Abstract
Background
Molecular phylogeny has resolved the liverworts as the earliest-divergent clade of land plants and mosses as the sister group to hornworts plus tracheophytes, with alternative topologies resolving the hornworts as sister to mosses plus tracheophytes less well supported. The tracheophytes plus fossil plants putatively lacking lignified vascular tissue form the polysporangiophyte clade.
Scope
This paper reviews phylogenetic, developmental, anatomical, genetic and paleontological data with the aim of reconstructing the succession of events that shaped major land plant lineages.
Conclusions
Fundamental land plant characters primarily evolved in the bryophyte grade, and hence the key to a better understanding of the early evolution of land plants is in bryophytes. The last common ancestor of land plants was probably a leafless axial gametophyte bearing simple unisporangiate sporophytes. Water-conducting tissue, if present, was restricted to the gametophyte and presumably consisted of perforate cells similar to those in the early-divergent bryophytes Haplomitrium and Takakia. Stomata were a sporophyte innovation with the possible ancestral functions of producing a transpiration-driven flow of water and solutes from the parental gametophyte and facilitating spore separation before release. Stomata in mosses, hornworts and polysporangiophytes are viewed as homologous, and hence these three lineages are collectively referred to as the ‘stomatophytes’. An indeterminate sporophyte body (the sporophyte shoot) developing from an apical meristem was the key innovation in polysporangiophytes. Poikilohydry is the ancestral condition in land plants; homoiohydry evolved in the sporophyte of polysporangiophytes. Fungal symbiotic associations ancestral to modern arbuscular mycorrhizas evolved in the gametophytic generation before the separation of major present-living lineages. Hydroids are imperforate water-conducting cells specific to advanced mosses. Xylem vascular cells in polysporangiophytes arose either from perforate cells or de novo. Food-conducting cells were a very early innovation in land plant evolution. The inferences presented here await testing by molecular genetics.
Keywords: Bryophytes, cuticle, homoiohydry, mycorrhizas, phylogeny, poikilohydry, polysporangiophytes, shoot apical meristem (SAM), stomata, stomatophytes, tracheophytes, vascular tissues
INTRODUCTION
Land plants are a monophyletic group sharing ancestry with charophycean algae (Qiu et al., 2006, 2007; Qiu, 2008; Finet et al., 2010) and are characterized primarily by a unique combination of features: a multicellular parenchymatous organization, a life cycle in which a haploid sexual gametophyte alternates with a diploid asexual sporophyte, zygote/embryo development within the parental gametophyte and sporopollenin-coated meiospores (Graham and Wilcox, 2000; Niklas and Kutschera, 2010).
After over a century of controversy and following the molecular revolution, Bower's (1890) hypothesis of the alternation of generations evolved in the ancestor of land plants through a delay in meiosis and intercalation of a new diploid organism in an originally haplontic life cycle is now the accepted model. Consequently, the term ‘embryophytes’ is currently used as a formal name for land plants (Graham and Wilcox, 2000; Nicklas and Kutschera, 2010).
Microfossils interpreted as sporopollenin-coated spores in mid-Ordovician rocks (around 470 Mya) are considered to be the first record of a land flora (Wellman et al., 2003). It has been suggested that a pivotal event in embryophyte evolution, pre-dating the appearance of a multicellular sporophyte, was a delay in post-fertilization sporopollenin deposition in the zygote and parallel acceleration of zygotic meiosis, resulting in the development of sporopollenin-coated spores (Hemsley, 1994; Brown and Lemmon, 2011a). This interpretation might help in understanding the origin of a diversity of spore types in mid-Ordovician to Late Silurian microfossil assemblages, including naked and enveloped monads, dyads and tetrads (Edwards et al., 1998b; Wellman and Gray, 2000).
Molecular phylogenies indicate that the three extant bryophyte lineages (liverworts, mosses and hornworts) separated before the lineage ancestral to present-day tracheophytes. The liverworts are resolved as the earliest-divergent land plant clade. Mosses are the sister group to a clade formed by hornworts and tracheophytes, with alternative topologies resolving the hornworts as sister to mosses plus tracheophytes less well supported (Qiu et al., 2006, 2007; Qiu, 2008; Chang and Graham, 2011).
An obvious corollary to the scenario outlined above is the notion that land plants primarily had a strongly dimorphic alternation of generations, with a relatively conspicuous, dominant gametophyte and a sporophyte consisting essentially of a mass of archesporial tissue. A haustorial foot and a sporangial wall of sterile cells were probably early additions (Hemsley, 1994). The sporophyte underwent further structural elaboration in the bryophyte grade, essentially with the evolution of specialized devices to enhance spore release, but it remained a uniaxial structure permanently associated with and dependent on a dominant gametophyte. A shift from a gametophyte-dominated to a sporophyte-dominated life cycle has marked the evolution of polysporangiate plants, i.e. present-day tracheophytes and their extinct relatives (Kenrick and Crane, 1997a, b; Kenrick, 2000; Gerrienne and Gonez, 2011).
The seminal papers by Mishler and co-workers (Mishler and Churchill, 1984, 1985; Mishler et al., 1985) have deeply influenced current ideas about character evolution in early land plants. Since then, dramatic advances in phylogenetic analysis, molecular genetics, developmental biology and anatomy have produced a body of novel information that demands a re-appraisal of the topic. Notably, Kato and Akiyama (2005) recently challenged the widely accepted notion that the sporophyte vegetative body in tracheophytes is homologous with the seta of mosses (Mishler and Churchill, 1984) and interpreted it as a novel structure interpolated between the embryo and sporogenesis.
In the context of the novel information now available, this paper analyses gametophyte and sporophyte character evolution in early land plants and attempts to reconstruct possible scenarios for the divergence of major present-day lineages. A glossary of technical terms employed in this review is given in Table 1.
Table 1.
Glossary of technical terms
| Anticlinal division | Cell division along a plane perpendicular to the outer surface of the organ in which the cell lies |
| Apomorphy, apomorphic character | Innovative character distinctive of a monophyletic group (see below) |
| Archesporium, archesporial tissue | The spore-forming tissue in a sporangium |
| Calyptra | Archegonium-derived cap covering the developing sporangium in peristomate mosses (see below) |
| Clade | A monophyletic group (see below) |
| Columella | A column-like central mass of sterile cells in the sporangium of mosses and hornworts and of the extinct protracheophyte Horneophyton |
| Desiccation tolerance | The ability to survive cellular dehydration |
| Dichotomous branching | Apical division of an axis into two branches of similar (isotomous branching) or different (anisotomous branching) sizes |
| Elaters | Cells with spirally thickened walls with the function of facilitating the separation and dispersion of mature spores |
| Exine | The sporopollenin-containing cell-wall layer (see below) in embryophyte spores |
| Exosporic gametophyte | A gametophyte that develops outside the confines of the spore cell wall |
| Endosporic gametophyte | A gametophyte entirely, or almost entirely, growing within the confines of the spore cell wall |
| Homoiohydry | The ability of an organism to maintain a relatively stable water content independently of short-term fluctuations in water supply |
| Monophyletic group | An evolutionary lineage including an ancestor and all its descendants; a synonym is holophyletic group |
| Mucilage papillae | Glandular structures secreting a carbohydrate-rich mucilage |
| Oil bodies | Endoplasmic-reticulum-derived vesicles containing terpenoid compounds |
| Operculum | Sporangium lid of mosses (present only in the Sphagnopsida and peristomate mosses) |
| Orthologous genes, orthologues | Genes present in different species and originated from the same ancestral gene |
| Perine | Outermost spore wall layer deposited at a final stage of spore maturation |
| Peristomate mosses | Mosses possessing a peristome, i.e. a ring of projections that surround the aperture of the sporangium and control spore release |
| Polyphyletic character | A character independently evolved in separate lineages |
| Phyllids | Leaf-like gametophytic structures |
| Phyllotaxis | Geometry of distribution of leaves or leaf-like structures along an axis |
| Plesiomorphy, plesiomorphic character | Primitive character present both within and outside a monophyletic group |
| Poikilohydry | Ecological strategy characterized by the lack of control of water content associated with protoplasmic tolerance to desiccation in somatic tissues |
| Protocorm | Parenchymatous structure preceding the formation of a proper sporophyte shoot during embryo development in certain polysporangiophytes |
| Pyrenoid | Discrete chloroplast structure containing a high concentration of the CO2-fixing enzyme ribulose biphoshate carboxylase/oxygenase (Rubisco) |
| Sister groups | Two groups divergent from a common ancestor |
| Sporeling | Few-celled initial product of spore germination |
| Sporopollenin | Chemically complex polymeric substance present in the cell wall of spores in embryophytes and of resting cells in green algae |
| Terete | Cylindrical |
| Totipotent (stem) cell | A dividing cell that is able to generate all cell types present in the mature form of a multicellular organism |
| Unistratose | Made of a single cell layer |
THE PALEOBOTANICAL RECORD
Polysporangiophytes
The oldest land plant macrofossil is a leafless isotomously branched sporophyte, a few centimetres high, bearing terminal Cooksonia-type sporangia and dating back to the mid-Silurian, about 425 Mya (Edwards and Feehan, 1980). Of utmost importance for the reconstruction of the origins of tracheophytes also are the Early Devonian forms Aglaophyton and Horneophyton and an Early Devonian assemblage of multisporangiate plants referred to as rhyniopsids (Kenrick and Crane, 1997a, b). Kenrick and Crane (1991) created the informal group ‘polysporangiophytes’ to include fossil and living embryophytes with branched sporophytes producing multiple sporangia. Most known polysporangiate embryophytes have vascular cells, at least in the sporophyte. Based on the presence/absence and morphology of decay-resistant cell-wall thickenings, Kenrick and Crane (1991, 1997a, b) distinguished three basic types of vascular cells: cells with uniform cell walls, S-type cells and G-type cells. The first allegedly recall moss hydroids as they lack distinct cell-wall thickenings and are interpreted as non-lignified vascular cells. Although exhibiting sharply different morphologies, both S- and G-type vascular cells have prominent wall thickenings and are supposed to be lignified. ‘Hydroid-like’ vascular cells occur in Aglaophyton and Horneophyton, two taxa considered archetypal tracheophytes and collectively referred to as the ‘pro-tracheophytes’; S-type cells characterize a rhyniopsid assemblage including Sennicaulis, Rhynia, Huvenia and Stockmansella, collectively referred to as the ‘paratracheophytes’ by Gerrienne et al. (2006); G-type cells were first described in the Early Devonian zosterophyll Gosslingia breconensis and are now considered as a synapomorphy of eutracheophytes, a lineage encompassing most extinct and all extant tracheophytes (Kenrick and Crane, 1997a, b). Several further types of vascular cells have been described and interpreted as variants of the three basic types mentioned above (Edwards and Axe, 2000; Edwards et al., 2003). Cooksonia pertoni has a central strand of vascular tissue (Edwards et al., 1992) and is considered to be an archetypal eutracheophyte (Gerrienne et al., 2006); other specimens of cooksonioid affinity have putative vascular cells of uncertain interpretation or lack a distinct vascular strand (Edwards et al., 1992; Edwards, 1993; Kenrick and Crane, 1997b).
