Abstract
Background and Aims
Hymenophyllaceae (filmy ferns) are typically plants of shady, constantly moist habitats. They attain greatest species diversity and biomass in humid tropical montane forests and temperate hyperoceanic climates. This paper presents ecophysiological data bearing on their worldwide ecological niche space and its limits.
Methods
Chlorophyll fluorescence was used to monitor recovery in desiccation experiments, and for measurements of 95 % saturating irradiance [photosynthetic photon flux density (PPFD95 %)] of photosynthetic electron flow and other parameters, in the New Zealand Hymenophyllum sanguinolentum, and three species each of Hymenophyllum and Trichomanes from forests in Trinidad and Venezuela.
Key Results
Hymenophyllum sanguinolentum was comparable in desiccation tolerance and light responses with the European species. The more common species in the two tropical forests showed PPFD95 % >100 µmol m−2 s−1, and withstood moderate desiccation (–40 MPa) for several days. The four most shade-adapted species had PPFD95 % ≤51 µmol m−2 s−1, and were sensitive to even mild and brief desiccation (–22 MPa for 3 d).
Conclusions
Light and desiccation responses of filmy ferns can be seen as an integrated package. At low light and windspeed in humid forests, net radiation and saturation deficit are low, and diffusion resistance high. Water loss is slow and can be supported by modest conduction from the sub-stratum. With higher irradiance, selection pressure for desiccation tolerance increases progressively. With low light and high humidity, the filmy fern pattern of adaptation is probably optimal, and the vascular plant leaf with mesophyll and stomata offers no advantage in light capture, water economy or CO2 uptake. Trade-offs between light adaptation and desiccation tolerance, and between stem conduction and water absorption through the leaf surface, underlie adaptive radiation and niche differentiation of species within the family. Hymenophyllaceae are a rare example of an evolutionary shift of adaptive strategy from typical vascular plant adaptation to the poikilohydry most typical of bryophytes.
Keywords: Chlorophyll fluorescence, desiccation tolerance, Hymenophyllum, light saturation, poikilohydry, Trichomanes, tropical montane forest, temperate rainforest
INTRODUCTION
The filmy ferns (Hymenophyllaceae) are typically plants of shady, constantly humid forests, especially tropical montane forests where they attain their greatest abundance and diversity. They extend into temperate latitudes in high-rainfall oceanic regions, such as the Macaronesian islands and the Atlantic coastal regions of western Europe, southern Chile, New Zealand and south-east Australia. Their leaves are typically only one cell thick, they lack stomata and they provide a rare instance of a coherent vascular plant family committed to a poikilohydric mode of life. This paper presents ecophysiological data bearing on their worldwide ecological niche space and its limits.
Studies on aspects of the physiology that underlie their ecological preferences and limits have been sparse. Shreve (1911) in his paper on Hymenophyllaceae in Jamaica provided some experimental data on the water relations of these plants, and a perceptive discussion of the varied habitats of the species. Richards and Evans (1972) contributed useful comparative data on the light and desiccation responses of the two British Hymenophyllum species. Proctor (2003) found both the British Hymenophyllum species to have considerable tolerance of desiccation (comparable with bryophytes of similar habitats in this respect) and low, but not excessively low, levels of light saturation of photosynthesis. Of the two, H. tunbrigense was more shade adapted and less desiccation tolerant than H. wilsonii, traits consistent with the ecological and geographical distribution of the two species. Johnson et al. (2000) discussed the photosynthetic adaptation of the filamentous gametophyte of Trichomanes speciosum to the extremely low light levels of its deeply shaded rock-crevice habitats, and showed that it is able to function in far deeper shade than these Hymenophyllum species.
The availability of material collected in New Zealand in September 2001, and visits to Trinidad in October 2003 and to Venezuela in January 2004 provided the opportunity to examine the light and desiccation responses of a wider range of Hymenophyllaceae including a number of widespread tropical American species of Hymenophyllum and Trichomanes. The collecting sites spanned a range of habitat conditions, and the different species displayed a correspondingly wide range of ecophysiological responses. The purpose of this paper is to present some comparative data for these species, and to consider the results in relation to the habitat adaptation of the family in general, and to the broader physical, physiological and evolutionary context.
MATERIALS AND METHODS
Species and collecting sites are listed in Table 1.
Table 1.
