Abstract
Background and Aims
The response of forest herb regeneration from seed to temperature variations across latitudes was experimentally assessed in order to forecast the likely response of understorey community dynamics to climate warming.
Methods
Seeds of two characteristic forest plants (Anemone nemorosa and Milium effusum) were collected in natural populations along a latitudinal gradient from northern France to northern Sweden and exposed to three temperature regimes in growth chambers (first experiment). To test the importance of local adaptation, reciprocal transplants were also made of adult individuals that originated from the same populations in three common gardens located in southern, central and northern sites along the same gradient, and the resulting seeds were germinated (second experiment). Seedling establishment was quantified by measuring the timing and percentage of seedling emergence, and seedling biomass in both experiments.
Key Results
Spring warming increased emergence rates and seedling growth in the early-flowering forb A. nemorosa. Seedlings of the summer-flowering grass M. effusum originating from northern populations responded more strongly in terms of biomass growth to temperature than southern populations. The above-ground biomass of the seedlings of both species decreased with increasing latitude of origin, irrespective of whether seeds were collected from natural populations or from the common gardens. The emergence percentage decreased with increasing home-away distance in seeds from the transplant experiment, suggesting that the maternal plants were locally adapted.
Conclusions
Decreasing seedling emergence and growth were found from the centre to the northern edge of the distribution range for both species. Stronger responses to temperature variation in seedling growth of the grass M. effusum in the north may offer a way to cope with environmental change. The results further suggest that climate warming might differentially affect seedling establishment of understorey plants across their distribution range and thus alter future understorey plant dynamics.
Keywords: Anemone nemorosa, climate change, common garden, growth chambers, latitudinal gradient, local adaptation, Milium effusum, plant regeneration, range edges, recruitment, seedling establishment, temperature
INTRODUCTION
Early life-history stages are among the most critical phases in the life cycle of plants and important determinants of population dynamics. Assessing changes in the regeneration from seed in response to temperature variation is therefore key to forecast the likely response of plant communities to climate warming (Adler and HilleRisLambers, 2008; Hedhly et al., 2009; Shevtsova et al., 2009; Klady et al., 2011; Saatkamp et al., 2011; Walck et al., 2011). Temperature may affect seed set as well as germination rates and timing through direct effects on the mother plants and seeds (Hedhly et al., 2009), and warming may also affect the performance of the resulting seedlings. To maximize reproductive success, plants avoid exposure to unfavourable environmental conditions by shifting seedling emergence both spatially and temporally (Probert, 2000; Fenner and Thompson, 2005). The timing of seed germination and seedling emergence, as well as the seedling emergence rate are important estimates to assess population dynamics and persistence. Our understanding of the responses of early life-stages to climatic change factors, however, is still limited (Hovenden et al., 2008; De Frenne et al., 2009; HilleRisLambers et al., 2009).
The response of plant regeneration to temperature variation likely differs across the distribution range of species. Alpine or northern subarctic populations are subject to higher local environmental variability, for example in terms of microclimatic conditions, than populations of the same species growing in a temperate climate or lowland region (Armbruster et al., 2007; Scherrer and Körner, 2010; Graae et al., 2012). Accordingly, adult plant individuals in northern populations are generally more plastic in response to environmental heterogeneity than southern populations (Ghalambor et al., 2006; Lacey et al., 2010). Furthermore, vegetative and reproductive fitness, as well as population densities, generally decrease from the centre to the edge of the distribution range in many plant species (e.g. Jump and Woodward, 2003; for a recent review, see Sexton et al., 2009). An intraspecific shift from sexual to vegetative reproduction towards the north has also been found (Mooney and Billings, 1961; Houle and Babeux, 1994; Dorken and Eckert, 2001). Hence, forecasting plant-community responses to climate change requires understanding variation in early life-history stages in response to temperatures across the distribution range.
Plants with different phenologies and life forms generally respond differently to temperature variation (Walker et al., 2006; Sherry et al., 2007; De Frenne et al., 2009, 2011a, b; Elmensdorf et al., 2012). Sherry et al. (2007), for instance, showed that plants flowering before the summer heat peak in a North American tallgrass prairie tended to advance their phenology in response to warming, whereas later-flowering plants delayed their phenology. De Frenne et al. (2011a) also suggested that climate warming may have a stronger influence on spring-flowering understorey plants than on summer-flowering plants. It has also been shown that grasses generally respond more strongly than forbs to resource alterations such as nitrogen enrichment and temperature increases (Dormann and Woodin 2002; Walker et al., 2006; De Schrijver et al., 2011; Klady et al., 2011).
In spite of their importance for forest biodiversity and ecosystem functioning (Gilliam, 2007), there have been few experimental tests of the effects of climate change on herbaceous understorey plants. Svenning and Skov (2006) showed that the bioclimatic envelope of many herbaceous understorey plants might shift dramatically towards northern latitudes in Europe in the near future. Whether the future distribution of understorey plants will ever match their predicted bioclimatic envelope is doubtful, with potentially serious consequences for the long-term persistence of these species. Investigating the response of understorey plants to rising temperatures is thus highly relevant.
In this study, we assessed the regeneration from seed of two forest understorey plant species along a 1900- and a 2300-km-long latitudinal gradient. The study species were the spring-flowering geophyte Anemone nemorosa and the early summer-flowering grass Milium effusum. Divergent responses of these species are to be expected due to their contrasting phenology (early vs. later-flowering) and life forms (forb vs. grass) (De Frenne et al., 2011a).