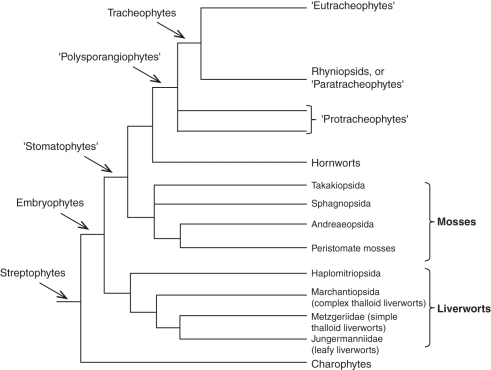
A morphological phylogenetic assessment (Kenrick and Crane, 1997b) has resolved the polysporangiophytes as a monophyletic group, with the rhyniopsids and eutracheophytes as sister groups within the tracheophyte clade, and the protracheophytes forming a paraphyletic group (Fig. 1). The transition from a sporophyte totally dependent on the gametophyte to a fully autonomous sporophyte must have been a long stepwise process that involved the development of an autonomous photosynthetic apparatus, water- and ion-absorbing structures, and vascular and mechanical tissues (Bateman et al., 1998). Mid Silurian (Cooksonia hemisphaerica) to Early Devonian fossils (e.g. Zosterophyllum, Horneophyton, Aglaophyton, Rhynia gwynne-vaughanii) of polysporangiate sporophytic axes document the presence of a cuticle, stomata and subepidermal parenchyma with air spaces (Kenrick and Crane, 1997b; Edwards, 1993; Edwards et al.,1998a, b; Taylor et al., 2009). Hence, these sporophytes were probably substantially autonomous from the gametophyte with regard to production of organic matter. The presence of rhizoids in Early Devonian sporophytic axes of Horneophyton (Kenrick and Crane, 1997b), Trichopherophyton, Rhynia and Aglaophyton (Edwards et al., 1998b) is rather an obvious sign of complete nutritional autonomy.
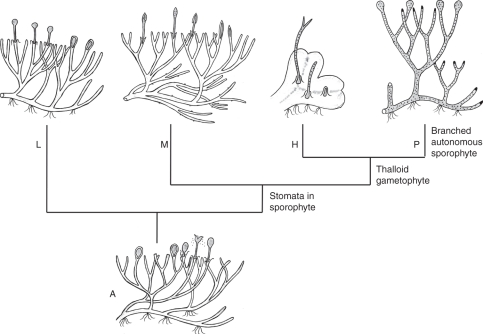
Fig. 1.
Cladogram of land plants (based on Kenrick and Crane, 1997a; Heinrichs et al., 2005; Forrest et al., 2006; Qiu et al., 2006; Cox et al., 2010; Gerrienne and Gonez, 2011).
Gametophytes have been identified and associated with their sporophytic counterparts for four Rhynie chert (Early Devonian) polysporangiophytes: the protracheophytes Aglaophyton major and Horneophyton lignieri, the rhyniopsid Rhynia gwynne-vaughanii, and Nothya aphylla, a taxon with ambiguous affinity in both the rhyniopsids and the zosterophylls (Taylor et al., 2009). These gametophytes consisted of a basal region with radiating erect axes, each terminated by an expanded structure bearing gametangia; the gametangiophore axes had stomata and a central strand of vascular tissue morphologically similar to that present in the corresponding sporophyte (Edwards et al., 1998a, b; Kerp et al., 2004; Taylor et al., 2005, 2009). The complete life cycles of Aglaophyton, Horneophyton and Rhynia have been reconstructed and it has been suggested that an isomorphic alternation of generations – at least in terms of relative structural complexity – is the plesiomorphic condition in polysporangiophytes, including basal eutracheophytes, and that the strongly heteromorphic pattern present in more advanced eutracheophyte taxa is derived (Kenrick and Crane, 1997a, b; Kenrick, 2000). As observed by Gerrienne and Gonez (2011), however, Lyonophyton and Remyophyton, the putative gametophytes respectively of Aglaophyton and Rhynia gwynne-vaughanii, were one order of magnitude smaller than their corresponding sporophytes, and hence the life cycle of these plants was already largely dominated by the sporophytic generation.
Bryophytes
Naked spore tetrads from Ordovician rocks possess a multilamellate layer interpreted as an indication of liverwort affinity (Wellman et al., 2003); strictly speaking, this is regarded as a character diagnostic of bryophytes sensu lato because a multilamellate layer also occurs in the spore wall of the early-divergent mosses Takakia and Sphagnum and in hornwort exines during development (Brown and Lemmon, 1990; Renzaglia et al., 1997). Thus, microfossil evidence indicates that plants with bryophytic affinities pre-dated the earliest known polysporangiophytes by about 50 Mya. In spite of this, the macrofossil record of bryophytes is very scarce and the earliest accepted specimens are from the Carboniferous (Edwards, 2000; Taylor et al., 2009). As an example, Muscites plumatus, from the Lower Carboniferous, is probably the oldest unequivocal moss fossil to date (Thomas, 1972). Unfortunately, the assessment of Silurian and Devonian macrofossils for bryophyte affinity is highly problematic because of the difficulty in demonstrating the unequivocal absence of sporophyte branching (Edwards, 2000). Among the best known candidates are Tortilicaulis transwallensis, a Late Silurian/Early Devonian fossil resembling the living moss Takakia, Sporogonites exuberans, an Early Devonian compression fossil with possible moss affinity, Pallaviciniites devonicus and Metzgeriothallus sharonae, respectively from the Late and Middle Devonian and both with probable affinity in the Pallaviciniales, Metzgeriidae (Hernick et al., 2008; Taylor et al., 2009, and references therein). There are no reliable fossil reports of hornworts prior to the Cretaceous (Taylor et al., 2009).
A phyletic tree of land plants, including major bryophyte lineages, is shown in Fig. 1.
GAMETOPHYTE AND SPOROPHYTE DEVELOPMENT IN THE BRYOPHYTE GRADE
The mature gametophyte is a thallus or a leafy shoot in liverworts, a leafy shoot in mosses and a thallus in hornworts (Fig. 2). In all three cases the gametophyte has indeterminate growth due to the activity of a totipotent apical cell. The geometry of division of the apical cell and its derivatives (merophytes) makes way for specific growth forms (Renzaglia et al., 2000, 2009; Crandall-Stotler et al., 2009). Thus, a tetrahedral apical cell in most leafy liverworts produces a terete axis bearing three rows of phyllids, one of which may be secondarily reduced or suppressed. Wedge- and lens-shaped apical cells in thalloid liverworts and in hornworts result in flattened plant bodies with clearly delimited dorsal and ventral sides. With the only exception of Takakia, the apical cell of the moss leafy shoot exhibits a unique ‘oblique’ pattern of segmentation in which each dividing septum is rotated by about 137 ° relative to the preceding one (Crandall-Stotler, 1984). Consequently, the leafy shoot in mosses has a spiral phyllotaxis, whereas in leafy liverworts the phyllids are three-ranked. The leafy shoot of Takakia has an isolateral tetrahedral apical cell with parallel segmentation and three irregular rows of phyllids, thus recalling the growth pattern of leafy liverworts (Schuster, 1966; K. S. Renzaglia and K. Mansouri, unpubl. res.).
Fig. 2.
Extant members of bryophyte lineages. (A, B) Monoclea forsteri (complex thalloid liverworts), male gametophytes (A) and mature sporophytes (B). (C) Schistochila alata (leafy liverworts), gametophytes. (D) Polytrichastrum formosum (mosses), mature gametophytes and sporophytes. (E) Phaeoceros carolinianus (hornworts), gametophytes and sporophytes.
The branching mechanism of the gametophyte in bryophytes is highly diverse. Branching by equal division of the apical cells (dichotomous branching) occurs in complex thalloid liverworts and in hornworts (Renzaglia, 1978; Crandall-Stotler, 1984), whereas in leafy and some simple thalloid liverworts lateral branches arise from cells of phyllid primordia or from epidermal or cortical cells of the stem (Crandall-Stotler et al., 2009). In mosses each derivative cell arising from the apical cell produces a branch primordium that can develop into a lateral branch some distance behind (Goffinet et al., 2009).
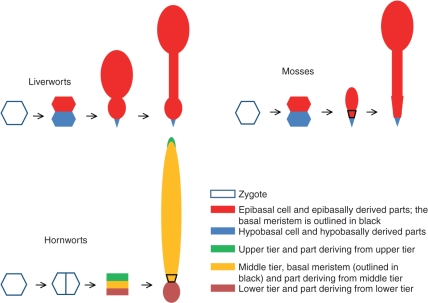
Embryo development in bryophytes has been discussed by Kato and Akiyama (2005); additional information can be found in Crandall-Stotler (1984), Ligrone et al. (1993) and Goffinet et al. (2009). A diagrammatic representation of embryo development in bryophytes is given in Fig. 3.
Fig. 3.
Diagrammatic representation of sporophyte development in liverworts, mosses and hornworts. The bicellular stage in hornworts is not in colour as the first two cells are not yet differentially determined. Different scale sizes are used for the zygote and early-embryo stages relative to later stages. Although most likely homologous with the foot in liverworts and mosses, the hornwort foot is illustrated in a different colour because differences in early embryo development do not distinguish epi- and hypobasally derived parts.
Briefly, in liverworts the first division of the zygote is transverse relative to the long axis of the archegonium and produces an epibasal and hypobasal cell. Subsequent development is rather variable in the different groups but usually the epibasal cell generates the sporangium, seta and foot, whereas the hypobasal cell gives rise to a small filamentous appendage referred to as the haustorium (Schertler, 1979). Sporophyte development proceeds by generalized cell division; meristematic activity occurs in a very early phase of development and subsequent growth, notably the elongation of the seta, only involves cell expansion.
As in liverworts, the first division of the zygote in mosses is transverse and produces an epibasal and hypobasal cell. The hypobasal cell undergoes a number of divisions producing an haustorial tip of one to few cells and the lower part of the foot; the epibasal cell divides anticlinally along two cutting sides to produce a number of derivative cells (about 12 in Physcomitrella patens, Sakakibara et al., 2008) that then divide in a regular fashion in all planes. When the sporophyte is still very short (about 0·5 mm long in P. patens), a meristematic area develops at the boundary between the epibasally and hypobasally derived cells. This area remains active for a while and produces the seta and the upper part of the foot, whereas the cells above give rise to the sporangium with little or no further somatic division. Although usually referred to as the intercalary meristem (Kato and Akiyama, 2005), this meristem produces cells only upwardly and therefore it is functionally a basal meristem. The sporophyte in the Sphagnopsida and in Andreaea (Andreaeopsida) lacks a distinct seta, due to the absence or precocious interruption of the activity of the basal meristem, and the foot is entirely or almost entirely of hypobasal derivation.