Species and collecting sites of filmy fern material used in this paper
| Species | Collecting site and date |
|---|---|
| Hymenophyllum fucoides (Sw.) Sw. (subgenus Hymenophyllum) | Common in the bryophyte mat on the floor of montane cloud forest, San Eusebio–La Carbonera Forest, Est. Mérida, Venezuela, 8 °38'30''N; 71 °24'33''W, alt. approx. 2400 m. M.C.F. Proctor, 17 January 2004. The only species at this site with serrate leaves (Ricardi and Marín, 1996). |
| Hymenophyllum hirsutum (L.) Sw. (subgenus Sphaerocionium) | In bryophyte mat covering small trunk base (and in other similar situations) in understorey of lower montane rainforest on crest of Northern Range, east of the Arima–Blanchisseuse road, Trinidad, 10 °43'N; 61 °18'W, alt. approx.. 900 m. M.C.F. Proctor, 22 October 2003. |
| Hymenophyllum polyanthos (Sw.) Sw. (subgenus Mecodium) | The most common filmy fern in the bryophyte mat on stumps, small trunks and branches in the understorey of lower montane rainforest on the crest of the Northern Range, east of the Arima–Blanchisseuse road, Trinidad, 10 °43'N; 61 °18'W, alt. approx. 900 m. M.C.F. Proctor, 22 October 2003. Specimen in my own collection. |
| Hymenophyllum sanguinolentum Hook. f. (subgenus Myrmecostylum) | Red beech (Nothofagus fusca) forest at Maruia Springs, Victoria Range, South Island, New Zealand, 42 °23'S; 172 °20'E, alt. approx. 834 m. J.G. Duckett, September 2001 |
| Trichomanes capillaceum L. | On rock outcrop in deep shade, constantly irrigated by trickling water, Monte Zerpa forest, Mérida, Est. Mérida, Venezuela, 8 °38'N; 71 °10'W, alt. approx. 2000 m, 22 January 2004. Usually on tree-fern bases, but certainly this species. |
| Trichomanes diaphanum Kunth. | Rather common in the bryophyte mat on small trunks in the understorey of lower montane rainforest on the crest of the Northern Range, east of the Arima–Blanchisseuse road, Trinidad, 10 °43'N; 61 °18'W, alt. approx. 900 m, 22 October 2003. Specimen in my own collection. |
| Trichomanes polypodioides L. | In bryophyte mat covering small stump in understorey of lower montane rainforest on crest of Northern Range, east of the Arima–Blanchisseuse road, Trinidad, 10 °43'N; 61 °18'W, alt. approx. 900 m. M.C.F. Proctor, 22 October 2003. |
| Trichomanes sp. | In spray of small waterfall, shaded by rock on which it was growing, in deep valley east of Cordillera de Mérida, approx. 70 km ENE of Mérida. Est. Barinas, Venezuela, 8 °50'14''N; 70 °34'30''W, alt. approx. 1500 m. M.C.F. Proctor, 19 January 2004. |
Names follow Baksh-Comeau (2000) and Brownsey and Smith-Dodsworth (1989); the subgenera of Hymenophyllum recognized by Ebihara et al. (2006) are given after the species. Ebihara et al. transfer Trichomanes capillaceum to a much enlarged Polyphlebium. The other three species belong in Trichomanes subgenus Trichomanes in their scheme.
Treatment of material following collection
The New Zealand H. sanguinolentum was returned to the UK by mail, and sprayed with deionized water on arrival. The material from Trinidad and Venezuela was brought back within a few days of collection in a fully hydrated state. All the material was maintained in closed polyethylene bags in ambient light in the laboratory, avoiding direct sun.
Physiological measurements
In general, measurements and methods were as in Proctor (2003), except that for the Venezuelan material an additional, less intense, desiccation treatment was added (KCl, 85 % relative humidity at 20 °C: approx. –22 MPa). The chlorophyll fluorescence parameter Fv/Fm, measured with a modulated chlorophyll fluorimeter (FMS-1, Hansatech, King's Lynn, UK), was used as a measure of recovery in the desiccation experiments. Response curves for relative electron transport rate [(RETR) calculated as photosynthetic photon flux density (PPFD) × ΦPSII] and other fluorescence parameters were used as measures of photosynthetic light responses. For an outline of chlorophyll fluorescence methods, see Maxwell and Johnson (2000) and Baker (2008).
Light response curves of RETR, 1 – qP and non-photochemical quenching (NPQ) were constructed for all the species. RETR provides a measure of photosynthetic electron flow (including photorespiration); the data were fitted by curves of the form y = A(1 – e−kx), where y is RETR and x is PPFD, A is the asymptote of the curve and k is a slope parameter. Ak is the initial slope, which should approximate to dark-adapted Fv/Fm, a useful test of fit. ‘Wild’ points at high irradiance judged likely to distort the saturation curve were omitted from the curve fitting, to avoid effects of photoinhibition, the non-saturating electron flow shown by many poikilohydric plants at high irradiance (Proctor, 2003, 2009; Marschall and Proctor, 2004; Proctor and Smirnoff, 2011), and the inherent increased variability of RETR at high PPFD. From the fitted curves, the irradiance at 95 % saturation (PPFD95 %) was calculated. The parameter 1 – qP gives an approximate measure of the reduction state of the first electron acceptor QA of photosystem II (PSII). The response curve is often quite well fitted by the same negative exponential curve as RETR, but a logistic curve on a logarithmic scale of PPFD (starting from 1 – qP = 0·0) generally gives a better fit. The parameters of the logistic curve give an asymptote, and the point at which 1 – qP reaches half its asymptotic value. The same form of curve generally gives a good fit to the non-photochemical quenching parameter NPQ, which largely reflects zeaxanthin-mediated photoprotection. In the context of the present data these fitted curves are empirical and descriptive, and they should be extrapolated only with caution.
RESULTS
Responses to desiccation
The widespread New Zealand species H. sanguinolentum (Fig. 1) was somewhat more desiccation tolerant than the European H. tunbrigense (Proctor, 2003), but its desiccation responses were essentially similar. It recovered quickly and apparently completely from 3 and 7 d desiccation, and the intensity of desiccation with which it was equilibrated (from –40 to –220 MPa) made little difference either to the final Fv/Fm or to mean absolute fluorescence yield (Fm). After 15 d desiccation at –40 MPa recovery was rather slower but still essentially complete. However, more intense desiccation led to progressively less complete recovery, and a significant 60 % decline in Fm at the lowest humidity (–220 MPa). After 30 d, the material kept at –114 MPa recovered slowly, and recovery was still incomplete after 48 h; at higher and lower humidities, recovery of Fv/Fm was initially promising, but then progressively declined. Material desiccated for 60 d showed little sign of life following re-wetting.
Fig. 1.
