricidal) anthers by bees (Endress, 1994). Extant Actinidia and Saurauia also have pollen-flowers and are pollinated by pollen-collecting bees (Soejarto, 1969; Schmid, 1978; Haber and Bawa, 1984; Cane, 1993). Little is known about pollination in Clethraceae, other than that Clethra alnifolia is bee pollinated (Reed, 2006). The distinctly poricidal anthers of Clethra (see Fig. 7C) indicate a buzz-pollination system, in which pollinating bees vibrate the stamens to release dry pollen from the anthers.
Whether the stomata that are present on the lower part of the ovary of Glandulocalyx were involved in nectar release cannot be established based on the fossil material. Most species of Actinidiaceae and Clethraceae apparently do not produce nectar (Dressler and Bayer, 2004; Schneider and Bayer, 2004), but Brown (1935) mentioned that Saurauia subspinosa produces nectar at the base of the corolla, without giving any further details. Similarly, Schmid (1978) reported small amounts of liquid on the adaxial surface of the petal bases of Actinidia chinensis. Brown (1938) described abundant nectar secretion from the lower part of the ovary in Clethra lancifolia, a condition that may be similar to that in Glandulocalyx. Among other Ericales, nectar production from the lower part of the ovary has been described for Fouquieriaceae and Polemoniaceae (Henrickson, 1972; Schönenberger, 2009).
In summary, the floral structure of G. upatoiensis strongly supports a phylogenetic relationship among core Ericales. Synapomorphies shared by Glandulocalyx and extant taxa in this clade include extrorse anther orientation in the bud, ventral anther attachment and possibly also late inversion (type A) of the anthers. Within core Ericales, the strongest similarities of Glandulocalyx are mainly with Actinidiaceae and in particular with the genus Saurauia. Shared, potentially synapomorphic, features of Saurauia and Glandulocalyx include protruding-diffuse and pendant placentae and the presence of free styles, a feature that also occurs in Actinidia. The same type of placentation is also present in Clethraceae. However, this family is distinct from Glandulocalyx (and Saurauia) in having consistently diplostemonous flowers with a symplicate style.
We conclude that placement of G. upatoiensis in Actinidiaceae is justified based on the information currently available, but stronger support for this, and for the phylogenetic placement of other fossil Ericales from the Late Cretaceous, will require a better understanding of floral structure and diversity among the diverse lineages of extant Ericales. Without secure structural synapomorphies to diagnose specific extant groups defined on the basis of molecular data, unequivocal systematic placement of even the best preserved fossil flowers will not be possible.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
P.R.C. and P.S.H. thank Andrew Drinnan, Jennifer Keller, Hallie Sims and Richard Lupia for help with fieldwork. John Brent, Fort Benning Military Reservation, provided access to the localities on Upatoi Creek and assisted with fieldwork. We are grateful to Kentaro Uesugi for assistance with the tomographic study at SPring-8. We used the X-ray micro-CT system at BL20B2 in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (proposal 2008A1027). Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract no. DE-AC02-06CH11357. We thank Nobuhito Nango and Kazutaka Nomura (Ratoc System Engineering) for their technical support in three-dimensional reconstruction software. We are grateful to Susanne Sontag and Susanne Pamperl for SEM preparations of extant floral material and help with graphics at the University of Vienna. We thank Peter K. Endress and an anonymous colleague for critically reviewing the manuscript. This work was supported by a grant-in-aid (18570083) from the Ministry of Education, Science, and Culture of Japan to M.T. This work was initiated with support from the Japan Society for the Promotion of Science (S-97128, S-98106) and the National Science Foundation (EAR-9614672) to P.R.C., and completed, in part, with financial support from the World Class University program of the National Research Foundation of Korea (R33-10089).