First, seeds of both species were wild-collected from plants growing along the latitudinal gradient and placed in growth chambers under three spring-temperature scenarios. By raising progeny in different spring-warming scenarios, the response of seedling establishment to spring temperature can be examined. Not only is spring a very important period for understorey plant growth and recruitment, but the IPCC (2007) also predicted that early-season warming in northern Europe will be greater than summer and autumn warming (relative increase of +4·3 °C in December–February and +3·1 °C in March–May vs. +2·7 °C in June–August and +2·9 °C in September–November by 2080–2099 compared with 1980–1999). Because seeds from this first experiment were wild-collected and directional selection may have favoured plant ecotypes adapted to local environmental conditions (Leimu and Fischer, 2008), we also performed a common-garden experiment. Common-garden experiments offer the opportunity to relocate ecotypes from a colder region into a warmer region with a longer growing season and can be used to study local adaptation and growth responses to temperature variation across a species' range (Haggerty and Galloway, 2011; Offord, 2011). Therefore, in a second experiment, we reciprocally transplanted adult individuals that originated from the same populations as in the first experiment in three common gardens (i.e. similar maternal environment) located into a southern, central and northern site along the same gradient (De Frenne et al., 2011a) and used the seeds produced in the common gardens. Seedling establishment was quantified by measuring the rate and percentage of seedling emergence, and seedling biomass in both experiments.
Specifically, we tested the following hypotheses: (a) emergence percentages and seedling growth are lower in plants from northern peripheral origin than from southern origin near the core of the distribution range; (b) seedling establishment of understorey plants with contrasting phenology and life form is differentially affected by temperature along a latitudinal transect; (c) seedling establishment is locally adapted, i.e. seedlings perform better when the mother plant has been transplanted closer to the home site.
MATERIALS AND METHODS
Study species
Two widespread European understorey plants were selected for this study: Anemone nemorosa (Ranunculaceae) is a spring-flowering geophyte while Milium effusum (Poaceae) is an early summer-flowering hemicryptophytic grass. The northern distribution edge of A. nemorosa is situated around 67 °N whereas M. effusum is distributed as far as 71 °N. Their southern range limits are around 38 − 40 °N (Hultén and Fries, 1986). The flowers of A. nemorosa are hermaphroditic and pollinated by insects, and selfing sometimes occurs. The species also propagates clonally with rhizomes. Milium effusum is wind pollinated (Tyler 2002), and also develops short stolons for re-sprouting. Regeneration from seed is the most important means of population persistence and spread for both species (Brunet and von Oheimb, 1998; Tyler, 2002).
Latitudinal gradient and in situ seed collection (first experiment)
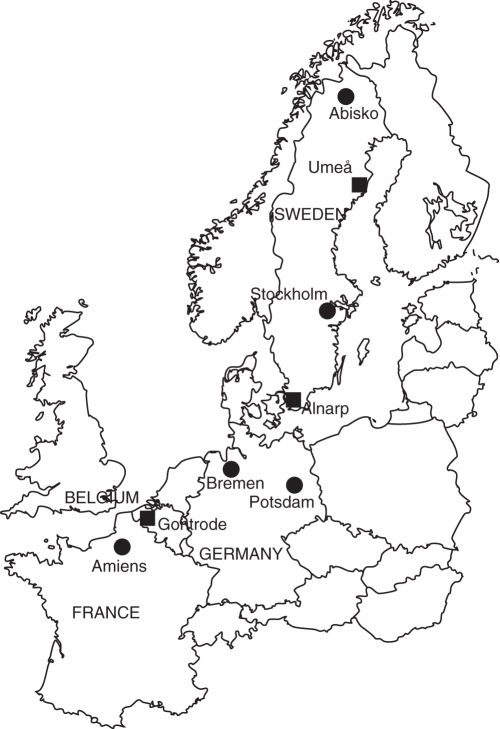
In situ seeds were collected in 2008 in seven (A. nemorosa) and eight (M. effusum) regions located along an approx. 1900-km (A. nemorosa) to 2300-km (M. effusum) latitudinal gradient from northern France via Belgium and Germany to northern Sweden (Fig. 1). Mean air temperatures between March and July 2008 were 12·8 °C in the southernmost (Amiens) and 6·6 °C and 2·7 °C in the northernmost (Umeå and Abisko) populations of A. nemorosa and M. effusum, respectively. More detailed temperature and growing degree-hour data along the latitudinal gradient are provided in De Frenne et al. (2011b). We randomly selected two populations of each species in each region, resulting in a total of 14 A. nemorosa and 16 M. effusum populations. Within each region, the populations were selected within an area of approx. 20 × 20 km2. All populations occurred in deciduous forests with no major recent disturbances in terms of management, grazing, etc. Within each population, the seeds of 15 randomly chosen individuals were collected at the time of seed maturity and pooled. In both species, a random subsample of 50 seeds was weighted per seed lot, and the mean seed mass was determined as the 50-seed mass divided by 50. The region- and species-specific seed collection dates are shown in the Appendix.
Fig. 1.
Map of northwestern Europe showing the latitudinal gradient along which seeds were sampled in situ in natural Anemone nemorosa and Milium effusum populations (both circles and squares; excluding Abisko for A. nemorosa) and the common gardens (squares) where ex situ seeds were collected. Two populations per region were sampled which resulted in 14 A. nemorosa and 16 M. effusum populations.