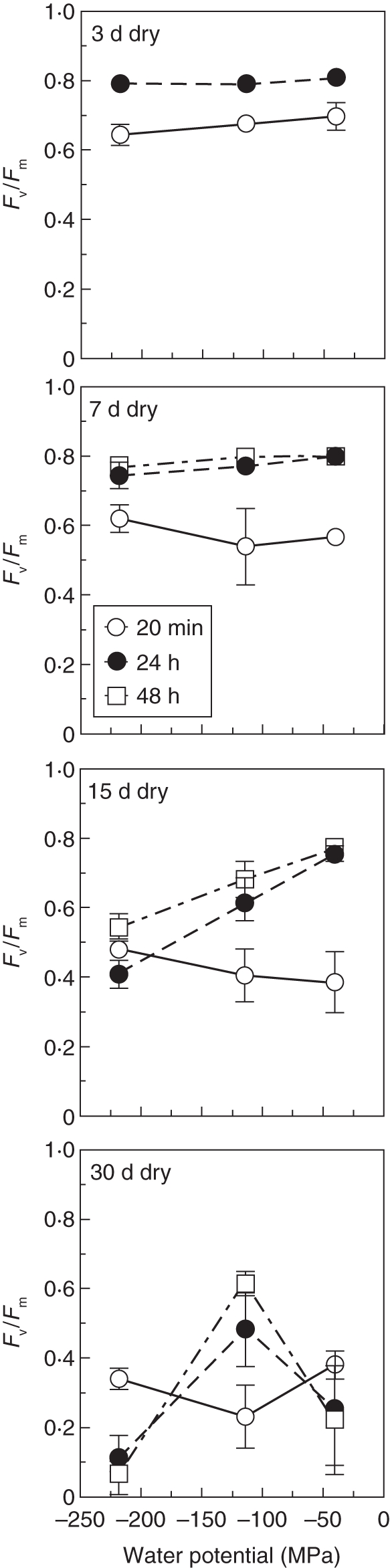
Recovery of Fv/Fm in Hymenophyllum sanguinolentum following 3, 7, 15 and 30 d desiccation in equilibrium with a range of water potentials. Means ± s.d., n = 3.
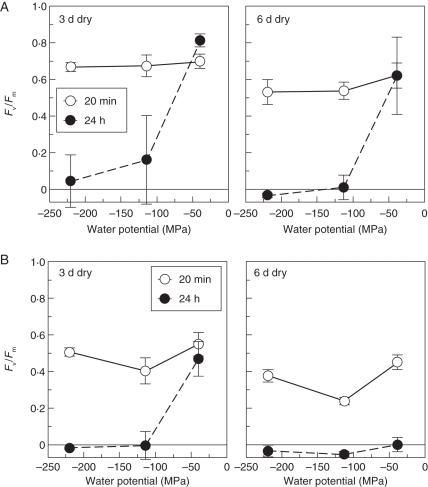
The Tropical American filmy ferns examined were all much more sensitive to desiccation (Fig. 2; Tables 2 and 3). The two most common species of Hymenophyllum in the understorey of the forests visited, H. fucoides in Venezuela and H. polyanthos in Trinidad, recovered well from moderate levels of drying (–40 MPa) for a few days. Hymenophyllum fucoides was the more tolerant of the two, surviving 6 d at –114 MPa with little apparent damage. Hymenophyllum polyanthos was progressively more damaged by more intense and longer desiccation, and H. fucoides showed a less marked trend in the same direction. The less common H. hirsutum in the Trinidadian forest was much more sensitive to drying, showing only partial recovery from 3 d at –40 MPa and virtually complete loss of photosynthetic function after more intense or longer desiccation. The figures (Table 2) for Trichomanes diaphanum rather closely match those for H. hirsutum; the unidentified Venezuelan Trichomanes sp. was a little more tolerant of drying than these two species, and T. polypodioides and T. capillaceum were more sensitive, with practically no tolerance of drying at all. Interestingly, in several species, notably T. polypodioides, apparent immediate recovery was followed by death within 24 h, suggesting that the photosynthetic system is not the most desiccation-sensitive part of the metabolism of the plant. In some cases apparent recovery of Fv/Fm may mask a large fall of total fluorescence yield, indicating degradation or loss of chlorophyll. It is therefore important not to rely on Fv/Fm alone, but to be aware also of possible changes in Fm (Table 3).
Fig. 2.
Recovery of Fv/Fm in (A) Hymenophyllum polyanthos and (B) Trichomanes diaphanum following 3 and 6 d desiccation in equilibrium with a range of water potentials. Means ± s.d., n = 3.
Table 2.