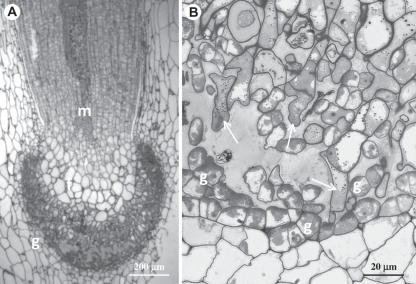
In hornworts the first division of the zygote is longitudinal relative to the long axis of the archegonium and subsequent divisions give rise to a three-tiered embryo. The lowest tier produces the haustorial foot and the top tier the tip of the sporophyte capsule; in both areas cell division ceases early in sporophyte development. In contrast, the middle tier gives rise to a meristematic area, referred to as the basal meristem, that remains active for an extended length of time producing sporangial tissue upwardly (Fig. 4).
Fig. 4.
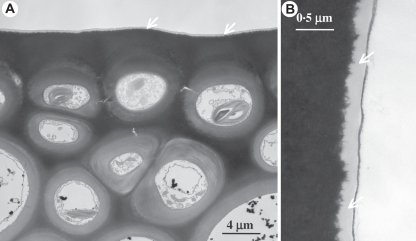
Details of the sporophyte in the hornwort Phaeomegaceros coriaceus. (A) Sporophyte base showing the foot (f) embedded in gametophyte tissue (g) and the persistent basal meristem (m). (B) Higher magnification of the placental region showing sporophytic haustorial cells (arrows) and gametophytic transfer cells (g).
It cannot be ruled out that the longitudinal first division of the hornwort zygote and the unique formative divisions of the embryo are related to the sunken nature of the archegonium in hornworts (Shaw and Renzaglia, 2004). Growth in length of the young embryo is inconsequential because it is surrounded by gametophytic tissue. In contrast, liverworts and mosses are the only extant land plants with superficial gametangia and the initial transverse divisions in the embryo establish a longitudinal axis that determines the three vertically elongating organs, namely foot, seta and capsule. Transverse division may also afford additional lateral support for the developing embryo in an exposed flask-like venter. Nevertheless, in all three bryophyte groups the nascent embryo consists of tiers of quadrants. Oblique periclinal division of quadrant cells produces an inner and an outer layer, respectively referred to as the endothecium and amphitecium. In liverworts the endothecium generates the archesporial tissue and the amphithecium the capsule wall. In the Sphagnopsida and hornworts the endothecium produces only a columella, whereas in the remaining mosses the endothecium forms both the sporogenous tissue and a columella.
GAMETOPHYTE AND SPOROPHYTE DEVELOPMENT IN TRACHEOPHYTES
The gametophytes of exosporic pteridophytes have a flattened thalloid anatomy if growing above ground and photosynthetically, or a tuber-like parenchymatous structure if subterranean. As in bryophytes, these gametophytes have an apical cell and often are indeterminate in growth. Early gametophyte growth involves the production of a spherical base, from which the mature form develops. Endosporic gametophytes, including those in seed plants, lack an apical cell, are highly reduced and have determinate growth (Brandes, 1973; Gifford and Foster, 1989).
Early embryogeny in tracheophytes is quite variable and we refer to Gifford and Foster (1989) and Johnson and Renzaglia (2009) for a detailed description. In seedless tracheophytes, zygotic division may be transverse or prone (nearly longitudinal) and the formative divisions and subsequent origin of embryonic regions are highly variable, even within phyla (Johnson and Renzaglia, 2009). A brief phase of generalized cell division produces a polarized embryo with a foot and, in many cases, a suspensor. Although not part of the embryo proper, the foot is the embryonic region that is universal in embryophytes. The embryo proper develops a shoot and in most cases a root primordium, each provided with an apical meristem. Palaeontological evidence suggests the root evolved secondarily, possibly independently in different lineages, by modification of rhizome-like shoots (Kenrick and Crane, 1997a, b; Raven and Edwards, 2001). Hence, the following description of sporophyte development will be restricted to the shoot.
The shoot apical meristem (SAM) is the structure that defines the polysporangiate lineage and is responsible for the transition from gametophyte to sporophyte dominance (Albert, 1999). The SAM includes one to several self-perpetuating totipotent stem cells referred to as the initial cells. The function of initial cells is strictly linked to their position and, if lost or experimentally removed, these are replaced in their function by neighbouring cells (Lyndon, 1998). A single initial is found in ferns (including Equisetum and Psilotaceae) and, among lycopsids, in Isoëtes and Stylites, whereas multiple initials characterize the SAM in seed plants and lycopods (Gifford and Foster, 1989; Philipson, 1990). The SAM in Selaginella has two short-lived apical initials that are constantly replaced (Harrison et al., 2007), as is also true of the impermanent apical initials in each layer of the complex SAM in seed plants (Bossinger et al., 1992; Furner and Pumfrey, 1992; Korn, 2001).
The branching pattern of the shoot axis in living polysporangiophytes is quite diverse but basically two types can be distinguished: apical (or dichotomous) branching and lateral branching. In apical branching the SAM bifurcates into two, more or less equal and divergent parts (Fig. 5). In lateral branching the main apex produces subordinate SAMs that may develop into shoots some distance behind (Sussex and Kerk, 2001; Harrison et al., 2007). Apical branching is predominant in ferns and lycopsids and is viewed as ancestral in polysporangiophytes, whereas lateral branching is characteristic of seed plants, although the distinction is not absolute (Philipson, 1990; Rothwell, 1995; Sussex and Kerk, 2001).
Fig. 5.
Dichotomously branched polysporangiate sporophyte of Psilotum nudum. In spite of its superficial resemblance to fossil rhyniopsids, Psilotum is firmly nested within the monilophytes along with the ferns and horsetails (Qiu et al., 2006, 2007).
After a phase of indeterminate vegetative growth, the SAM switches to reproductive growth and produces a sporangium or a sporangial cluster and annexed structures where present. During this phase the initial cell(s) are lost and growth becomes determinate (Lyndon, 1998). Palaeontological evidence reviewed by Kenrick and Crane (1997b) and Edwards et al. (1998b) suggests that this pattern of sporophyte development was already present in Early Devonian/Late Silurian polysporangiophytes, with a single or multiple apical initials responsible for indeterminate vegetative growth, including apical branching, followed by determinate reproductive growth. When considered in this light, early polysporangiophytes differed from bryophytes only in the vegetative growth and branching from the SAM prior to the production of terminal sporangia (cf. the capsules of bryophytes). Based on circumstantial fossil evidence, Rothwell (1995) assumed that the branched sporophytes of cooksonioid plants were determinate like moss sporophytes. If true, this character would provide important support for an independent origin of these plants.
STOMATA AND HOMOIOHYDRY
Stomata are one of the most important anatomical characters linking mosses, hornworts and tracheophytes (Paton and Pearce, 1957). Among mosses, stomata or stoma-like structures are found in the sporophyte of the Sphagnopsida (an early-divergent clade) and peristomate mosses (a late-divergent clade), but are lacking in the Takakiopsida and Andreaeopsida, both resolved as early-divergent clades (Fig. 1; Cox et al., 2010; Volkmar and Knoop, 2010; Chang and Graham, 2011). Stomata are of regular occurrence in the hornwort sporophyte, except Nothothylas and a lineage including Megaceros, Nothoceros and Dendroceros (Renzaglia et al., 2007, 2009). Stomata are lacking in liverworts.
The stomata are scattered throughout the mature sporangium in the hornworts and, except the operculum, in the Sphagnopsida, whereas they are usually gathered in the capsule neck, i.e. the transition region between the seta and the spore-containing part, in peristomate mosses (Fig. 6A). Bryophyte stomata usually are anomocytic in structure, i.e. they lack specialized subsidiary cells (Fig. 6A–C and D); however, sunken stomata associated with specialized epidermal cells occur in the moss Orthotrichum anomalum (Fig. 6B). Stomata exhibit a remarkable morphological diversity in mosses (Fig. 6A–D) whereas they are fairly uniform in hornworts (Fig. 6D).
Fig. 6.
Diversity of stomatal morphology in bryophytes. (A) Young sporangium of the moss Bryum capillare with numerous stomata scattered in the neck region. (B) Detail of sporangial wall in the moss Orthotrichum anomalum, showing a sunken stoma overarched by projections from adjacent epidermal cells. (C) Non-functional stomata in the moss Sphagnum fimbriatum. (D) Densely arranged stomata raised above the epidermis in the sporangium of the moss Polytrichastrum formosum. (E) A stoma in the sporangial wall of Anthoceros punctatus; the guard cells are covered with hydrophobic material that possibly prevents water entry into the substomatal cavity.
With the possible exception of early forms of uncertain interpretation (Edwards et al., 1996) and some living lycopsids and angiosperms for which the astomatous condition is certainly derived (Kenrick and Crane, 1997b), stomata have been a regular feature of the sporophyte of polysporangiophytes since the Late Silurian (Kenrick and Crane, 1997b; Edwards et al., 1998a, b). As mentioned above, stomata have also been reported in gametophytes of Devonian polysporangiophytes (Taylor et al., 2005, 2009). Stomata are not known to occur in the gametophyte of extant land plants.
Stomata in hornworts and mosses, with the exception of the Sphagnopsida (Duckett et al., 2009, 2010), have substomatal cavities and are associated with underlying photosynthetic tissue and air spaces. In contrast to tracheophytes, where intercellular spaces are gas-filled from the outset, substomatal intercellular spaces are liquid-filled before stomatal opening in hornworts (Duckett et al., 2010). Limited evidence indicates that bryophyte stomata are able to function in response to variation in water content (Paton and Pearce, 1957). Early studies have reported abscissic acid (ABA)-induced stomatal closure in the moss Funaria (Garner and Paolillo, 1973) and the hornwort Anthoceros punctatus (Hartung et al., 1987). The stomata in Sphagnopsida are non-functional, although their number per capsule is usually much higher than in any other moss. It has been suggested that their function is to facilitate capsule drying after spore maturation (Duckett et al., 2009).
Based on biomechanical considerations, it has been inferred that the stomata in bryophytes and early-divergent polysporangiophytes, including both extinct Devonian forms and present-day lower tracheophytes, have a closing/opening mechanism essentially based on vertical displacement of guard cells; in contrast, stomatal functioning in angiosperms, notably in grasses, also involves horizontal displacement of guard cells (Beerling and Franks, 2009). Stomata in the lycopsid Lycopodium and the fern Pteridium have recently been found to lack the ABA-mediated and epidermal cell-mediated responses to water fluctuations present in angiosperms, and hence it has been inferred that, unlike angiosperm stomata, they function as passive hydraulic valves (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012). In contrast, nearly concomitant work (Chater et al., 2011; Ruszala et al., 2011) has reported that the lycopsid Selaginella and the mosses Physcomitrella and Funaria have stomatal responses to ABA and CO2 comparable with those in the angiosperm Arabidopsis. The same group also demonstrated that a homologue of the ABA-regulatory protein kinase OST1 of Arabidopsis is essential for stomatal response to ABA in the moss Physcomitrella and that the moss gene can rescue an Arabidopsis mutant lacking OST1 activity (Chater et al., 2011).