Ex situ seed collection (second experiment)
Adult individuals (i.e. rhizome fragments in A. nemorosa and whole root clumps in M. effusum) were collected in each of the natural populations along the latitudinal gradient between May and August 2008 and reciprocally transplanted in three common-garden sites near Gontrode (Belgium), Alnarp (southern Sweden) and Umeå (northern Sweden) in September 2008 (Fig. 1). Hence, the maternal plants for the ex situ experiment originated from the same populations as used for the in situ study. The adult individuals were transplanted in 1·5-L pots (four replicate pots per population and per species in each common garden) filled with standard potting soil. The potting soil consisted of a 2 : 1 volume mixture of peat and coconut fibres that was assumed to resemble litter-rich forest soil and had the following characteristics: pH-KCl of 5·5; ammonium acetate-EDTA extractable P of 0·35 mg kg−1 dry soil and KCl extractable NO3– of 2·68 mg kg−1 dry soil. Moreover, a 15–8–11–2·5 N : P : K : Mg fertilizer containing trace elements (Osmocote Exact Lo.Start 16–18M; Scotts International BV, Geldermalsen, The Netherlands) was added at an application rate of 6 g fertilizer L−1 potting soil at the beginning of the experiment. The same pots, potting soil, fertilizer and experimental design were used in the three common gardens. The pots were then placed in a randomized design in forest ecosystems characteristic of the region where the common garden was established (in terms of tree-species composition and overstorey canopy cover). More details on the design of the common-garden sites and detailed microclimatic data are provided in De Frenne et al. (2011a).
The ex situ seeds were then collected from the transplanted mother plants in 2009 in each of the three common-garden sites at the time of seed maturity; again, the exact collection dates were site-dependent (Appendix), seeds were pooled per population × common garden combination and seed mass was determined as described above. Anemone nemorosa had to be omitted from the ex situ analyses because individuals produced no or only few seeds in the common gardens in 2009 (of 56 individuals in each common garden, only eight produced seeds in Belgium, one in southern Sweden and four in northern Sweden).
Growth-chamber experiments: seed sowing and measurements
The seeds were germinated in temperature- and light-controlled growth chambers. The in situ and ex situ seeds were handled in exactly the same way. Seeds of A. nemorosa were stored in moistened white sand at room temperature between seed collection and the start of the experiment (for approx. 12 weeks) to avoid desiccation and viability loss (Ali et al., 2007). Distilled water was added when needed to the sand-seed mixture. Seeds of M. effusum were stored at room temperature until sowing; short-term desiccation poses no threat to the viability of its seeds (Thompson, 1980). On 1 Sepember 2008 (in situ seeds of both species) and 1 September 2009 (ex situ seeds of M. effusum), the seeds were sown in containers filled with potting soil (same soil–fertilizer mixture as used in the common gardens). The containers were subdivided into 28 cells of 17 cm3 each. In each cell, ten seeds were sown. Each population (2008) and population × common garden combination (2009) was randomly assigned to a cell (seven replicates per population and per treatment). The containers were then placed in growth chambers (Sanyo MLR-351 Versatile Environmental Test Chamber; Osaka, Japan) and exposed to the same seasonal cycle in both years (starting on 1 September): (a) autumn simulation at 10 °C for 8 weeks (continuous light); (b) winter simulation at 2 °C for 11 weeks (8 h light, 16 h darkness); (c) early spring simulation at 10 °C for 2 weeks (8 h light, 16 h darkness).
In the in situ experiment, the containers were then (after the 2 weeks at 10 °C mentioned above) randomly assigned to three different growth chambers for 6 weeks representing three possible spring scenarios across the species' range: cool (10 °C), intermediate (15 °C) or warm (20 °C) with 12 h light and 12 h darkness in all cases. In the ex situ experiment, the containers were placed in a growth chamber with an intermediate 15 °C spring scenario (12 h light, 12 h darkness) for 6 weeks. The number of growing degree-hours >5 °C at the end of the experiment (following Lindsey and Newman, 1956) calculated for the three scenarios amounted to 6960, 12240 and 17520 °C-hours, respectively. These values are within the range of growing degree-hours in the species' natural habitats between northern France and northern Sweden (seed maturity of A. nemorosa and M. effusum occurs at approx. 8000–12 000 °C-hours and approx. 12 000–18 000 °C-hours, respectively; De Frenne et al., 2011b). Thus, provenances were grown in environments similar to their natural habitats. Photosynthesis photon flux density in the centre of the growth chambers was always set at 112 µmol m−2 s−1 during light and at 0 µmol m−2 s−1 during darkness. In both experiments, the containers were rotated weekly within each growth chamber and watered as necessary.
The number of seedlings (visible emerged shoot) was recorded weekly from the start until the end of the experiment. On 10–12 March 2009 (in situ seeds) and 11–12 March 2010 (ex situ seeds) the final emergence percentage (calculated as the number of seedlings at the end of the experiment divided by the original seed input) was determined and their above-ground biomass was harvested. All biomass samples were dried for 48 h at 60 °C and weighed. Subsequently, the above-ground biomass per cell was divided by the number of seedlings to determine the biomass per seedling. Additionally, in the case of A. nemorosa, above-ground and below-ground biomass was separated for one randomly chosen seedling per cell and dried as above to determine below-ground biomass and root : shoot ratio (in the case of M. effusum, it was impossible to determine below-ground biomass due to difficulties of separating fine roots and potting soil). Finally, the mean emergence time (MET) was calculated as (adapted from Deines et al., 2007; Milbau et al., 2009):
where ni is the number of emerged seedlings within consecutive time intervals, ti is the time between the beginning of the experiment and the end of a time interval (in weeks), and N is the final number of emerged seedlings.