Recovery of Fv/Fm in some tropical American Hymenophyllaceae (means ± s.d.; n = 3 unless stated otherwise)
| Species | Recovery time | –22 MPa | –40 MPa | –114 MPa | –220 MPa |
|---|---|---|---|---|---|
| 3 d desiccation | |||||
| Hymenophyllum fucoides | 20 min | 0·749 ± 0·036 | 0·716 ± 0·027 | 0·692 ± 0·021 | – |
| 24 h | 0·861 ± 0·015 | 0·845 ± 0·014 | 0·825 ± 0·005 | – | |
| Hymenophyllum hirsutum | 20 min | – | 0·520 ± 0·032 | 0·522 ± 0·130 | 0·526 ± 0·158 |
| 24 h | – | 0·420 ± 0·049 | 0·079 ± 0·126 | 0·080 ± 0·098 | |
| Hymenophyllum polyanthos | 20 min | – | 0·698 ± 0·039 | 0·672 ± 0·059 | 0·667 ± 0·024 |
| 24 h | – | 0·813 ± 0·035 | 0·160 ± 0·241 | 0·043 ± 0·144 | |
| Trichomanes capillaceum | 20 min | 0·125 ± 0·106 | 0·015 ± 0·077 | 0·049 ± 0·044 | – |
| 24 h | 0·096 ± 0·166 | –0·018 ± 0·016 | –0·033 ± 0·030 | – | |
| Trichomanes diaphanum | 20 min | – | 0·548 ± 0·064 | 0·403 ± 0·072 | 0·505 ± 0·022 |
| 24 h | – | 0·469 ± 0·094 | –0·004 ± 0·077 | –0·019 ± 0·006 | |
| Trichomanes polypodioides | 20 min | – | 0·216 ± 0·082 | 0·204 ± 0·012 | 0·293 ± 0·042 |
| 24 h | – | 0·031 ± 0·003 | 0·028 ± 0·012 | 0·050 ± 0·029 | |
| Trichomanes sp. | 20 min | 0·620 ± 0·054 | 0·523 ± 0·070 | 0·156 ± 0·079 | – |
| 24 h | 0·577 ± 0·172 | 0·243 ± 0·222 | 0·181 ± 0·214 | – | |
| 6 d desiccation | |||||
| Hymenophyllum fucoides | 20 min | 0·696 ± 0·032 | 0·622 ± 0·042 | 0·625 ± 0·029 | – |
| 24 h | 0·840 ± 0·006 | 0·815 ± 0·012 | 0·748 ± 0·031 | – | |
| Hymenophyllum hirsutum | 20 min | – | 0·348 ± 0·051 | 0·191 ± 0·080 | 0·295 ± 0·071 |
| 24 h | – | 0·084 ± 0·067 | 0·067 ± 0·063 | –0·022 ± 0·039 | |
| Hymenophyllum polyanthos | 20 min | – | 0·621 ± 0·069 | 0·537 ± 0·046 | 0·530 ± 0·068 |
| 24 h | – | 0·619 ± 0·210 | 0·009 ± 0·067 | –0·034 ± 0·012 | |
| Trichomanes capillaceum | 20 min | 0·284 ± 0·194 | – | 0·000 (n = 1) | – |
| 24 h | 0·096 ± 0·166 | – | –0·026 (n = 1) | – | |
| Trichomanes diaphanum | 20 min | – | 0·450 ± 0·040 | 0·236 ± 0·021 | 0·375 ± 0·034 |
| 24 h | – | –0·002 ± 0·039 | –0·054 ± 0·013 | –0·036 ± 0·032 | |
| Trichomanes polypodioides | 20 min | – | –0·022 ± 0·020 | 0·039 ± 0·024 | 0·216 ± 0·020 |
| 24 h | – | –0·039 ± 0·010 | –0·025 ± 0·006 | 0·014 ± 0·019 | |
| Trichomanes sp. (n = 2) | 20 min | – | 0·384 ± 0·034 | – | – |
| 24 h | – | –0·079 ± 0·036 | – | – |
For localities see Table 1.
Table 3.
Recovery of absolute fluorescence yield (Fm; arbitrary units) in the same material as Table 2 (means ± s.d.; n = 3 unless stated otherwise
| Species | Recovery time | –22 MPa | –40 MPa | –114 MPa | –220 MPa |
|---|---|---|---|---|---|
| 3 d desiccation | |||||
| Hymenophyllum fucoides | 20 min | 204 ± 15 | 208 ± 66 | 195 ± 60 | – |
| 24 h | 307 ± 82 | 324 ± 75 | 325 ± 39 | – | |
| Hymenophyllum hirsutum | 20 min | – | 121 ± 47 | 139 ± 41 | 134 ± 19 |
| 24 h | – | 35 ± 13 | 16 ± 4 | 24 ± 6 | |
| Hymenophyllum polyanthos | 20 min | – | 230 ± 97 | 246 ± 42 | 247 ± 60 |
| 24 h | – | 416 ± 70 | 89 ± 19 | 90 ± 20 | |
| Trichomanes capillaceum | 20 min | 28 ± 8 | 43 ± 7 | 74 ± 42 | – |
| 24 h | 35 ± 22 | 51 ± 10 | 75 ± 16 | – | |
| Trichomanes diaphanum | 20 min | – | 208 ± 19 | 168 ± 49 | 221 ± 26 |
| 24 h | – | 89 ± 39 | 43 ± 7 | 69 ± 12 | |
| Trichomanes polypodioides | 20 min | – | 152 ± 28 | 157 ± 45 | 237 ± 19 |
| 24 h | – | 152 ± 24 | 142 ± 11 | 144 ± 7 | |
| Trichomanes sp. | 20 min | 163 ± 28 | 144 ± 71 | 66 ± 11 | – |
| 24 h | 130 ± 96 | 39 ± 32 | 19 ± 9 | – | |
| 6 d desiccation | |||||
| Hymenophyllum fucoides | 20 min | 215 ± 73 | 148 ± 19 | 150 ± 7 | – |
| 24 h | 417 ± 22 | 373 ± 56 | 292 ± 62 | – | |
| Hymenophyllum hirsutum | 20 min | – | 92 ± 34 | 62 ± 7 | 74 ± 17 |
| 24 h | – | 19 ± 5 | 15 ± 2 | 14 ± 1 | |
| Hymenophyllum polyanthos | 20 min | – | 197 ± 29 | 208 ± 5 | 217 ± 53 |
| 24 h | – | 220 ± 175 | 69 ± 5 | 78 ± 6 | |
| Trichomanes capillaceum | 20 min | 21 ± 12 | – | 30 (n = 1) | – |
| 24 h | 27 ± 9 | – | 39 (n = 1) | – | |
| Trichomanes diaphanum | 20 min | – | 155 ± 55 | 171 ± 69 | 139 ± 6 |
| 24 h | – | 54 ± 30 | 54 ± 22 | 45 ± 5 | |
| Trichomanes polypodioides | 20 min | – | 120 ± 18 | 164 ± 39 | 197 ± 18 |
| 24 h | – | 121 ± 17 | 133 ± 20 | 130 ± 19 | |
| Trichomanes sp. (n = 2) | 20 min | – | 69 ± 28 | – | – |
| 24 h | – | 43 ± 7 | – | – |
For localities see Table 1.