In line with Kenrick and Crane (1997b), the most parsimonious interpretation of the information summarized above, pending further developmental and genetic studies, is that stomata are an apomorphy of the progenitor of the moss/hornwort/polysporangiophyte lineage and have been lost several times independently. Accordingly, we henceforth adopt the name ‘stomatophytes’ (Kenrick and Crane, 1997b; Bateman et al., 1998) for the three lineages collectively. An analysis of the occurrence and expression patterns in lower land plants of orthologues of genes known to control stomatal development in Arabidopsis (Bergmann et al., 2004; Sugano et al., 2010) is likely to provide further clues on stomatal evolution.
A cutinized epidermis, stomata, gas-filled intercellular spaces (air-spaces) and vascular tissue are the essential features underlying the homoiohydric strategy (Raven, 2002). The cuticle of tracheophytes is a composite extracellular matrix covering all aerial primary parts of the sporophyte; it not only insulates the plant, thereby preventing dehydration, but also performs important roles in the control of plant growth and interfacial interactions (Bargel et al., 2006; Jeffree, 2006). The biochemistry and permeability properties of the cuticle have been investigated extensively and numerous genes involved in cutin and wax biosynthesis have been identified in angiosperms (Schreiber, 2010).
A coating structure somewhat similar to the cuticle in tracheophytes is present in both the gametophyte and the sporophyte (Fig. 7) of bryophytes, as well as in charophycean algae (Cook and Graham, 1998). Bryophytes are poikilohydric plants that express somatic desiccation tolerance in both the gametophyte and the sporophyte, with relatively few desiccation-sensitive taxa restricted to habitats where water is available throughout the life cycle (Oliver et al., 2005; Proctor et al., 2007). In general the cuticle-like coats in bryophytes are simpler in structure than tracheophyte cuticles (Cook and Graham, 1998) and do not appear to function as efficient barriers against dehydration (Proctor, 1979). However, it has recently been shown that a multi-layered cuticle-like coat, presumably with significant waterproofing properties, is present on the calyptra of Funaria hygrometrica (Budke et al., 2011). Although water-repellent waxy deposits are relatively common in mosses and liverworts, these are likely to function in preventing waterlogging of photosynthetic tissues rather than reducing water loss (Proctor, 1979; Pressel and Duckett, 2011; Fig. 6E).
Fig. 7.
Transmission electron micrographs of sporophyte seta in the moss Oedipodium griffithianum. (A) Transverse section showing thick-walled peripheral cells. The arrows point to a cuticle-like coat on the external surface. (B) At higher magnification the cuticle-like coat shows a bilayered structure (arrows).
Most probably, somatic desiccation tolerance is plesiomorphic in bryophytes; in contrast, somatic desiccation tolerance is rare and unevenly scattered in present-day polysporangiophytes, and hence it is considered to be a derived condition that evolved several times independently, probably by deployment of mechanisms underlying desiccation tolerance in spores and seeds (Proctor and Tuba, 2002; Alpert, 2005; Oliver et al., 2005; Watkins et al., 2007; Rensing et al., 2008; Farrant and Moore, 2011).
The fossil record provides extensive evidence for the occurrence of stomata and underlying air-spaces in early-divergent polysporangiophytes (Kenrick and Crane, 1997b; Edwards et al., 1998a; Taylor et al., 2009). Indirect evidence of homoiohydry for ancient plants such as Cooksonia pertonii, Aglaophyton, Horneophyton and Rhynia gwynne-vaughanii comes from their relatively large sizes and lack of efficient mechanical tissues, indicating dependence on turgor pressure for support (Bateman et al., 1998). More direct evidence is available for the Early Devonian trimerophyte Psilophyton dawsonii and the zosterophylls Zosterophyllum myretonianum and Sawdonia ornata. Flash-pyrolysis mass spectrometry of compression fossils of these plants has revealed the presence of carbon chains probably derived from cutan, a polymer present in the cuticle of modern tracheophytes and much more resistant to decay than cutin (Nip et al., 1986). In combination with anatomy, this was considered compelling evidence of homoiohydry (Edwards et al., 1998b).
Investigating the possible occurrence in bryophytes of orthologues of genes involved in cutin biosynthesis in Arabidopsis and their expression in either generation might provide major insights into the evolution of land plants and the interlinked origins of stomata, cuticle and homoiohydry.
GAMETOPHYTE AND SPOROPHYTE ELABORATION IN MAJOR LAND PLANT CLADES
In spite of apparent similarities, the three bryophyte lineages present several major divergences in anatomy and development, in part already discussed in the preceding sections.
Distinctive characteristics of liverworts include centrosome-like structures named polar organizers (Brown and Lemmon, 2007, 2011b), oil bodies (Asakawa, 1995; Duckett and Ligrone, 1995) and elaters (Crandall-Stotler et al., 2009). The seta of the liverwort sporophyte lacks mechanical and vascular tissues, elongates by cellular expansion after spore maturation and rapidly degenerates after spore release, which is a one-time process.
Mosses diverge from the rest of land plants in several characters, including septate multicellular rhizoids, filamentous protonemata, a spiral leafy organization of the mature gametophyte and a unique segmentation pattern of the apical cell. These signature features of mosses are lacking in Takakia, the probable sister taxon to other mosses (Volkmar and Knoop, 2010). A seta is present in Takakia, Andreaeobryum and peristomate mosses, but is lacking in Sphagnopsida and Andreaea, two other early-divergent moss lineages. Where present, the seta elongates before spore maturation, is provided with a robust mechanical tissue (Fig. 7) and usually contains a central strand of vascular tissue (Hébant, 1977; Renzaglia et al., 1997; Ligrone et al., 2000). Because of major differences in anatomy and development, independent evolution of the seta in liverworts and mosses cannot be excluded.
In many hornworts the chloroplast contains a pyrenoid and presents a unique arrangement of the thylakoid system (Vaughn et al., 1992; Renzaglia et al., 2009). Hornworts also have a unique type of placenta, with gametophytic transfer cells and sporophytic haustorial cells (Fig. 3; Ligrone et al., 1993). A seta is lacking and sporangial growth by means of a persistent basal meristem affords a more extended reproductive season than in mosses or liverworts. The consistent association with nitrogen-fixing cyanobacteria and the presence in most genera of a pyrenoid-based CO2-concentrating mechanism (Smith and Griffiths, 1996; Renzaglia et al., 2009) suggest that a dominant theme in the early evolution of hornworts was adaptation to nutrient-poor wet habitats.
The last common ancestor of present-day land plants
The model proposed by Mishler and Churchill (1985) was a liverwort-like dorsiventral thalloid gametophyte with unicellular rhizoids and a moss-like uniaxial sporophyte. The occurrence of a tetrahedral apical cell in the basal liverworts Haplomitrium and Treubia and the basal moss Takakia (Renzaglia, 1982; Shaw and Renzaglia, 2004) is more compatible with a radially symmetrical archetypal gametophyte. Although these three bryophytes have a similar apical cell, their phyllids are sharply different in anatomy and development, and hence they most probably evolved independently. We infer that the last common ancestor of present-day land plants had a leafless, axial gametophyte bearing unicellular rhizoids and mucilage papillae (Fig. 8A). Vascular tissue, if present, was a central strand of perforate cells as in Takakia and Haplomitrium. Gametophyte axes bore terminal sporophytes consisting of a foot, a short seta and a sporangium. As in modern liverworts, the seta probably elongated by cellular swelling after spore maturation to enable the mature sporangium to emerge from gametophytic involucres, and spore release was a one-time process. Meiosis was probably monoplastidic, a condition considered plesiomorphic for extant land plants (Renzaglia et al., 1993).
Fig. 8.
Reconstruction of the divergence of the liverwort (L), moss (M), hornwort (H) and polysporangiophyte (P) lineages from a common ancestor (A) represented as a dichotomously branched, leafless gametophyte with rhizoid-producing horizontal axes and erect photosynthetic axes bearing simple terminal sporophytes. Mosses, hornworts and polysporangiophytes (M, H and P) form the stomatophyte clade. Splitting of mature sporangia is shown in (A) and (H). The gametophyte generation has been omitted for the polysporangiophyte lineage (P). Black dots on sporophytes in M, H and P are stomata. Cladogram based on Qiu et al. (2006).
Liverworts
Diverging from the hypothetical ancestor described above, the liverworts originally retained a similar anatomy (Fig. 8L) but with adaptation to diverse habitats they evolved leafy and thalloid forms several times independently (Fig. 2A–C; Forrest et al., 2006; Crandall-Stotler et al., 2009; Shaw et al., 2011). Changes in growth form involved transitions to different apical cell geometries (Crandall-Stotler et al., 2009), with the tetrahedral cell plesiomorphic or atavistic. Liverwort radiation also involved repeated loss of ancestral fungal associations and evolution of novel fungal symbioses (Pressel et al., 2010). The liverworts retained the putative ancestral type of seta elongating by cellular expansion after spore maturation. We view elaters and oil bodies as apomorphies of liverworts, the latter possibly related to desiccation tolerance (Pressel et al., 2009). Pseudoelaters in hornworts are considered not to be homologous with liverwort elaters (Kenrick and Crane, 2007b). Further comparative analysis of the cytokinetic apparatus of land plants probably is needed to evaluate the evolutionary significance of polar bodies in liverworts (Brown and Lemmon, 2007, 2011b).
Stomatophytes
The stomatophyte lineage was marked by the appearance of stomata in the sporangial wall. Other likely apomorphies of stomatophytes include a moss-like seta developing from a basal meristem, lost in hornworts and replaced by the sporophyte shoot in polysporangiophytes, a columella, lost in most polysporangiophytes (Kenrick and Crane, 2007b) but retained in mosses and hornworts, a perine layer in mature spores (Renzaglia et al., 2007) and polar transport of auxin in the sporophyte (Poli et al., 2003; Fujita et al., 2008), both lost or modified in hornworts. Seta elongation preceding sporangial development permitted expression of mechanical and vascular tissues, thus affording the sporangium a more robust and durable support and possibly enhancing water and nutrient uptake from the parental gametophyte.