Data analysis
The effects of the latitude of origin and temperature treatment (in situ) or of the latitude of origin and common-garden site (ex situ) on the seedling traits (i.e. emergence percentage, mean emergence time, and biomass and root : shoot ratio) were analysed with linear mixed-effect models, using the lme-function of the nlme-library in R 2·13·0 (R Development Core Team, 2011). To account for potential maternal effects of seed mass on seedling establishment (e.g. Moles and Westoby, 2002), seed mass data were included in the models as covariate. ‘Region’ and ‘population’ nested within ‘region’ were added to the models as random-effect terms to account for potential autocorrelation of populations from the same region and replicates within populations. The effects of the latitudinal home-away distance [i.e. the absolute value of the latitudinal difference between the latitude of origin (LO) and the latitude of the common-garden transplant site (LCG); i.e. ΔLatitude = |LO – LCG|] were analysed with a similar mixed-effect model. Emergence percentage data were arcsine square-root transformed, and above-ground biomass data of M. effusum and below-ground biomass and root : shoot ratio data of A. nemorosa were log10-transformed prior to the analyses.
RESULTS
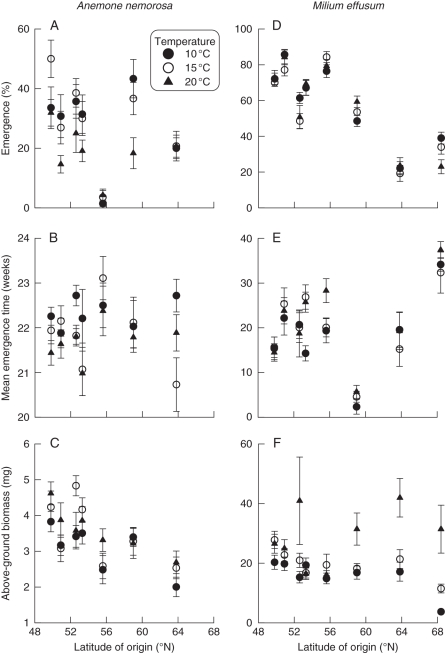
In the in situ experiment, both the emergence percentage (significant only for M. effusum) and above-ground seedling biomass (for both species) decreased with increasing latitude of seed origin (Table 1 and Fig. 2) indicating lower seed quality and seedling performance for individuals of northern origin. Rising spring temperatures consistently increased the above-ground seedling biomass of both species, the below-ground biomass and root : shoot ratio of A. nemorosa, and decreased and (marginally) increased the emergence time in A. nemorosa and M. effusum, respectively (Table 1). Temperature did not consistently affect emergence percentages in A. nemorosa (Table 1 and Fig. 2A). Significant interaction terms were present for M. effusum, indicating a differential response to temperature depending on the latitude of seed origin (Table 1). More specifically, the above-ground seedling biomass increased more with increasing temperature in M. effusum seedlings from northern than from southern origin (Fig. 2F). No such pattern could be seen in A. nemorosa. Seed mass significantly increased the biomass of A. nemorosa seedlings and the emergence time of M. effusum seedlings (Table 1).
Table 1.
The effects of the latitude of seed origin and spring temperature treatment on seedling traits of (A) Anemone nemorosa and (B) Milium effusum in the in situ experiment
| Latitude |
Temperature |
Latitude × temperature |
Seed mass |
|||||
|---|---|---|---|---|---|---|---|---|
| d.f. | F-value | d.f. | F-value | d.f. | F-value | d.f. | F-value | |
| (A) A. nemorosa | ||||||||
| Emergence (%) | 5 | 0·91 n.s. | 267 | ↕7·26** | 267 | 0·55 n.s. | 6 | 2·10 n.s. |
| Emergence time | 5 | 0·02 n.s. | 200 | ↓5·38** | 200 | 2·25 n.s. | 6 | 0·54 n.s. |
| Above-ground biomass | 5 | ↓25·48** | 200 | ↑3·93* | 200 | 0·12 n.s. | 6 | ↑7·95* |
| Below-ground biomass | 5 | 4·05 n.s. | 196 | ↑15·98*** | 196 | 1·80 n.s. | 6 | ↑4·89 (*) |
| Root : shoot ratio | 5 | ↑5·15 (*) | 199 | ↑21·45*** | 199 | 0·80 n.s. | 6 | 0·01 n.s. |
| (B) M. effusum | ||||||||
| Emergence (%) | 6 | ↓23·29** | 334 | 1·05 n.s. | 334 | 3·46* | 7 | 2·74 n.s. |
| Emergence time | 6 | 0·70 n.s. | 328 | ↑2·70 (*) | 328 | 1·74 n.s. | 7 | ↑18·85** |
| Above-ground biomass | 6 | ↓7·23* | 324 | ↑34·98*** | 324 | 28·02*** | 7 | 1·35 n.s. |
Seed mass was included as a covariate in each of the models.
Results from linear mixed-effect models with ‘region’ and ‘population’ nested within ‘region’ as random effects. n.s., P > 0·1; (*), P < 0·1; *, P < 0·05; **, P < 0·01; ***, P < 0·001. d.f., Denominator degrees of freedom. Directions of the effect are reported if P < 0·1: ↓ and ↑ indicate a negative and positive effect, respectively. In the case of the temperature scenarios, ↕ specifies mixed effects (i.e. a negative or positive effect for the 15 °C or 20 °C scenario compared with the 10 °C scenario).
Fig. 2.