A notable feature in Table 2, especially in the more sensitive species or with more intense desiccation, is that Fv/Fm often recovers apparently well after 20 min re-wetting, but collapses to low levels within 24 h. It is striking that some of the more sensitive species (e.g. T. polypodioides) retain their apparently healthy bright green colour 20 min after re-wetting, but show only limited recovery of Fv/Fm and subsequently discolour and fail to survive.
Light responses
Figure 3 shows light–response curves of RETR, 1 – qP and NPQ for Trichomanes capillaceum, and some photosynthetic parameters for all the species are set out in Table 4. Hymenophyllum sanguinolentum stood out as having by far the highest PPFD95 %, and the highest values for (1 – qP)50 and NPQ50, followed (but not closely) by H. fucoides. Then follow H. polyanthos, the most common filmy fern in the forest understorey in Trinidad, and the Trichomanes species from a relatively well lit but constantly wet situation in Venezuela. Finally, H. hirsutum and the three remaining Trichomanes species form a deep shade group, with PPFD95 % values from 37 to 51 µmol m−2 s−1 and correspondingly low figures for (1 – qP)50 and NPQ50.
Fig. 3.
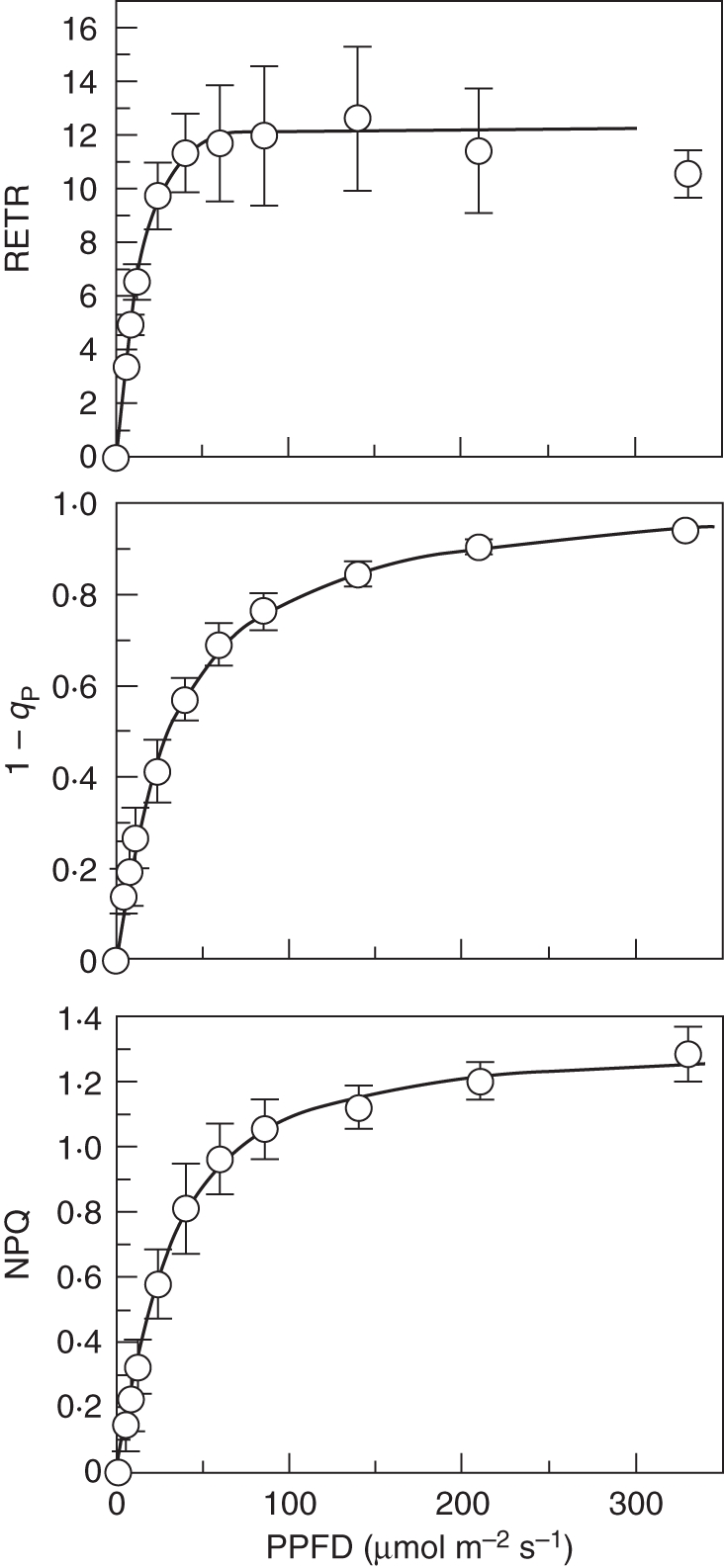
Response curves of relative electron flow, 1 – qP and NPQ for Trichomanes capillaceum. Negative exponential saturation curve fitted to RETR; logistic curves fitted to the other two parameters. Means ± s.d., n = 3.
Table 4.