Mosses
Our model for the ancestral moss (Fig. 8M) is a leafless dichotomously branched gametophyte resembling that proposed by Kenrick (2000). The presence of a likely primitive tetrahedral apical cell and distinctive terete phyllids aligned in three rows in Takakia is in line with molecular analyses resolving this (plus Sphagnopsida) as the earliest divergent moss lineage (Fig. 1; Cox et al., 2010; Volkmar and Knoop, 2010; Chang and Graham, 2011). Following the divergence of Takakia, the evolution of mosses involved several major innovations in the anatomy and reproductive biology of the gametophyte, notably (1) an apical cell with a novel geometry of division, (2) unistratose phyllids and spiral phyllotaxis, and (3) the intercalation of a juvenile filamentous phase (the protonema) and production of multiple gametophores from each spore. Unicellular rhizoids, present in all other embryophytes, perhaps never existed in moss phylogeny and were ancestrally supplanted in function with leafless underground axes in Takakia (Grubb, 1970) and septate multicellular rhizoids in peristomate mosses. The lack of rhizoids in the mature gametophyte of the Sphagnopsida is probably a derived condition as the protonemal phase in Sphagnum includes rhizoid-like septate filaments (Goode et al., 1993). A vascularized seta developing before the sporangium is viewed as a plesiomorphy in mosses that was independently lost in the Sphagnopsida and in Andreaea among the Andreaeopsida. Evolution of a peristome, almost certainly polyphyletic (Shaw et al., 2011), provided a means to disperse spores for an extended length of time in response to moisture fluctuations.
The hornwort-polysporangiophyte lineage
The divergence of the hornwort-polysporangiophyte lineage probably involved a transition to a thalloid gametophyte (Fig. 8H, P). Further possible apomorphies included the pattern of sporeling growth, sunken archegonial venters and internal embryo establishment (Renzaglia et al., 2000), and xylan-containing secondary cell walls (Carafa et al., 2005). A thalloid gametophyte probably was an essential condition for the evolution of self-sustaining sporophytes in the polysporangiophyte lineage. Developing in a location adjacent to the substrate and not on an upright axis, the sporophyte could establish independence by producing a creeping rhizome or a positively geotropic axis (Fig. 9).
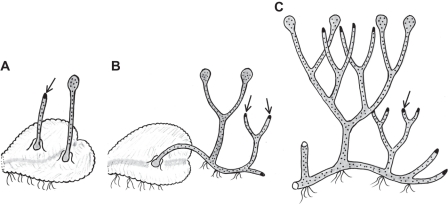
Fig. 9.
Hypothetical stages in the evolution of polysporangiophytes. (A) Unbranched sporophyte permanently attached to a thalloid gametophyte. (B) Branched sporophyte retaining attachment to the gametophyte but also producing rhizoid-bearing axes. (C) Profusely branched sporophyte attaining autonomy early in development. The gametophyte generation is omitted in C. Black dots on sporophyte axes and sporangia are stomata. Arrows point to apical meristem in sporophyte vegetative axes.
Hornworts
A major innovation of the hornwort gametophyte were mucilage-filled intercellular spaces colonized by Nostoc symbionts through epidermal clefts (Renzaglia et al., 2009). The lack of water-conducting cells (Fig. 10) probably is a reduction. Sporophyte innovations included a change in the orientation of zygote division from transverse to longitudinal, the evolution of a persistent basal meristem and the loss of the seta. The role of polar transport of auxin as a morphogenetic mechanism is attenuated in the hornwort sporophyte (Poli et al., 2003), possibly due to reduced axial polarization. Further details on hornwort evolutionary trends may be found in Renzaglia et al. (2009) and references therein.
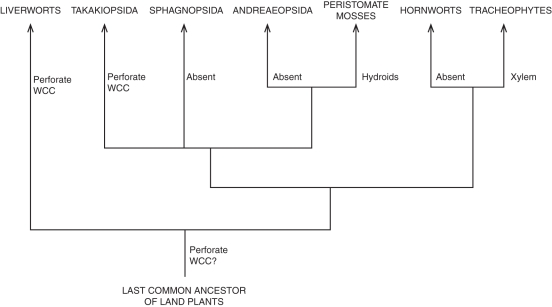
Fig. 10.
Distribution of water-conducting cells (WCC) in embryophytes. Cladogram based on Qiu et al. (2007) and Cox et al. (2010).
Polysporangiophytes
The key innovation marking the divergence of the polysporangiate lineage was the evolution of the SAM in the sporophyte. This introduced a reiterative indeterminate developmental pattern that transiently replaced the pre-existing determinate embryonic growth. Shifting to the reproductive mode, the SAM lost the self-perpetuating initial cell(s), thus reverting to a bryophyte-like embryonic growth, and sporangial development terminated axial growth (Fig. 8P). Although profoundly modified as a result of the elaboration of a diversity of complex forms, this developmental pattern is always recognizable in its essentials in present-day polysporangiophytes. With the evolution of the SAM, the ancestral seta was replaced with a vegetative body, the sporophyte shoot, that combined anatomical characters of the sporangium (the stomata and possibly photosynthetic tissue) and seta (vascular and mechanical tissue). Because of this and in disagreement with Kato and Akiyama (2005), we believe that homology of the sporophyte shoot with a part of the moss sporophyte, not necessarily the seta, cannot presently be ruled out.
The evolutionary transition from embryonic meristematic activity to a SAM with two alternative developmental programmes must have involved extensive changes in gene expression. Because the SAM was a new acquisition in polysporangiophytes, either novel regulatory genes evolved or pre-existing genes were co-opted from the gametophyte meristem, or both processes occurred in tandem (Shaw et al., 2011). A recent analysis (Szövényi et al., 2011) has shown that the proportion of generation-biased gene expression is lower in the moss Funaria (approx. 2·5 % of the total genome for both generations) than in Arabidopsis (approx. 5 and 25 % of the total genome for gametophyte and sporophyte, respectively). This suggests that extensive gene sharing between generations is the ancestral condition in land plants and that elaboration of the sporophyte and reduction of the gametophyte enhanced genetic divergence of the two generations during polysporangiophyte evolution. Evidence from genetic research suggests that the evolution of a persistent apical meristem in the sporophyte of polysporangiophytes and possibly also the delay in sporangial development involved duplication and re-programming of a diversity of genes whose ancestral forms are found in both basal and advanced land plant lineages and in several cases also in charophycean algae or even deeper in the eukaryote phyletic tree (Floyd and Bowman, 2007). Among these are class 1 Knox genes, which code for transcription factors essential for the function and maintenance of the indeterminate shoot apical meristem in angiosperms and perform similar functions in gymnosperms and ferns (Floyd and Bowman, 2007). Functional analysis in the moss Physcomitrella patens and the fern Ceratopteris richardii has revealed that class 1 KNOX orthologues (MKN genes) are essential for sporophyte development whereas they are not expressed in the gametophytes (Sano et al., 2005; Singer and Ashton, 2007; Sakakibara et al., 2008). Likely candidates for the evolution of the mechanism controlling the transition from vegetative to reproductive development are MIKC-type MADS box genes (Tanabe et al., 2005; Floyd and Bowman, 2007; Quodt et al., 2007; Zobell et al., 2010). Hortologues of MIKC genes appear to have a major role in gametangial development in charophycean algae, liverworts and mosses, whereas different members of the MIKC gene family in angiosperms control either the gametophyte or floral development. Possibly involved in the evolution of a SAM and delay of meiosis also are AML genes; these control vegetative meristem activity and meiosis in Arabidopsis (Kaur et al., 2006) and are related to the mei2 gene whose product is essential for meiosis in fungi and basal eukaryotes (Jeffares et al., 2004). Comparative functional dissection of development regulatory genes in bryophytes and ferns is likely to produce further insights on the evolution of the mechanisms underpinning sporophyte development in higher plants.
An obvious advantage of a vegetative body interposed between syngamy and sporogenesis was an amplification of the sporophyte photosynthetic potential and consequent lower dependence on the gametophyte and/or more copious spore production. It is possible, however, that the sporophyte vegetative body initially evolved as a replacement of the seta for elevating and supporting the sporangium, and photosynthetic competence was progressively strengthened as the sporophyte sizes increased. The latter option is consistent with the hypothesis by Boyce (2008) of cooksonioid axes as branched sporophytes still lacking photosynthetic tissue and dependent on the gametophyte.
We view the ancestor of polysporangiophytes as a bryophyte-like plant with a thalloid gametophyte and uniaxial sporophyte (Fig. 9A). This points to the SAM and the SAM-derived vegetative body as defining characters of polysporangiophytes preceding sporophyte branching (Fig. 9B). Sporophyte branching might have evolved by deployment of a pre-existing gametophytic mechanism. However, because the functioning of the apical meristem appears to be controlled by different gene sets in the gametophyte and sporophyte (Sano et al., 2005; Singer and Ashton, 2007; Sakakibara et al., 2008), it is likely that an iterative branching mechanism evolved de novo in the sporophyte. Subsequent evolution of anisotomous branching gave way to elaboration of lateral sporangial clusters from which all locations of sporangia in extant tracheophytes may be derived. The increasing structural complexity of the sporophyte involved a positive feedback on auxin-mediated morphogenesis, thus promoting the evolution of the sophisticated mechanism present in modern tracheophytes (Kieffer et al., 2010). The diagrammatic reconstruction of polysporangiophyte evolution presented in Fig. 9 assumes that sporophyte branching and development of rhizoids were strictly inter-linked and preceded complete autonomy. An analysis of the evolution of sporophyte branching in early land plants has been produced by Rothwell (1995).
The sporophyte meristems
Mishler and Churchill (1985) suggested an independent origin of the basal meristems in mosses and hornworts. The more parsimonious hypothesis of homology implies that a basal meristem present in the stomatophyte ancestor was retained in the moss and hornwort lineages. The distinctive growth pattern of the hornwort sporophyte might have originated from an ‘invasion’ of the sporangium by the basal meristem. The archesporium-generating capsular region was incorporated into the basal meristem, resulting in the continuous upward production of embryonic endothecial and amphithecial cells and the loss of the seta. A fundamental difference between the hornwort basal meristem and SAM is that the first produces only sporangial tissue whereas the latter generates a vegetative body interpolated between the zygote and sporogenesis. Whether the basal meristems in mosses and hornworts and the SAM in polysporangiophytes have a common origin (hence they share a genetic signature) or evolved independently is an issue open to investigation.
Looking for bryophyte fossils
The evolutionary transitions described above presumably spanned the long hiatus between Ordovician sporopollenin-coated meiospores (Wellman et al., 2003) and the Late Silurian first land plant macrofossils (Edwards and Feehan, 1980). The leafless vascularized plants proposed herein as the putative ancestors to all land plants (Fig. 8A), liverworts (Fig. 8L) and mosses (Fig. 8M) could produce fossils closely similar, in the absence of obvious gametangia, to some of the fossils referred to as the cooksonioid assemblage (Gerrienne et al., 2002; Taylor et al., 2009), with structures interpreted as sporangia actually being gametangiophores or whole sporophytes. This might provide a novel perspective in assessing the bryophyte affinity of Silurian macrofossils (Edwards et al., 1998b; Edwards, 2000).