Relationships between seedling traits and latitude of origin of seeds collected in natural populations of the forest herbs Anemone nemorosa (left; A–C) and Milium effusum (right; D–F); the seeds were exposed to three different spring-temperature treatments (10 °C, 15 °C, 20 °C, as indicated). The data were averaged across the two populations within a region (only for this figure, not for statistical analyses). Bars denote s.e.
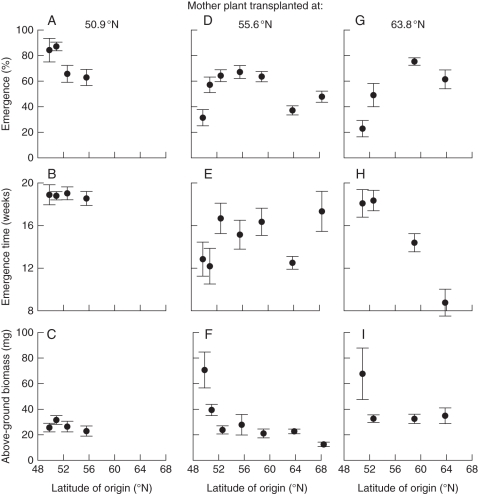
In the ex situ experiment, the biomass of M. effusum seedlings decreased significantly with the latitude of seed origin in each of the three common gardens; similar to the results of the in situ experiment, above-ground seedling biomass significantly decreased from 52 ± 10 mg in the southern populations to 12 ± 2 mg in the northernmost populations (Table 2A and Fig. 3). Conversely, the common-garden transplant site of the mother plant significantly affected seed quality and seedling performance in terms of emergence and above-ground biomass of M. effusum. Across all provenances, the above-ground biomass in seedlings where the mother plant was grown in the southern transplant sites was lower than the biomass in seedlings where the mother plant was grown in the northern sites (Table 2A and Fig. 3C, F, I). The significant interaction between common-garden site and latitude of origin for the emergence percentage and timing again indicates differential behaviour in each transplant site depending on the latitude of origin (Table 2A). The emergence percentage was significantly higher for the seeds produced by the mother plants that were transplanted closer to the home site (i.e. with lower ΔLatitude), suggesting local adaptation (Table 2B and Fig. 3A, D, G). Conversely, the above-ground seedling biomass increased with increasing ΔLatitude (Table 2B). Seed mass positively affected the emergence percentage of M. effusum in the second experiment (Table 2).
Table 2.
Effects of (A) the latitude of origin of the mother plant, common-garden transplant site and their interaction and (B) the latitudinal home-away distance (i.e. the absolute value of the latitudinal difference between the latitude of origin and the latitude of the common-garden transplant site; ΔLatitude), common-garden transplant site and their interaction on seedling traits of ex situ seeds of Milium effusum collected in three common-garden transplant sites located along a latitudinal gradient
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Latitude |
Common garden |
Latitude × common garden |
Seed mass |
|||||
| d.f. | F-value | d.f. | F-value | d.f. | F-value | d.f. | F-value | |
| Emergence (%) | 5 | 0·40 n.s. | 151 | ↓15·41*** | 151 | 11·77*** | 151 | ↑24·65*** |
| Emergence time | 5 | 0·57 n.s. | 147 | ↓10·76*** | 147 | 12·07*** | 147 | 0·01 n.s. |
| Abovegr. biomass | 5 | ↓15·01* | 144 | ↑10·36*** | 144 | 2·02 n.s. | 144 | 0·77 n.s. |
| (B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| ΔLatitude |
Common garden |
ΔLatitude × common garden |
Seed mass |
|||||
| d.f. | F-value | d.f. | F-value | d.f. | F-value | d.f. | F-value | |
| Emergence (%) | 150 | ↓53·72*** | 150 | ↓4·92*** | 150 | 1·35 n.s. | 150 | ↑8·32** |
| Emergence time | 146 | 0·58 n.s. | 146 | ↓14·11*** | 146 | 6·48** | 146 | 0·71 n.s. |
| Abovegr. biomass | 143 | ↑6·35* | 143 | ↑5·79** | 143 | 0·72 n.s. | 143 | 0·57 n.s. |
Seed mass was included as a covariate in each of the models.
Results from linear mixed-effect models with ‘region’ and ‘population’ nested within ‘region’ as random effects. n.s., P > 0·1; *, P < 0·05; **, P < 0·01; ***, P < 0·001. d.f., Denominator degrees of freedom. The direction of the effects is reported if P < 0·1: ↓ and ↑ indicate a negative and positive effect, respectively. In the case of the effect of the common gardens, ↓ and ↑ indicate a northward decrease and increase, respectively.
Fig. 3.
Seedling traits of the understorey forest plant Milium effusum along a latitudinal gradient: (A–C) ex situ collected seeds from the southernmost common garden (Belgium, 50·9°N); (D–F) ex situ collected seeds from the central common garden (southern Sweden, 55·5°N); (G–I) ex situ collected seeds from the northernmost common garden (northern Sweden, 63·8°N). The data were averaged across the two populations within a region (only for this figure; not for statistical analyses). Bars represent s.e.
DISCUSSION
By applying a combination of raising progeny in different spring-warming scenarios and using seeds collected from adult individuals that were reciprocally transplanted in three common gardens, we were able to assess the effects of the maternal environment as well as direct temperature effects on the seedling establishment of the characteristic understorey plants Anemone nemorosa and Milium effusum along a latitudinal gradient. We found decreasing emergence percentages and seedling growth from the centre to the northern edge of the distribution range for both species. Furthermore, elevated spring temperatures had positive effects on the growth of the seedlings which suggests that spring warming might enhance seedling growth and, in turn, improve seedling survival and plant regeneration. Biomass of M. effusum seedlings from northern origin increased more with increasing spring temperature than in those from southern origin. Milium effusum populations close to the range edge thus seemed to respond more strongly to variation in temperature. Finally, the emergence percentage decreased with increasing home-away distance in seeds from the transplant experiment, confirming our hypothesis and suggesting that the maternal plants were best adapted to their home environment.