Photosynthetic parameters of some Hymenophyllaceae from chlorophyll fluorescence measurements
| Species, and dark-adapted Fv/Fm | Locality | PPFD95 % (μmol m−2 s−1) | (1 – qP)max | PPFD (1 – qP)50 (μmol m−2 s−1) | NPQmax | PPFD NPQ50 (μmol m−2 s−1) |
|---|---|---|---|---|---|---|
| Hymenophyllum fucoides Fv/Fm= 0·829 ± 0·007 | San Eusebio forest. Mérida, Venezuela | 212 ± 42 | 0·86 ± 0·03 | 138 ± 9 | 2·23 ± 0·20 | 207 ± 21 |
| Hymenophyllum hirsutum Fv/Fm= 0·833 ± 0·032 | Northern Range, Trinidad | 51 ± 9 | 0·98 ± 0·01 | 42 ± 1 | 1·11 ± 0·03 | 36 ± 2 |
| Hymenophyllum polyanthos Fv/Fm= 0·843 ± 0·014 | Northern Range, Trinidad | 112 ± 24 | 0·95 ± 0·02 | 85 ± 4 | 1·85 ± 0·07 | 83 ± 5 |
| H. sanguinolentum Fv/Fm= 0·824 ± 0·028 | Maruia Springs, New Zealand | 567 ± 60 | 0·96 ± 0·05 | 455 ± 61 | 4·95 ± 0·19 | 483 ± 37 |
| Trichomanes sp. Fv/Fm= 0·810 ± 0·010 | Est. Barinas, Venezuela | 111 ± 30 | 0·97 ± 0·01 | 78 ± 2 | 1·03 ± 0·08 | 97 ± 10 |
| Trichomanes diaphanum Fv/Fm= 0·787 ± 0·019 | Northern Range, Trinidad | 49 ± 6 | 1·00 ± 0·01 | 43 ± 1 | 1·27 ± 0·03 | 25 ± 2 |
| Trichomanes capillaceum Fv/Fm= 0·833 ± 0·013 | Monte Zerpa forest. Mérida, Venezuela | 47 ± 3 | 1·03 ± 0·02 | 32 ± 2 | 1·31 ± 0·02 | 28 ± 1 |
| Trichomanes polypodioides Fv/Fm= 0·813 ± 0·013 | Northern Range, Trinidad | 37 ± 2 | 0·97 ± 0·01 | 33 ± 1 | 1·68 ± 0·03 | 15 ± 1 |
The PPFD95 % figures are the mean ± s.d. of estimates calculated from three independent replicate curves. The last four columns are means ± s.e. parameters of logistic curves fitted to the whole data. See text for further explanation.
Overall, the PPFD95 % values range from about 30 % of full-sunlight irradiance for H. sanguinolentum to about 2 % of full sunlight for T. polypodioides. The asymptote of 1 – qP for almost all the species approximated to 1·0, and 50 % of this limiting value is reached at approx. 0·7–0·9 of PPFD95 %. NPQ is more variable, the asymptotes (based on extrapolation but probably broadly realistic) ranging from almost 5 for H. sanguinolentum to figures between 1 and 2 for most of the tropical American species.
DISCUSSION
In terms of desiccation tolerance, the present data span much of the range between the two west-European species of Hymenophyllum (Proctor 2003) and what is most often perceived as the norm for the family. The temperate H. sanguinolentum, one of the most common and most widespread species in New Zealand (Holloway, 1923a), falls into place beside the two temperate European species, with desiccation tolerance between that of H. tunbrigense and H. wilsonii, and PPFD95 % nearly matched by H. wilsonii from Cornwall. Probably further measurements at different sites and seasons would show it to be as variable as the European species. Holloway (1923b) noted that H. sanguinolentum and Trichomanes [Hymenophyllum] reniforme ‘occur abundantly on the scoria-blocks on the slopes of Rangitoto Island [Auckland] in the full blaze of the sun’, and Brownsey and Smith-Dodsworth (1989) describe H. sanguinolentum as ‘A very drought-resistant filmy fern’. Hymenophyllum wilsonii and H. sanguinolentum represent the light- and desiccation-tolerant extreme of the adaptive space of the Hymenophyllaceae examined so far, and there may yet be more desiccation-tolerant species amongst the rich filmy fern floras of New Zealand and Chile. These species show a pattern of poikilohydric adaptation which they share with the bryophytes that grow in profusion in the same habitats.
The filmy ferns at the shade-tolerant extreme pose a particular set of questions. Johnson et al. (2000) found that the filamentous gametophyte of T. speciosum was growing for much of the time it at an irradiance <1 µmol m−2 s−1. In situ chlorophyll fluorescence measurements suggested light saturation at approx. 4–5 µmol m−2 s−1. The gametophyte in situ showed virtually no non-photochemical quenching, in contrast to the sporophyte, or gametophyte material that had been maintained in the laboratory. Their oxygen electrode measurements (at saturating CO2) probably do not realistically reflect responses in the field. The T. speciosum gametophyte can persist in drier, colder and darker habitats than the sporophyte, which itself is generally a plant of deeply shaded humid habitats, though sometimes is found in more open situations (Ratcliffe et al., 1993).
Of the tropical American Hymenophyllaceae investigated, none came near to matching the extreme shade tolerance of the T. speciosum gametophyte. Two species of Hymenophyllum stood out from the others, with PPFD95 % >100, H. polyanthos and H. fucoides; the most widespread species in the Trinidadian and Venezuelan forests visited. Shreve (1911) considered H. polyanthos to be the most common filmy fern in Jamaica and the most capable of resisting desiccation; he listed H. fucoides as another common species in the same habitat group. In the Venezuelan material, these two species were matched only by a Trichomanes species collected in a relatively open situation kept moist by spray from a small waterfall, with PPFD95 % of 111. The remaining species had PPFD95 % levels between 37 and 51 and were more sensitive to desiccation. Surprisingly, H. hirsutum, regarded by Shreve as one of the most tolerant of desiccation and high light in Jamaica, was more sensitive on both counts in the material from Trinidad, and fell into this group. From general considerations, a plant would be expected usually to be photosynthesizing at irradiances somewhat but not excessively below light saturation, so these PPFD95 % figures may reflect ambient irradiances commonly up to about 25 µmol m−2 s−1, around 1·5 % of full sunlight. In the San Eusebio forest the irradiance at 2 m varies around the year between about 1 and 2 % of the irradiance above the canopy (León-Vargas et al., 2006). All the filmy ferns investigated showed significant NPQ – up to 1·0–1·7 for the more shade-adapted species (cf. the findings of Johnson et al. for T. speciosum), and higher values for the species with photosynthesis saturating at higher irradiances, reaching nearly 5 in H. sanguinolentum. The filmy ferns thus have a significant degree of photoprotection, and the curves for 1 – qP do not suggest that the QA pool is likely to become over-reduced at any irradiance likely to be encountered in the field.