LITERATURE CITED
- Agardh JG. Theoria systematis plantarum. Lund: C. W. K. Gleerup; 1858. [Google Scholar]
- Albach DC, Soltis PS, Soltis DE, Olmstead RG. Phylogenetic analysis of asterids based on sequences of four genes. Annals of the Missouri Botanical Garden. 2001;88:163–212. [Google Scholar]
- Anderberg AA. The circumscription of the Ericales, and their cladistic relationships to other families of higher dicotyledons. Systematic Botany. 1992;17:660–675. [Google Scholar]
- Anderberg AA. Cladistic interrelationships and major clades of the Ericales. Plant Systematics and Evolution. 1993;184:207–231. [Google Scholar]
- Anderberg AA, Rydin C, Källersjö M. Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. American Journal of Botany. 2002;89:677–687. doi: 10.3732/ajb.89.4.677. [DOI] [PubMed] [Google Scholar]
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Artopoeus A. Über den Bau und die Öffnungsweise der Antheren und die Entwicklung der Samen der Erikaceen. Flora. 1903;92:309–345. [Google Scholar]
- Barkman TJ, Lim SH, Salleh KM, Nais J. Mitochondrial DNA sequences reveal the photosynthetic relatives of Rafflesia, the world's largest flower. Proceedings of the National Academy of Sciences, USA. 2004;101:787–792. doi: 10.1073/pnas.0305562101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. American Journal of Botany. 2010;97:1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- Brown EGS. The floral mechanism of Saurauia subspinosa Anth. Transactions and Proceedings of the Botanical Society of Edinburgh. 1935;31:485–497. [Google Scholar]
- Brown WH. The bearing of nectaries on the phylogeny of flowering plants. Proceedings of the American Philosophical Society. 1938;79:549–595. [Google Scholar]
- Cane JH. Reproductive role of sterile pollen in Saurauia (Actinidiaceae), a cryptically dioecious neotropical tree. Biotropica. 1993;25:493–495. [Google Scholar]
- Christopher RA. Palynostratigraphy of the basal Cretaceous units of the eastern Gulf and southern Atlantic Coastal Plains. In: Arden DD, Beck BF, Morrow E, editors. Proceedings of the Second Symposium on the Geology of the Southeastern Coastal Plain. Atlanta: Georgia Geological Survey; 1982. pp. 10–23. [Google Scholar]
- Conran JG. Roridulaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004. pp. 339–342. [Google Scholar]
- Copeland HF. Further studies on Monotropoideae. Madroño. 1941;6:97–144. [Google Scholar]
- Copeland HF. Observations on the structure and classification of the Pyroleae. Madroño. 1947;9:33–64. [Google Scholar]
- Crepet WL. Timing in the evolution of derived floral characters: Upper Cretaceous (Turonian) taxa with tricolpate and tricolpate derived pollen. Review of Palaeobotany and Palynology. 1996;90:339–359. [Google Scholar]
- Crepet WL. The fossil record of angiosperms: requiem or renaissance? Annals of the Missouri Botanical Garden. 2008;95:3–33. [Google Scholar]
- Cronquist A. An integrated system of classification of flowering plants. New York: Columbia University Press; 1981. [Google Scholar]
- Dahlgren R. General aspects of angiosperm evolution and macrosystematics. Nordic Journal of Botany. 1983;3:119–149. [Google Scholar]
- Dickison WC. Observations on the floral morphology of some species of Saurauia, Actinidia, and Clematoclethra. Journal of the Elisha Mitchell Scientific Society. 1972;88:43–54. [Google Scholar]
- Diels L. Roridulaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. 18a. Leipzig: W: Engelmann; 1930. pp. 346–348. [Google Scholar]
- Dressler S, Bayer C. Actinidiaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004. pp. 14–19. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Friis EM. Actinocalyx gen.nov., sympetalous angiosperm flowers from the Upper Cretaceous of southern Sweden. Review of Palaeobotany and Palynology. 1985;45:171–183. [Google Scholar]