The emergence percentage and seedling biomass of the study plants decreased in peripheral populations with colder climate compared with southern populations situated within the core of the distribution range. This is consistent with the findings obtained for many other plants (e.g. Jump and Woodward, 2003; Sexton et al., 2009) and might be one of the causes for the current range limits observed in these species. Reduced emergence percentages and seedling growth in northern peripheral populations may reflect that northern plants tend to invest more in clonal reproduction (e.g. Dorken and Eckert, 2001) and possibly put less effort in, for instance, seed production. This is partly supported by our finding of an increasing root : shoot ratio towards the north in A. nemorosa. Since the decrease in seedling performance was generally unrelated to seed-mass differences, possibly a latitudinal decline in seed nutrient concentrations forms the basis for the reduced seedling growth for northern provenances. It has previously been found that the seed nitrogen concentration in A. nemorosa more than doubled between plants growing at 63·8°N and plants growing at 49·8°N (De Frenne et al., 2011c) which may account for the decreasing performance of northern seedlings, irrespective of seed mass effects. Hence, further research could aim at disentangling whether decreasing sexual reproductive fitness from the centre to the edge of the distribution range of plant species (Dorken and Eckert, 2001) might be caused by differential seed provisioning strategies within species. We believe that intraspecific differences in dormancy are most likely not biasing the recruitment success of these study species in the context of the present study. First, A. nemorosa seeds from different provenances germinate very consistently at cooler temperatures following warm stratification (Mondoni et al., 2008). Secondly, M. effusum seeds collected in exactly the same populations and year, and sown under field conditions in forests along a latitudinal gradient still showed lower seedling emergence with increasing latitude of seed origin 3 years after sowing (De Frenne et al., 2011a) which suggests that the pattern of northwards decreasing seedling emergence in the growth chambers likely reflects patterns observed in the field.
Apart from the seedling emergence percentage, the timing of seedling emergence is also a key factor in the potential reproductive success of plants, especially in terms of the predicted changes in phenology across the globe. Although we found no significant linear latitudinal relationship in emergence time in the first, i.e. in situ, experiment, the northernmost M. effusum seedlings still emerged approx. 20 weeks after the southernmost seedlings. Thompson (1980) already suggested that M. effusum seeds may germinate in autumn in southern populations, whereas seeds stay dormant during autumn and winter in northern Europe. Hence, delayed seedling emergence in M. effusum until the next spring seems to be an adaptive strategy in northern populations to avoid the harsh winter conditions, whereas growing conditions in the south are favourable enough to build-up a growth advantage before the winter. Secondly, although spring warming shortened the emergence time of A. nemorosa and thus has the potential to lengthen the growing season and positively affect the regeneration success, all seedlings emerged after the winter treatment and showed limited geographical variation. An expansion of the length of the first growing season of A. nemorosa seedlings might provide them with an important survival advantage (see also Milbau et al., 2009).
The emergence percentage significantly decreased with increasing distance between the latitude of origin and the transplant site (ΔLatitude), which suggests that the maternal plants were best adapted to their home environment. This may have been related to the lower adult plant performance and seed size when the maternal plants were transplanted farther from their home site (see also De Frenne et al., 2011a). Populations of widespread plant species generally display adaptation to the local site conditions due to divergent natural selection (Leimu and Fischer, 2008). The transgenerational local adaptation observed here cannot be attributed to soil properties or competitive interactions because all mother plants were grown principally without competition in exactly the same soil in the three common gardens. Rather, this pattern must be due to adaptation to other local factors, including climate, photoperiod or canopy cover (De Frenne et al., 2011a). Yet, the seedling biomass was generally the highest for the southern provenances, irrespective of the transplant site of the mother plant, and thus the maternal environmental effect seemed to disappear during the development of the progeny (Teng et al., 2009).
In conclusion, we showed that both the maternal environment as well as the temperature directly experienced by the offspring differentially affected the seedlings across the distribution range of the two study species. Spring warming had a positive effect on the growth of understorey seedlings. The larger biomass growth response of seedlings of the grass M. effusum originating from northern populations might enable these populations to cope with more extreme microclimatic heterogeneity in the northern subarctic, than in temperate regions (e.g. Armbruster et al., 2007; Lacey et al., 2010; Graae et al., 2012) and possibly with the climatic changes projected for the coming decades (Hof et al., 2011; Walck et al., 2011). Other studies investigating seed production, germination and/or seedling establishment of plants were inconclusive, showing both positive (Milbau et al., 2009; Klady et al., 2011) and negative (Hovenden et al., 2008; Shevtsova et al., 2009) effects of rising temperatures on sexual plant reproduction. Of course, the impact of changing temperatures on germination and seedling establishment also depends on other factors including water and nutrient availability, herbivory and competition (Ali et al., 2007; Shevtsova et al., 2009; reviewed by Walck et al., 2011). We demonstrated that the question of whether rising temperatures will favour or constrain plant regeneration in the future is not solely a species-dependent issue. Seedling establishment may also show wide intraspecific variation across broad geographical areas. Hence, these phenomena should be incorporated into predictions of future population dynamics across species' ranges. Our results further indicate that climate warming might affect the seedling establishment and regeneration success of plants across their distribution range and thus differentially change understorey plant dynamics across Europe in the near future.