The tropical filmy ferns in this investigation were generally more sensitive to desiccation than the European and New Zealand Hymenophyllum species examined, and this may reflect the equability of the humid tropics compared with the seasonality of temperate regions. There are indeed wetter and drier seasons in the equatorial zone, but these are generally not extreme, and are moderated at altitude (where tropical filmy ferns reach their greatest species number and biomass) by cloud cover and cloudwater deposition; frequency is more important than amount of precipitation for poikilohydric plants (León-Vargas et al., 2006). With the forest floor receiving only 1–2 % of full daylight, the short-wave solar irradiance is only some 10–20 W m−2. Thermal infrared emission at 15 °C (of the order of 400 W m−2) changes by about 5 W m−2 K−1 (Monteith and Unsworth, 1990), so even modest temperature differences between ground, trunks and canopy will result in long-wave energy exchanges comparable with the short-wave (light) radiation income during the day. Solar radiation is not the dominant factor it is in less shaded situations. The other driver of evaporation, saturation deficit, is also small at the high humidities prevailing close to the forest floor (approx. 0·1 kPa at 95 % relative humidity and 20 °C) and, at the low windspeeds inside the forest, diffusion resistance to heat and mass transfer will be high; a rough calculation suggests around 200 s m−1. We may thus accept the conclusion of Shreve (1911) from his experiments that ‘The extremely low water loss from surface-dry leaves in a very moist atmosphere can be met, therefore, by root absorption and conduction in all but the most pronouncedly moisture-loving species.’ At the low irradiances prevailing in filmy fern habitats, CO2 uptake by unistratose leaves should never be diffusion limited; the PPFD95 % of 567 µmol m−2 s−1 in the New Zealand H. sanguinolentum, which tolerates relatively unshaded conditions, is probably close to the maximum attainable by a unistratose leaf. In bryophytes the liquid-phase diffusion resistance becomes a limitation only above an irradiance of about 250 µmol m−2 s−1 PPFD in thalloid liverworts (one surface), and even bryophytes with overlapping unistratose leaves seldom exceed a PPFD95 % of 1000 µmol m−2 s−1 (Marschall and Proctor, 2004; Proctor, 2005).
The filmy fern pattern of adaptation, with photosynthesis saturating at low irradiance and generally low levels of desiccation tolerance, is an integrated package adapted to more or less constantly shady humid environments. In the deep shade of the forest floor or rock-crevice habitats, light is limiting for most plants. However, with the deep shade comes high humidity, low windspeed and low net radiation income – minimizing evaporation, and nullifying the advantages of the typical vascular plant leaf with its ventilated mesophyll and stomatal control over water loss. The most desiccation-sensitive filmy fern leaf is not more sensitive to drying out than typical vascular plant mesophyll tissue. The difference is that the typical mesophyll is provided with an external water supply and protected from equilibrating with the air by a waterproof epidermis and stomata. If neither water supply nor evaporation is a problem, then the filmy fern leaf has the simple and elegant solution to all its needs – but in all but the most constantly humid environments it also requires the fall-back option of some degree of desiccation tolerance. Obviously there is scope for trade-offs between saturation irradiance for photosynthesis and desiccation tolerance, and between conduction of water via the stems and uptake of water through the leaves. This underlies the leafy liverwort-like adaptive radiation of the family, spanning a range from the relatively desiccation-tolerant H. sanguiolentum and H. wilsonii at one extreme to the desiccation-sensitive and shade-adapted species of humid evergreen forests at the other – and more subtly the niche differentiation between the species in any one site.
The central ecological niche of the filmy ferns is in shady humid forests, especially in the tropics, where they are particularly concentrated at moderate altitudes. Thus in Jamaica three times as many species occur at 1500 m as at 300 m (Shreve, 1911). Annual mean temperatures at these altitudes are ‘temperate’ rather than ‘tropical’, and comparable climatic conditions are found at lower altitudes in temperate hyperoceanic regions, such as Atlantic western Europe, southern Chile, south-east Australia, and New Zealand. The seasonal shifts of temperature in oceanic temperate regions are no greater than the altitudinal range of temperature on tropical mountains, but winter-deciduous tree cover imposes greater challenges to light and desiccation tolerance and may ultimately limit filmy fern distribution polewards.
There are two basic strategies by which plants have adapted to life on land – the vascular plant strategy and the poikilohydric desiccation-tolerant strategy exemplified by bryophytes (Proctor and Tuba 2002). Which of these strategies is optimal is closely bound up with scale, and there is good reason to think that, while the vascular strategy is optimal for a large green land plant (taller than a few centimetres), the poikilohydric strategy is optimal for a small one (Proctor et al., 2007). There is a limited ‘size window’ around 1–10(–20) cm, where both strategies are viable, and within which most filmy ferns lie. Most major phyletic groups of land plants have remained faithful to one or other of these adaptive strategies throughout the fossil record. The small, poikilohydric ones (with a dominant gametophyte generation) we call bryophytes. With those we contrast the larger vascular plants, the lycophytes, and the complex clade including the ferns and horsetails, the gymnosperms and their Mesozoic offshoot the angiosperms, in all of which the sporophyte generation is dominant. We may well ask why poikilohydry and desiccation tolerance are so predominantly, and yet not entirely, associated with the haploid, gametophyte generation. The answer may lie largely in evolutionary history, and be partly a matter of scale. The liverworts and the mosses are a pair of eminently successful groups, numbering about 5000 and 13 000 species, respectively. They occupy many ecological niches which are too cold, too dry, too nutrient poor or simply too small for vascular plants, and few habitats are without them. In some tundra and bog areas and often on rock surfaces and the bark of trees they are the dominant plants.