- Friis EM, Crane PR, Pedersen KR. Early flowers and angiosperm evolution. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Haber WA, Bawa KS. Evolution of dioecy in Saurauia (Dilleniaceae) Annals of the Missouri Botanical Garden. 1984;71:289–293. [Google Scholar]
- Henrickson J. A taxonomic revision of the Fouquieriaceae. Aliso. 1972;7:439–537. [Google Scholar]
- Hermann PM, Palser BF. Stamen development in the Ericaceae. I. Anther wall, microsporogenesis, inversion, and appendages. American Journal of Botany. 2000;87:934–957. [PubMed] [Google Scholar]
- Hunter GE. Revision of Mexican and Central American Saurauia (Dilleniaceae) Annals of the Missouri Botanical Garden. 1966;53:47–89. [Google Scholar]
- Igersheim A. The character states of the Caribbean monotypic endemic Strumpfia (Rubiaceae) Nordic Journal of Botany. 1993;13:545–559. [Google Scholar]
- Igersheim A, Cichocki O. A simple method for microtome sectioning of prehistoric charcoal specimens, embedded in 2-hydroxyethyl methacrylate (HEMA) Review of Palaeobotany and Palynology. 1996;92:389–393. [Google Scholar]
- Kavaljian LG. The floral morphology of Clethra alnifolia with some notes on C. acuminata and C. arborea. Contributions from the Hull Botanical Laboratory 632. Botanical Gazette. 1952;113:392–413. [Google Scholar]
- Keller JA, Herendeen PS, Crane PR. Fossil flowers and fruits of the Actinidiaceae from the Campanian (Late Cretaceous) of Georgia. American Journal of Botany. 1996;83:528–541. [Google Scholar]
- Kubitzki K. Cyrillaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004a. pp. 114–116. [Google Scholar]
- Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004b. [Google Scholar]
- Kubitzki K. Sarraceniaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004c. pp. 422–425. [Google Scholar]
- Leslie AB, Herendeen PS, Crane PR. Upatoia barnardii gen. et sp. nov., an araucarian pollen cone with in situ pollen from the Late Cretaceous (Santonian) of Georgia, USA. Grana. 2009;48:128–135. [Google Scholar]
- Luna I, Ochoterena H. Phylogenetic relationships of the genera of Theaceae based on morphology. Cladistics. 2004;20:223–270. doi: 10.1111/j.1096-0031.2004.00024.x. [DOI] [PubMed] [Google Scholar]
- Lupia R, Schneider H, Moeser GM, Pryer KM, Crane PR. Marsileaceae sporocarps and spores from the Late Cretaceous of Georgia, U.S.A. International Journal of Plant Sciences. 2000;161:975–988. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2010 Version 2·74. http://mesquiteproject.org . [Google Scholar]
- Magallón S. Using fossils to break long branches in molecular dating: a comparison of relaxed clocks applied to the origin of angiosperms. Systematic Biology. 2010;59:384–399. doi: 10.1093/sysbio/syq027. [DOI] [PubMed] [Google Scholar]
- Magallón S, Castillo A. Angiosperm diversification though time. American Journal of Botany. 2009;96:349–365. doi: 10.3732/ajb.0800060. [DOI] [PubMed] [Google Scholar]
- Magallón S, Crane PR, Herendeen PS. Phylogenetic pattern, diversity and diversification of eudicots. Annals of the Missouri Botanical Garden. 1999;86:297–372. [Google Scholar]
- Magallón-Puebla S, Herendeen PS, Crane PR. Quadriplatanus georgianus gen. et sp. nov.: staminate and pistillate platanaceous flowers from the Late Cretaceous (Coniacian-Santonian) of Georgia, USA. International Journal of Plant Sciences. 1997;158:373–394. [Google Scholar]
- Martínez-Millán M, Crepet WL, Nixon KC. Pentapetalum trifasciculandricus gen. et sp. nov., a thealean fossil flower from the Raritan Formation, New Jersey, USA (Turonian, Late Cretaceous) American Journal of Botany. 2009;96:933–949. doi: 10.3732/ajb.0800347. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Knox EM. The comparative morphology of the stamen in the Ericaceae. Transactions and Proceedings of the Botanical Society of Edinburgh. 1926;29:243–281. [Google Scholar]