ACKNOWLEDGEMENTS
We are grateful to the staff at the Abisko Scientific Research Station for accommodation, facilities and assistance, as well as to Luc Willems, Greet De bruyn, Rob Dhondt, Thomas Westin and Emma Holmström for laboratory and field assistance, and to Jake Alexander, Pat Heslop-Harrison, Mick Hanley and two anonymous reviewers for extensive remarks on earlier drafts. This work was supported by the Research Foundation – Flanders (FWO) by funding the scientific research network FLEUR (www.fleur.ugent.be); the Kempe Foundation; the Petra and Karl Erik Hedborg Foundation; and by an EU Transnational Access Program ATANS Grant (grant number Fp6 506004). This paper was written while P.D.F. and A.D.S. held post-doctoral fellowships from the FWO.
APPENDIX
Seed collection dates (first, i.e. in situ experiment: 2008; second, i.e. ex situ experiment: 2009; in Julian days) along the latitudinal gradient.
| Region | City | Latitude (°N) | Longitude (°E) | Seed collection dates in situ |
Seed collection dates ex situ | |
|---|---|---|---|---|---|---|
| A. nemorosa | M. effusum | M. effusum† | ||||
| France | Amiens | 49·8 | 2·1 | 140 | 157 | |
| Belgium | Gontrode | 50·9 | 3·8 | 130 | 157 | 162 |
| NE Germany | Potsdam | 52·6 | 13·0 | 136 and 137 | 162 and 164 | |
| NW Germany | Bremen | 53·3 | 9·2 | 142 | 165 | |
| S Sweden | Alnarp | 55·6 | 13·3 | 141 | 170 and 171 | 173 |
| C Sweden | Stockholm | 59·0 | 17·5 | 157 | 198 | |
| N Sweden | Umeå | 63·8 | 20·0 | 176 | 187 and 198 | 195 |
| N Sweden | Abisko | 68·4 | 18·8 | –* | 235 and 239 | |
* A. nemorosa has no natural occurrences near Abisko.
† A. nemorosa had to be omitted from the ex situ analyses because individuals produced only few seeds in the common gardens in 2009 (see text).
LITERATURE CITED
- Adler PB, HilleRisLambers J. The influence of climate and species composition on the population dynamics of ten prairie forbs. Ecology. 2008;89:3049–3060. doi: 10.1890/07-1569.1. [DOI] [PubMed] [Google Scholar]
- Ali N, Probert R, Hay F, Davies H, Stuppy W. Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research. 2007;17:155–163. [Google Scholar]
- Armbruster WS, Rae DA, Edwards ME. Topographic complexity and terrestrial biotic response to high-latitude climate change: variance is as important as the mean. In: Orbćk JB, Kallenborn R, Tombre I, Hegseth EN, Falk-Petersen S, Hoel AH, editors. Arctic alpine ecosystems and people in a changing environment. Berlin: Springer; 2007. pp. 105–117. [Google Scholar]
- Brunet J, von Oheimb G. Colonization of secondary woodlands by Anemone nemorosa. Nordic Journal of Botany. 1998;18:369–377. [Google Scholar]
- De Frenne P, Kolb A, Verheyen K, et al. Unravelling the effects of temperature, latitude and local environment on the reproduction of forest herbs. Global Ecology and Biogeography. 2009;18:641–651. [Google Scholar]
- De Frenne P, Brunet J, Shevtsova A, et al. Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Global Change Biology. 2011a;17:3240–3253. [Google Scholar]
- De Frenne P, Graae BJ, Kolb A, et al. An intraspecific application of the leaf-height-seed ecology strategy scheme to forest herbs along a latitudinal gradient. Ecography. 2011b;34:132–140. [Google Scholar]
- De Frenne P, Kolb A, Graae BJ, et al. A latitudinal gradient in seed nutrients of the forest herb Anemone nemorosa. Plant Biology. 2011c;13:493–501. doi: 10.1111/j.1438-8677.2010.00404.x. [DOI] [PubMed] [Google Scholar]
- De Schrijver A, De Frenne P, Ampoorter E, et al. Cumulative nitrogen input drives species loss in terrestrial ecosystems. Global Ecology and Biogeography. 2011;20:803–816. [Google Scholar]
- Deines L, Rosentreter R, Eldridge DJ, Serpe MD. Germination and seedling establishment of two annual grasses on lichen-dominated biological soil crusts. Plant and Soil. 2007;295:23–35. [Google Scholar]
- Dorken ME, Eckert CG. Severely reduced sexual reproduction in northern populations of a clonal plant. Journal of Ecology. 2001;89:339–350. [Google Scholar]
- Dormann CF, Woodin SJ. Climate change in the Arctic: using plant functional types in a meta-analysis of field experiments. Functional Ecology. 2002;16:4–17. [Google Scholar]
- Elmensdorf SC, Henry GHR, Hollister RD, et al. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters. 2012;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integrative and Comparative Biology. 2006;46:5–17. doi: 10.1093/icb/icj003. [DOI] [PubMed] [Google Scholar]
- Gilliam FS. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57:845–858. [Google Scholar]
- Graae BJ, De Frenne P, Kolb A, et al. On the use of weather data in ecological studies along altitudinal and latitudinal gradients. Oikos. 2012;121:3–19. [Google Scholar]
- Haggerty BP, Galloway LF. Response of individual components of reproductive phenology to growing season length in a monocarpic herb. Journal of Ecology. 2011;99:242–253. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends in Plant Science. 2009;14:30–36. doi: 10.1016/j.tplants.2008.11.001. [DOI] [PubMed] [Google Scholar]
- HilleRisLambers J, Harpole WS, Schnitzer S, Tilman D, Reich PB. CO2, nitrogen, and diversity differentially affect seed production of prairie plants. Ecology. 2009;90:1810–1820. doi: 10.1890/07-1351.1. [DOI] [PubMed] [Google Scholar]