TOWARDS SPOROPHYTE AUTONOMY: STOMATA, RHIZOIDS AND FUNGAL ASSOCIATIONS
Stomata
We have seen that in mosses and hornworts stomata are found in the sporophyte but are lacking in the gametophyte. As both generations are poikilohydric, water relationships cannot account for such a major difference. Indeed, homoiohydry would be a senseless choice for a sporophyte permanently dependent on a poikilohydric gametophyte for water uptake. Anatomical constraints do not appear to be important either: the stem (but not the phyllids) of the mature gametophyte in mosses, the thalloid gametophyte in hornworts and the putative axial gametophyte of their common ancestor could provide the three-dimensional organization necessary for stomatal development and functioning. We infer that stomata evolved in the ancestral stomatophyte in response to a selection pressure that operated on the sporophyte but not the gametophyte.
The bryophyte sporophyte needs to divert water and nutrients from the parental gametophyte. Transfer cells at the sporophyte/gametophyte interface actively pump solutes from the gametophyte, with passive flow of water following ion transport (Ligrone et al., 1993). Stomatal transpiration might be expected to decrease the water potential of the sporangial tissue (Raven, 2002), thereby enhancing water and solute uptake from the gametophyte; conversely, stomatal closure under water shortage would prevent the sporophyte from dehydrating earlier than the gametophyte. This is the same as saying that stomata may confer the sporophyte of mosses and hornworts a degree of homoiohydry relative to the gametophytic counterpart. Unfortunately, as far as we are aware, no data are currently available on sporophyte/gametophyte water relationships in bryophytes to corroborate or reject this hypothesis. Interestingly, in mosses and hornworts the sporangium usually becomes free of gametophytic protective involucres early in development, thus permitting transpiration, whereas the astomatous sporangia of liverworts are enclosed within the gametophyte until spore maturation. Physiological benefits from stomata in mosses and hornworts certainly are affected by ecological and anatomical factors (Raven, 2002) and apparently in several circumstances they have not been significantly restrictive in evolutionary terms, thus allowing for repeated loss.
In line with considerations by Vaizey (1887), Ligrone and Gambardella (1988) and Edwards et al. (1998a), we suggest that a primordial function of stomata in the sporangium of the archetypal stomatophyte was the production of a transpiration-driven gradient of water potential that enhanced water and solute uptake from the parental gametophyte. This mechanism would be particularly effective if associated with vascular tissue (Raven, 1993), which is consistent with the hypothesis that the seta of the ancestral stomatophyte was vascularized. A second function of ancestral stomata, not at odds with the former notion, might be facilitating sporangial desiccation prior to spore discharge (Duckett et al., 2009). By permitting air to penetrate the inner tissue of the capsule, the occurrence of openings in the capsule epidermis would facilitate separation of maturing spores. The mechanism underlying localized cell-wall splitting and air-space development, knowledge of which is still in its infancy (Roland, 1978; Jeffree et al., 1984; Raven, 1996), probably evolved in parallel with stomata at the dawn of stomatophyte divergence. Control of CO2 balance, a second major function of modern stomata (Brodribb et al., 2009), probably was a later addition associated with the evolution of a photosynthetic tissue in the sporangium of early stomatophytes.
Originally a sporangial specialization, the stomata were later also expressed in the sporophyte vegetative body in polysporangiophytes and became one of its most distinctive features. Figure 9 assumes that the evolution of stomata in the vegetative body preceded sporophyte branching, but the opposite is also possible. A further critical innovation paving the way to homoiohydry was minimization of non-stomatal transpiration by a cuticle with low permeability to water, which presumably appeared in the polysporangiophyte lineage during the transition from a gametophyte-dependent to an independent sporophyte (Fig. 9). The combination of an indeterminate vegetative body, nutritional autonomy and homeohydry gave the polysporangiophyte sporophyte access to an entirely new adaptive zone, thus triggering the Late Silurian/Early Devonian radiation (Bateman et al., 1998; Gensel, 2008).
If stomata were originally a sporophytic character, as their distribution in present-day stomatophytes suggests, their occurrence in gametophytes of extinct Devonian plants should be regarded as a derived condition, possibly an evolutionary attempt of the gametophytic generation to follow the sporophyte towards homoiohydry. In contrast, the evolution of present-day polysporangiate lineages generally involved a reduction of the gametophyte. Relative to ancestral forms, modern polysporangiophytes apparently also underwent a structural simplification of the sporangia, including the almost universal loss of stomata, that instead were present in the sporangia of Early Devonian polysporangiophytes such as Aglaophyton major, Nothia aphylla and Cooksonia pertoni (Edwards et al., 1998a)
Rhizoids
In the bryophyte grade, rhizoid formation is restricted to the gametophyte. The demonstration that rhizoid development in the Arabidopsis sporophyte and Physcomitrella gametophyte is under the control of homologous genes suggests again that rhizoids evolved in the sporophyte by deployment of gametophytic genes (Menand et al., 2007). As discussed above, the available palaeontological evidence does not permit us to establish whether the ability to produce rhizoids pre-dated sporophyte branching or appeared afterwards. A possible transitional stage was the development of a mycorrhizal protocorm as in certain living lycopods (Gifford and Foster, 1989; Duckett and Ligrone, 1992). It has been suggested that the elongate haustorial cells in the sporophyte foot of hornworts (Fig. 3) are evolutionary precursors to sporophyte rhizoids (Campbell, 1924). Developmental affinity of these cell types can now be tested experimentally by investigating the expression of AtRHD6 orthologues (Menand et al., 2007) in hornworts.
Fungal associations
With the appearance of rhizoids and the establishment of direct interactions with the substrate, the sporophyte developed mutualistic associations with fungi that enhanced the ability to absorb mineral nutrients. As for other characteristics, this probably involved deployment of a condition already present in the gametophyte. In fact, symbiotic associations with fungi are common in the gametophytes (but absent in the sporophytes) in liverworts and hornworts and are found in both generations in basal tracheophytes (Read et al., 2000; Pressel et al., 2010). Recent research has revealed that the DMI1, DMI3 and IPD3 genes, known to be required for mycorrhiza formation in angiosperms, also occur in liverworts, mosses and hornworts, and hence were probably present in the last common ancestor of land plants (Wang et al., 2010). Moreover, the DMI3 genes from the liverworts Haplomitrium and Treubia and the hornwort Phaeoceros recovered the mycorrhizal phenotype in a transformed mutant of the angiosperm Medicago truncatula (Wang et al., 2010). Fungal endophytes in Haplomitrium and Treubia, the earliest extant liverwort lineages, and in the early-divergent simple thalloid liverwort Allisonia and the similarly early-divergent complex thalloid Neohodgsonia have recently been identified as members of the Endogonaceae, an earlier fungal lineage than the Glomeromycota, previously considered to be the earliest mycorrhiza-forming fungi (Bidartondo et al., 2011). These endogonaceous fungal endophytes form inter- and intracellular structures (Carafa et al., 2003; Duckett et al., 2006) closely resembling those described in Early Devonian fossils, namely the gametophyte and sporophyte of the protracheophyte Aglaophyton (Remy et al., 1994; Taylor et al., 2005) and the sporophyte of Nothia aphylla (Krings et al., 2007). Fungal endophytes in the gametophyte of later-divergent liverworts and in hornworts have been identified as Glomeromycota (Ligrone et al., 2007; Bidartondo et al., 2011; M. I. Bidartondo et al., Department of Biology, Imperial College London, unpubl. res.). We infer that symbiotic relationships with endogonaceous and/or glomeromycotean fungi were present in the gametophyte of the archetypal ancestor of land plants (Fig. 6A), were vertically inherited by the gametophytes of liverworts, hornworts and polysporangiophytes, and in the last-named they were expressed also in the sporophyte when this became autonomous, thus originating the fundamental symbiosis known as the arbuscular mycorrhizas (Smith and Read, 2008). It is noteworthy in this context that the fungal associations in the gametophytes and sporophytes of Psilotum and Tmesipteris are virtually identical (Duckett and Ligrone, 2005). The absence of mutualistic associations with fungi in mosses and in a number of liverwort clades is interpreted as a result of loss (Renzaglia et al., 2007; Pressel et al., 2010), possibly linked to adaptation to saxicolous and epiphytic habitats incompatible with fungal symbiosis and/or an increased efficiency in direct uptake of mineral nutrients.
VASCULAR TISSUES
Water-conducting cells (WCCs) and lignin
The plesiomorphic type of WCCs in embryophytes are cells with plasmodesma-derived perforations; these have been reported in the gametophyte of both early- (Haplomitrium) and later-divergent (Pallaviciniales, Metzgeriidae) liverwort clades and in both generations in the early-divergent moss Takakia (Renzaglia et al., 1997; Ligrone et al., 2000). Internal WCCs are absent in the Sphagnopsida and Andreaeopsida, whereas peristomate mosses typically have imperforate WCCs referred to as hydroids. The developmental pattern of hydroids involve obliteration of plasmodesmata during final differentiation (Ligrone et al., 2000). The cell walls of hydroids in the leafy shoot of polytrichopsid mosses consist of thin areas with a ‘hydrolysed’ appearance scattered among thickened areas; in contrast, the hydroids in bryopsid mosses have uniform thin cell walls (Ligrone et al., 2000). Immunocytochemical evidence suggests that the thickened cell wall areas in the hydroids of polytrichopsid mosses are not secondary cell walls (Ligrone et al., 2002; Carafa et al., 2005).
The occurrence of lignin in the bryophyte grade is a controversial issue. Early studies based on oxidative degradation and 13C nuclear magnetic resonance spectroscopy have concluded that no compound referable to as lignin was present in mosses or liverworts (reviewed by Ligrone et al., 2008). In contrast, using extraction by thioacidolysis, a recent study (Espiñeira et al., 2011) detected lignin in the liverwort Marchantia but not in Physcomitrella. This is consistent with the absence in Physcomitrella of CYP84, an enzyme needed for the synthesis of S-lignol (Rensing et al., 2008). An immunocytochemical analysis demonstrated the occurrence of lignin-related epitopes in bryophytes; these epitopes did not have a tissue-specific localization in bryophytes, except an apparent higher frequency in cell-wall thickenings in hornwort pseudoeleaters, whereas in tracheophytes they were specifically localized in secondary cell walls, essentially in tracheary elements and sclerenchyma cells (Ligrone et al., 2008). The overall picture suggests that the cell walls of bryophytes contain lignin-related polyphenolic compounds that possibly confer protection against UV irradiation and attack by micro-organisms but, unlike lignins, are not involved in mechanical strengthening.