There has been little trespass of these adaptive types into the phyletic territory of the other. Interestingly, the ferns have poikilohydric gametophytes, but predominantly vascular-adapted sporophytes. Watkins et al. (2007) have pointed out that gametophytes of some tropical epiphytic ferns are very different in form from the stereotype textbook fern prothallus of Dryopteris filix-mas, and take their place as full desiccation-tolerant members of the bryophyte-dominated community of the upper branches. Hymenophyllaceae share in this diversity; filamentous and thalloid gametophytes, with or without gemmae, occur within the family. There are some striking and well known desiccation-tolerant fern sporophytes, such as Ceterach officinarum, Notholaena marantae and Polypodium polypodioides, but these function as mainstream vascular plants when water is not limiting. The filmy ferns stand out as the most notable example of a family that has shifted its whole adaptive commitment across the divide between the homoiohydry of the vascular plants and the poikilohydry most typically seen in bryophytes.
ACKNOWLEDGEMENTS
I am indebted to Professor Jeff Duckett for the New Zealand material of Hymenophyllum sanguinolentum, to Dr David Stradling and Dr Victor Quesnel for guidance in the field in Trinidad, to Dr Yelitza León for guidance in the field in Venezuela, to Brian O'Shea for GPS latitudes and longitudes of the Venezuelan sites, and to two commendably thorough anonymous reviewers whose comments materially improved this paper.
LITERATURE CITED
- Baker NR. Chlorophyll fluorescence: a probe for photosynthesis in vivo. Annual Review of Plant Biology. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Baksh-Comeau YS. Checklist of pteridophytes of Trinidad and Tobago. Fern Gazette. 2000;16:11–122. [Google Scholar]
- Brownsey PJ, Smith-Dodsworth JC. New Zealand ferns and allied plants. Auckland, New Zealand: David Bateman; 1989. [Google Scholar]
- Ebihara A, Dubuisson J-Y, Iwatsuki K, Hennequin S, Ito M. A taxonomic revision of Hymenophyllaceae. Blumea. 2006;51:221–280. [Google Scholar]
- Holloway JE. Studies in the New Zealand Hymenophyllaceae. Part 1. The distribution of the species in Westland, and their growth-forms. Transactions of the New Zealand Institution. 1923a;54:577–618. [Google Scholar]
- Holloway JE. Studies in the New Zealand Hymenophyllaceae. Part 2. The distribution of the species throughout the New Zealand biological region. Transactions of the New Zealand Institution. 1923b;55:67–94. [Google Scholar]
- Johnson GN, Rumsey FJ, Headley AD, Sheffield E. Adaptations to extreme low light in the fern Trichomanes speciosum. New Phytologist. 2000;148:423–431. doi: 10.1046/j.1469-8137.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- León-Vargas Y, Engwald S, Proctor MCF. Microclimate, light adaptation and desiccation tolerance of epiphytic bryophytes in two Venezuelan cloud forests. Journal of Biogeography. 2006;33:901–913. [Google Scholar]
- Marschall M, Proctor MCF. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Annals of Botany. 2004;95:593–603. doi: 10.1093/aob/mch178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Monteith JL, Unsworth MH. Principles of environmental physics. 2nd edn. London: Edward Arnold; 1990. [Google Scholar]
- Proctor MCF. Comparative ecophysiological measurements on the light responses, water relations and desiccation tolerance of the filmy ferns Hymenophyllum wilsonii Hook. and H. tunbrigense (L.) Smith. Annals of Botany. 2003;91:717–727. doi: 10.1093/aob/mcg077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF. Why do Polytrichaceae have lamellae? Journal of Bryology. 2005;27:221–229. [Google Scholar]
- Proctor MCF. Desiccation tolerance in some British ferns. Fern Gazette. 2009;18:216–234. [Google Scholar]
- Proctor MCF, Smirnoff N. Ecophysiology of photosynthesis in bryophytes: major roles for oxygen photoreduction and non-photochemical quenching at high irradiance in mosses with unistratose leaves? Physiologia Plantarum. 2011;141:130–140. doi: 10.1111/j.1399-3054.2010.01424.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. Poikilohydry and homoiohydry: antithesis or spectrum of possibilities? New Phytologist. 2002;156:327–349. doi: 10.1046/j.1469-8137.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. Desiccation tolerance in bryophytes: a review. Bryologist. 2007;110:595–621. [Google Scholar]
- Ratcliffe DA, Birks HJB, Birks HH. The ecology and conservation of the Killarney Fern Trichomanes speciosum in Britain and Ireland. Biological Conservation. 1993;66:231–247. [Google Scholar]
- Ricardi M, Marín M. Sinopsis de la flora pteridológica del bosque La Carbonera–San Eusebio, Mérida (Venezuela) Plantula. 1996;1:55–64. [Google Scholar]
- Richards PW, Evans GB. Biological Flora of the British Isles. Hymenophyllum. Journal of Ecology. 1972;60:245–268. [Google Scholar]
- Shreve F. Studies on Jamaican Hymenophyllaceae. Botanical Gazette. 1911;51:184–209. [Google Scholar]
- Watkins JE, Mack MC, Sinclair TR, Mulkey SS. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist. 2007;176:708–717. doi: 10.1111/j.1469-8137.2007.02194.x. [DOI] [PubMed] [Google Scholar]