- Hof C, Levinsky I, Araujo MB, Rahbek C. Rethinking species' ability to cope with rapid climate change. Global Change Biology. 2011;17:2987–2990. [Google Scholar]
- Houle G, Babeux P. Variations in rooting ability of cuttings and in seed characteristics of 5 populations of Juniperus communis var. depressa from subarctic Quebec. Canadian Journal of Botany. 1994;72:493–498. [Google Scholar]
- Hovenden MJ, Wills KE, Chaplin RE, et al. Warming and elevated CO2 affect the relationship between seed mass, germinability and seedling growth in Austrodanthonia caespitosa, a dominant Australian grass. Global Change Biology. 2008;14:1633–1641. [Google Scholar]
- Hultén E, Fries M. Atlas of North European vascular plants: north of the Tropic of Cancer I–III. Königstein: Koeltz Scientific Books; 1986. [Google Scholar]
- IPCC. Climate change: the physical science basis. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Jump AS, Woodward FI. Seed production and population density decline approaching the range-edge of Cirsium species. New Phytologist. 2003;160:349–358. doi: 10.1046/j.1469-8137.2003.00873.x. [DOI] [PubMed] [Google Scholar]
- Klady RA, Henry GHR, Lemay V. Changes in high Arctic tundra plant reproduction in response to long-term experimental warming. Global Change Biology. 2011;17:1611–1624. [Google Scholar]
- Lacey EP, Lovin ME, Richter SJ, Herington DA. Floral reflectance, color, and thermoregulation: what really explains geographic variation in thermal acclimation ability of ectotherms? American Naturalist. 2010;175:335–349. doi: 10.1086/650442. [DOI] [PubMed] [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PloS ONE. 2008;3:e4010. doi: 10.1371/journal.pone.0004010. http://dx.doi.org/10.1371/journal.pone.0004010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey AA, Newman JE. Use of official weather data in spring time – temperature analysis of an Indiana phenological record. Ecology. 1956;37:812–823. [Google Scholar]
- Milbau A, Graae BJ, Shevtsova A, Nijs I. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany. 2009;104:287–296. doi: 10.1093/aob/mcp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Westoby M. Seed addition experiments are more likely to increase recruitment in larger-seeded species. Oikos. 2002;99:241–248. [Google Scholar]
- Mondoni A, Probert R, Rossi G, Hay F, Bonomi C. Habitat-correlated seed germination behaviour in populations of wood anemone (Anemone nemorosa L.) from northern Italy. Seed Science Research. 2008;18:213–222. [Google Scholar]
- Mooney HA, Billings WD. Comparative physiological ecology of Arctic and Alpine populations of Oxyria digyna. Ecological Monographs. 1961;31:1–29. [Google Scholar]
- Offord CA. Pushed to the limit: consequences of climate change for the Araucariaceae: a relictual rain forest family. Annals of Botany. 2011;108:347–357. doi: 10.1093/aob/mcr135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert RJ. The role of temperature in the regulation of seed dormancy and germination. In: Fenner M, editor. The ecology of regeneration in plant communities. Wallingford, UK: CABI Publishing; 2000. pp. 261–292. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. www.r-project.org . [Google Scholar]
- Saatkamp A, Affre L, Dutoit T, Poschlod P. Germination traits explain soil seed persistence across species: the case of Mediterranean annual plants in cereal fields. Annals of Botany. 2011;107:415–426. doi: 10.1093/aob/mcq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer D, Korner C. Infra-red thermometry of Alpine landscapes challenges climatic warming projections. Global Change Biology. 2010;16:2602–2613. [Google Scholar]
- Sexton JP, Mcintyre PJ, Angert AL, Rice KJ. Evolution and ecology of species range limits. Annual Review of Ecology Evolution and Systematics. 2009;40:415–436. [Google Scholar]
- Sherry RA, Zhou XH, Gu SL, et al. Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences of the USA. 2007;104:198–202. doi: 10.1073/pnas.0605642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova A, Graae BJ, Jochum T, et al. Critical periods for impact of climate warming on early seedling establishment in subarctic tundra. Global Change Biology. 2009;15:2662–2680. [Google Scholar]
- Svenning JC, Skov F. Potential impact of climate change on the northern nemoral forest herb flora of Europe. Biodiversity and Conservation. 2006;15:3341–3356. [Google Scholar]
- Teng N, Jin B, Hao H, Ceulemans R, Kuang T, Lin J. No detectable maternal effects of elevated CO2 on Arabidopsis thaliana over 15 generations. PloS ONE. 2009;4:e6035. doi: 10.1371/journal.pone.0006035. http://dx.doi.org/10.1371/journal.pone.0006035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PA. Germination strategy of a woodland grass – Milium effusum L. Annals of Botany. 1980;46:593–602. [Google Scholar]
- Tyler T. Geographic structure of genetic variation in the widespread woodland grass Milium effusum L.: a comparison between two regions with contrasting history and geomorphology. Genome. 2002;45:1248–1256. doi: 10.1139/g02-079. [DOI] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, et al. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the USA. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]