Xylem vascular cells in tracheophytes have a continuous imperforate primary cell wall and a secondary cell wall interrupted by pits of varying size, morphology and arrangement; unlike perforations in bryophyte vascular cells, pit development in xylem has no direct relationship with plasmodesmata (Barnett, 1982). Dissolution of transverse walls in vessel elements generates conduits up to several metres long, but pits along lateral walls are always closed by a pit membrane derived from the primary cell wall (Evert, 2006). Because of their basically imperforate anatomy, xylem vascular cells recall moss hydroids. The notion that the two cell types are homologous, formally introduced by Mishler et al. (1985) along with the assumption of homology of the sporophyte shoot in tracheophytes with the moss seta, is now widespread in the botanical community. Obstacles to the hypothesis of homology of the two types of vascular cells arise not only from differences in morphology and cytochemistry, notably the lack of lignified secondary cell walls and xylan-associated epitopes in hydroids (Carafa et al., 2005), but also from taxonomic distribution (Fig. 10). Hydroids are an apomorphy of peristomate mosses, i.e. the most advanced moss lineage; the Sphagnopsida and Andreaeopsida lack internal WCCs, whereas Takakia has perforate WCCs distinctly different from both hydroids and xylem vascular cells in general morphology and development (Ligrone et al., 2000).
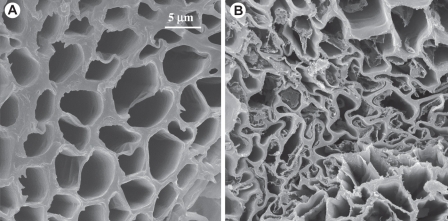
Owing to the lack of a rigid lignified cell wall, the hydroids are able to collapse during water stress, thus avoiding cavitation, but they promptly refill with water and expand upon rehydration (Fig. 11). Arguably, this property makes hydroids a highly effective type of vascular cell in desiccation-tolerant plants. We have no information on the behaviour of perforate WCCs under water-stress, but it might be expected that because of the presence of pervious pores these cells are more prone than hydroids to loss of function by cavitation, particularly so for the WCCs with thickened cell walls present in the Pallaviciniales (Ligrone and Duckett, 1996a). It is noteworthy that Takakia and the few liverwort species having this type of vascular cells are confined to wet habitats and their aerial vegetative body does not survive desiccation (Grubb, 1970). Considering that the basalmost moss clade with hydroids is the Polytrichopsida (Cox et al., 2010; Ligrone and Duckett, 2011), which typically consists of large-size mosses, we suggest that hydroids evolved in mosses as a solution combining desiccation tolerance with relatively large body sizes (Proctor, 2007). Hydroids might have arisen either by modification of pre-existing perforate WCCs or, more likely, independently after the loss of perforate WCCs (Fig. 9). The absence of hydroids in several advanced lineages of peristomate mosses, e.g. the Orthotrichaceae and Hookeriales (Hébant, 1977), is most likely a reduction. An alternative system to hydroids is the hyalocysts, water-storing dead cells that evolved in the Sphagnopsida with adaptation to bog habitats (Lewis, 1988).
Fig. 11.
Transverse sections of the leafy shoot in the moss Polytrichastrum formosum showing reversible collapse and expansion of hydroids: (A) hydrated state, (B) dehydrated state.
From the arguments presented above, we infer that WCCs in the last common ancestor of land plants, if present, were of the Haplomitrium-like perforate type and were restricted to the gametophyte. A vascular system of perforate cells probably was present in both the gametophyte and the sporophyte of the ancestral stomatophyte, much as in present-day Takakia. The anatomy of vascular cells with uniform longitudinal walls in Early Devonian polysporangiophytes (Edwards et al., 2003) is compatible with that of Takakia-like perforate WCCs. Conversely, the microporate cell-wall layers described in vascular cells in early-divergent tracheophytes (Kenrick and Crane, 1991; Edwards and Axe, 2000; Edwards et al., 2003) have no likely counterpart in perforate WCCs or any other type of WCCs in living plants. Xylem evolved in the sporophyte of polysporangiophytes when the transition to homoiohydry and larger body sizes required the walls of vascular cells to be reinforced against compression forces and sealed against the increased risk of cavitation due to internal negative pressure (Niklas, 2000). WCCs are absent in complex thalloid and most simple thalloid liverworts. Considering the derived status assigned to the Pallaviciniales in liverwort phylogeny (Heinrichs et al., 2005; Forrest et al., 2006), we view the perforate WCCs in this clade as an instance of independent evolution.
It is probably appropriate to remark here that the basal meristem in the sporophyte of mosses is transient and therefore cell differentiation is able to produce a continuous strand of vascular cells connecting the foot to the sporangium; the same probably was true for the stomatophyte ancestor. In contrast, the persistent basal meristem in the hornwort sporophyte is likely to act as a bottleneck by permitting water and solutes from the foot to move upwards only by diffusion, a process that is several orders of magnitude slower than mass flow through vascular tissue. Hence, vascular tissue above the basal meristem would be useless. This might explain why the hornwort sporophyte never evolved vascular tissue in spite of its relatively large sizes and seemingly appropriate anatomy. The anatomical conservativeness of the hornwort sporophyte relative to its equivalent in mosses and polysporangiophytes (Renzaglia et al., 2009) may be, at least in part, a consequence of constrictions inherent to its distinctive growth pattern.
Food-conducting cells (FCCs)
The highly fragmentary fossil record of phloem-like cells renders analysis of FCC evolution far more problematic than for WCCs. Ultrastructural studies have revealed that FCCs characterized by a distinctive cytological organization are widespread in bryophytes (Ligrone and Duckett, 1994a, b, 1996b, 1998; Ligrone et al., 2000; Edwards et al., 2003; Pressel et al., 2008). Their key attributes include specialized plasmodesmata in the end walls, alignment of plastids, mitochondria and endoplasmic reticulum-derived vesicles along longitudinal arrays of endoplasmic microtubules, breakdown of the tonoplast, mixing of the vacuolar and cytoplasmic contents, and, in some instances (e.g. polytrichalean mosses), nuclear breakdown. Apart from the endoplasmic microtubules, these are all features common to sieve elements. This highly distinctive bryophytic FCC cytology has been found in complex liverwort thalli (Ligrone and Duckett, 1994a) and perhaps more significantly in the stems of Haplomitrium (Edwards et al., 2003) at the base of the tree of extant land plants, but not in simple thalloid or leafy liverworts. FCC cytology is also absent in hornworts but ubiquitous in both generations in peristomate mosses (Ligrone and Duckett, 1994b, 1996b), including rhizoids and caulonematal filaments (Pressel et al., 2008). It also characterizes the central cells in Sphagnum stems (Ligrone and Duckett, 1994a) but was not found in Andreaea and its occurrence in Takakia has yet to be explored. Overall this distribution strongly suggests that FCCs were an ancestral feature of land plants and functionally just as important as WCCs. Absences are almost certainly secondary losses. As for other characters considered in this analysis, identification of sieve-element-specific genes and a search for these in bryophytes is likely to provide useful information for assessing evolutionary relationships of FCCs in land plants.
CONCLUSIONS
Performed in the framework of molecular phylogeny, the present analysis is an attempt to explore some of the most recalcitrant issues in land plant evolution and to reconstruct the sequence of events that shaped the major present-day lineages of land plants. The main conclusions of this work are summarized below.
Sporophyte elaboration in land plants involved extensive deployment of gametophytic structures and mechanisms, as well as a number of major innovations.
The last common ancestor of land plants probably was a leafless axial gametophyte bearing morphologically simple unisporangiate sporophytes. Water-conducting vascular tissue, if present, was restricted to the gametophyte and consisted of perforate cells as in Haplomitrium and Takakia.
Stomata in mosses, hornworts and polysporangiophytes probably are homologous; the monophyletic lineage encompassing these three groups is therefore referred to as the ‘stomatophytes’.
Stomata are a sporophyte innovation, possibly with the ancestral functions of producing a controlled transpiration-driven flow of water and solutes from the parental gametophyte and facilitating the separation of maturing spores before release.
The evolution of stomata/air spaces and sporophyte vascularization, the latter probably by deployment of vascular tissue from the gametophyte, were pivotal to the divergence of the stomatophyte lineage.
Determinate sporophyte development based on embryonic meristematic activity is the ancestral condition in land plants, still present in modern liverworts and mosses.
An indeterminate sporophyte body (the sporophyte shoot) developing from an apical meristem (SAM) is the fundamental innovation of polysporangiophytes. Homology of the SAM with the basal meristem in mosses and hornworts is a possibility open to investigation.
Poikilohydry is the ancestral condition in land plants; homeohydry evolved in the sporophyte of polysporangiophytes.
Symbiotic associations with fungi first evolved in the gametophyte generation before the separation of major lineages and were acquired by the sporophyte when this developed rhizoids and established a direct contact with the substrate.
Hydroids are an imperforate type of WCC evolved in advanced (peristomate) mosses; hydroids are not homologous to xylem vascular cells.
Xylem vascular cells evolved in the sporophyte of polysporangiophytes, either from pre-existing perforate vascular cells or de novo, in parallel with the establishment of homoiohydry.
Food-conducting cells first evolved in the gametophyte generation at the dawn of land plant evolution.
Some of the above interpretations and inferences may turn out to be incorrect, but the main scope of the present analysis is to raise the attention of prospective investigators on numerous previously unexplored congruences (and some incongruences) in palaeontological, morphological, developmental, genetic and phylogenetic data. A notion that we feel needs to be emphasized is that fundamental characters of land plants primarily appeared in the bryophyte grade then to be passed on to polysporangiophytes; hence, living bryophytes hold the key to a better understanding of the early evolution of land plants.
ACKNOWLEDGEMENTS
This work was supported by grant ‘Orto Botanico’ from Provincia di Caserta (Italy). We thank Dr Marco Vigliotti (Dipartimento di Scienze ambientali, SUN, Italy) for assistance in the preparation of the figures.