- Matthews JR, Taylor DW. The structure and development of the stamen in Erica hirtiflora. Transactions and Proceedings of the Botanical Society of Edinburgh. 1926;29:235–242. [Google Scholar]
- Nixon KC, Crepet WL. Late Cretaceous fossil flowers of ericalean affinity. American Journal of Botany. 1993;80:616–623. [PubMed] [Google Scholar]
- Paterson BR. Studies of floral morphology in the Epacridaceae. Botanical Gazette. 1961;122:259–279. [Google Scholar]
- Pyykkö M. Placentation in the Ericales. I. Pyrolaceae and Monotropaceae. Annales Botanici Fennici. 1969;6:255–268. [Google Scholar]
- Reed SM. Reproductive biology of Clethra alnifolia. Horticultural Science. 2006;41:567–570. [Google Scholar]
- Renner SS. Floral biological observations on Heliamphora tatei (Sarraceniaceae) and other plants from Cerro de la Neblina in Venezuela. Plant Systematics and Evolution. 1989;163:21–29. [Google Scholar]
- Schmid R. Actinidiaceae, Davidiaceae, and Paracryphiaceae: systematic considerations. Botanische Jahrbücher für Systematik. 1978;100:196–204. [Google Scholar]
- Schnarf K. Bemerkungen zur Stellung der Gattung Saurauia im System. Sitzungsberichte der Akademie der Wissenschaften Wien, Abteilung I. 1924;133:17–28. [Google Scholar]
- Schneider JV, Bayer C. Clethraceae. In: Kubitzki K, editor. The families and genera of vascular plants. Volume VI. Flowering plants – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004. pp. 69–73. [Google Scholar]
- Schönenberger J. Comparative floral structure and systematics of Fouquieriaceae and Polemoniaceae (Ericales) International Journal of Plant Sciences. 2009;170:1132–1167. [Google Scholar]
- Schönenberger J, Friis EM. Fossil flowers of ericalean affinity from the Late Cretaceous of Southern Sweden. American Journal of Botany. 2001;88:467–480. [PubMed] [Google Scholar]
- Schönenberger J, Anderberg AA, Sytsma KJ. Molecular phylogenetics and patterns of floral evolution in the Ericales. International Journal of Plant Sciences. 2005;166:265–288. [Google Scholar]
- Schönenberger J, von Balthazar M, Sytsma KJ. Diversity and evolution of floral structure among early diverging lineages in the Ericales. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:437–448. doi: 10.1098/rstb.2009.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreve F. The development and anatomy of Sarracenia purpurea. Botanical Gazette. 1906;42:107–123. [Google Scholar]
- Soejarto DD. Aspects of reproduction in Saurauia. Journal of the Arnold Arboretum. 1969;50:180–196. [Google Scholar]
- Soltis D, Soltis P, Chase M, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Stevens PF. onwards. Angiosperm Phylogeny Website. 2001 Version 9, June 2008 [and more or less continuously updated since]. http://www.mobot.org/mobot/research/apweb/ [Google Scholar]
- Stevens PF, Luteyn J, Oliver EGH, et al. Ericaceae. In: Kubitzki K, editor. The families and genera of vascular plants. VI. Flowering plants. – dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales. Berlin: Springer-Verlag; 2004. pp. 145–194. [Google Scholar]
- Strauss SY, Zangerl AR. Plant–insect interactions in terrestrial ecosystems. In: Herrera CM, Pellmyr O, editors. Plant–animal interactions: an evolutionary approach. Oxford: Blackwell Publishing; 2002. pp. 77–106. [Google Scholar]
- Thomas JL. The genera of the Cyrillaceae and Clethraceae of the Southeastern United States. Journal of the Arnold Arboretum. 1961;42:96–106. [Google Scholar]
- Uphof JCT. Sarraceniaceae. In: Engler A, Harms H, editors. Die natürlichen Pflanzenfamilien. 2nd edn. 17b. Leipzig: W: Engelmann; 1936. pp. 704–727. [Google Scholar]
- Vogel S. Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ, editor. The pollination of flowers by insects. London: Academic Press; 1978. pp. 89–96. [Google Scholar]
- Zhang X-P, Anderberg AA. Pollen morphology in the ericoid clade of the order Ericales, with special emphasis on Cyrillaceae. Grana. 2002;41:201–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.