LITERATURE CITED
- Albert VA. Shoot apical meristems and floral patterning: an evolutionary perspective. Trends in Plant Science. 1999;4:84–86. [Google Scholar]
- Alpert P. The limits and frontiers of desiccation-tolerant life. Integrative and Comparative Biology. 2005;45:685–695. doi: 10.1093/icb/45.5.685. [DOI] [PubMed] [Google Scholar]
- Asakawa Y. Chemical constituents of the Bryophytes. In: Herz W, Kirby GW, Moore RE, Steglich W, editors. Progress in the chemistry of organic natural products. Vol. 65. Vienna: Springer; 1995. pp. 1–562. [DOI] [PubMed] [Google Scholar]
- Bargel H, Koch K, Cerman Z, Neinhuis C. Structure-function relationships of the plant cuticle and cuticular waxes – a smart material? Functional Plant Biology. 2006;33:893–910. doi: 10.1071/FP06139. [DOI] [PubMed] [Google Scholar]
- Barnett JR. Plasmodesmata and pit development in secondary xylem elements. Planta. 1982;155:251–260. doi: 10.1007/BF00392724. [DOI] [PubMed] [Google Scholar]
- Bateman RM, Crane PR, DiMichele WA, et al. Early evolution of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation. Annual Review of Ecology and Systematics. 1998;29:263–292. [Google Scholar]
- Beerling DJ, Franks PJ. Evolution of stomatal function in ‘lower’ land plants. New Phytologist. 2009;183:921–925. doi: 10.1111/j.1469-8137.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Read DJ, Trappe JM, Merckx , Ligrone R, Duckett JG. The dawn of symbiosis between plants and fungi. Biology Letters. 2011;7:574–577. doi: 10.1098/rsbl.2010.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger G, Maddaloni M, Motto M, Salamini F. Formation and cell lineage patterns of the shoot apex of maize. Plant Journal. 1992;2:311–320. [Google Scholar]
- Boyce CK. How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology. 2008;34:179–194. [Google Scholar]
- Bower FO. On anthithetic as distinct from homologous alternation of generations in plants. Annals of Botany. 1890;4:347–370. [Google Scholar]
- Brandes H. Gametophyte development in ferns and bryophytes. Annual Review of Plant Physiology. 1973;24:115–128. [Google Scholar]
- Brodribb TJ, McAdam SAM. Passive origins of stomatal control in vascular plants. Science. 2011;331:582–585. doi: 10.1126/science.1197985. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Field TS. Evolution of stomata responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytologist. 2009;183:839–847. doi: 10.1111/j.1469-8137.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Sporogenesis in bryophytes. In: Blackmore S, Knox RB, editors. Microspores: evolution and ontogeny. London: Academic Press; 1990. pp. 55–94. [Google Scholar]
- Brown RC, Lemmon BE. The pleomorphic plant MTOC: an evolutionary perspective. Journal of Integrative Plant Biology. 2007;49:1142–1153. [Google Scholar]
- Brown RC, Lemmon BE. Spores before sporophytes: hypothesizing the origin of sporogenesis at the algal-plant transition. New Phytologist. 2011a;90:875–881. doi: 10.1111/j.1469-8137.2011.03709.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Dividing without centrioles: innovative MTOCs organize mitotic spindles in bryophytes, the earliest extant lineages of land plants. AoB PLANTS. 2011b;2011 doi: 10.1093/aobpla/plr028. http://dx.doi.org/doi:10.1093/aobpla/plr028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. A hundred-year-old question: is the moss calyptra covered by a cuticle? A case study of Funaria hygrometrica. Annals of Botany. 2011;107:1279–1286. doi: 10.1093/aob/mcr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DH. A remarkable development of the sporophyte in Anthoceros fusiformis. Annals of Botany. 1924;38:473–483. doi: 10.1126/science.58.1503.307. [DOI] [PubMed] [Google Scholar]
- Carafa A, Duckett JG, Ligrone R. Subterranean gametophytic axes in the primitive liverwort Haplomitrium harbour an unique type of endophytic association with aseptate fungi. New Phytologist. 2003;160:185–197. doi: 10.1046/j.1469-8137.2003.00849.x. [DOI] [PubMed] [Google Scholar]
- Carafa A, Duckett JG, Knox JP, Ligrone R. Distribution of cell-wall xylans in bryophytes and tracheophytes: new insights into basal relationships of land plants. New Phytologist. 2005;168:231–240. doi: 10.1111/j.1469-8137.2005.01483.x. [DOI] [PubMed] [Google Scholar]
- Chang Y, Graham SW. Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set. American Journal of Botany. 2011;98:839–849. doi: 10.3732/ajb.0900384. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. Regulatory mechanisms controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology. 2011;21:1025–1029. doi: 10.1016/j.cub.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Cook ME, Graham LE. Structural similarities between surface layers of selected charophycean algae and bryophytes and the cuticles of vascular plants. International Journal of Plant Science. 1998;159:780–787. [Google Scholar]
- Cox CJ, Goffinet B, Wickett NJ, Boles SB, Shaw AJ. Moss diversity: a molecular phylogenetic analysis of genera. Phytotaxa. 2010;9:175–195. [Google Scholar]
- Crandall-Stotler B. Musci, hepatics and anthocerotes – an essay on analogues. In: Schuster RM, editor. New manual of bryology. Vol. 2. Nichinan: Hattori Botanical Laboratory; 1984. pp. 1093–1129. [Google Scholar]
- Crandall-Stotler B, Stotler RE, Long DG. Morphology and classification of the Marchantiophyta. In: Goffinet B, Shaw J, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2009. pp. 1–54. [Google Scholar]
- Duckett JG, Ligrone R. A light and electron microscope study of the fungal endophytes in the sporophyte and gametophyte of Lycopodium cernuum with observations on the gametophyte-sporophyte junction. Canadian Journal of Botany. 1992;70:58–72. [Google Scholar]
- Duckett JG, Ligrone R. The formation of catenate foliar gemmae and the origin of oil bodies in the liverwort Odontoschisma denudatum (Mart.) Dum. (Jungermanniales): a light and electron microscope study. Annals of Botany. 1995;76:405–419. [Google Scholar]
- Duckett JG, Ligrone R. A comparative cytological analysis of fungal endophytes in the sporophyte rhizomes and vascularized gametophytes of Tmesipteris and Psilotum. Canadian Journal of Botany. 2005;83:1443–1456. [Google Scholar]
- Duckett JG, Carafa A, Ligrone R. A highly differentiated glomeromycotean association with the mucilage-secreting, primitive antipodean liverwort Treubia (Treubiaceae): clues to the origins of mycorrhizas. American Journal of Botany. 2006;93:797–813. doi: 10.3732/ajb.93.6.797. [DOI] [PubMed] [Google Scholar]
- Duckett JG, Pressel S, P'ng KMY, Renzaglia KS. Exploding a mith: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytologist. 2009;183:1053–1063. doi: 10.1111/j.1469-8137.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- Duckett JG, Pressel S, P'ng KMY, Renzaglia KS. The function and evolution of stomata in bryophytes. Field Bryology. 2010;101:38–40. [Google Scholar]
- Edwards D. Cells and tissues in the vegetative sporophyte of early land plants. New Phytologist. 1993;125:225–247. doi: 10.1111/j.1469-8137.1993.tb03879.x. [DOI] [PubMed] [Google Scholar]
- Edwards D. The role of Mid-Palaeozoic mesofossils in the detection of early bryophytes. Philosophical Transactions of the Royal Society London. 2000;B 355:733–755. doi: 10.1098/rstb.2000.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Axe L. Novel conducting tissues in Lower Devonian plants. Botanical Journal of the Linnean Society. 2000;134:383–399. [Google Scholar]
- Edwards D, Feehan J. Records of Cooksonia-type sporangia from late Wenlock strata in Ireland. Nature. 1980;287:41–42. [Google Scholar]
- Edwards D, Davies KL, Axe L. A vascular conducting strand in the early land plant Cooksonia. Nature. 1992;357:683–685. [Google Scholar]
- Edwards D, Abbot GD, Raven JA. Cuticles of early land plants: a palaeoecophysiological evaluation. In: Kersteins G, editor. Plant cuticles: an integrated functional approach. Oxford: Bios; 1996. pp. 1–31. [Google Scholar]
- Edwards D, Kerp H, Hass H. Stomata in early land plants: an anatomical and ecophysiological approach. Journal of Experimental Botany. 1998a;49:255–278. [Google Scholar]
- Edwards D, Wellman CH, Axe L. The fossil record of early land plants and interrelationships between primitive embryophytes: too little and too late? In. In: Bates JW, Ashton NW, Duckett JG, editors. Bryology for the twenty-first century. Leeds: Maney and British Bryological Society; 1998b. pp. 15–43. [Google Scholar]
- Edwards D, Axe L, Duckett JG. Diversity in conducting cells in early land plants and comparisons with extant bryophytes. Botanical Journal of the Linnean Society. 2003;141:297–347. [Google Scholar]
- Espiñeira J, Novo Uzel E, Gòmez Ros LV, et al. Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biology. 2011;13:59–68. doi: 10.1111/j.1438-8677.2010.00345.x. [DOI] [PubMed] [Google Scholar]
- Evert RF. Esau's plant anatomy. Hoboken: Wiley; 2006. [Google Scholar]
- Finet C, Timme RE, Delwiche CF, Marlétaz F. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Current Biology. 2010;20:2217–2222. doi: 10.1016/j.cub.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Floyd S, Bowman JL. The ancestral developmental tool kit of land plants. International Journal of Plant Sciences. 2007;168:1–35. [Google Scholar]
- Farrant JM, Moore JP. Programming desiccation tolerance: from plants to seeds to resurrection plants. Current Opinion in Plant Biology. 2011;14 doi: 10.1016/j.pbi.2011.03.018. 349–345. [DOI] [PubMed] [Google Scholar]
- Forrest LL, Davis EC, Long DG, Crandall-Stotler BJ, Clark A, Hollingsworth ML. Unraveling the evolutionary history of the liverworts (Marchantiophyta): multiple taxa, genomes and analyses. Bryologist. 2006;109:303–334. [Google Scholar]
- Fujita T, Sakaguchi H, Hiwatashi Y, et al. Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evolution and Development. 2008;10:176–186. doi: 10.1111/j.1525-142X.2008.00225.x. [DOI] [PubMed] [Google Scholar]
- Furner IJ, Pumfrey JE. Cell fate in the shoot apical meristem of Arabidopsis thaliana. Development. 1992;115:755–764. [Google Scholar]
- Garner DLB, Paolillo DJ. On the functioning of stomata in Funaria. Bryologist. 1973;76:423–427. [Google Scholar]
- Gensel PG. The earliest land plants. Annual Review of Ecology, Evolution and Systematics. 2008;39:459–477. [Google Scholar]
- Gerrienne LE, Gonez P. Early evolution of life cycles in embryophytes: a focus on the fossil evidence of gametophyte/sporophyte size and morphological complexity. Journal of Systematics and Evolution. 2011;49:1–16. [Google Scholar]
- Gerrienne P, Bergamaschi S, Pereira E, Rodrigues MAC, Steemans P. An Early Devonian flora, including Cooksonia, from the Paranà Basin (Brasil) Review of Palaeobotany and Palinology. 2001;116:19–38. [Google Scholar]
- Gerrienne P, Dilcher DL, Bergamaschi S, Milagres I, Pereira E, Rodrigues MAC. An exceptional specimen of the early land plant Cooksonia paranensis, and a hypothesis on the life cycle of the earliest eutracheophytes. Review of Palaeobotany and Palynology. 2006;142:123–130. [Google Scholar]
- Gifford EM, Foster AS. Morphology and evolution of vascular plants. New York: W.H. Freeman and Co; 1989. [Google Scholar]
- Goffinet B, Buck W, Shaw J. Morphology, anatomy and classification of the Bryophyta. In: Goffinet B, Shaw J, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2009. pp. 55–138. [Google Scholar]
- Goode JA, Stead AD, Duckett JG. Experimental studies of protonemal morphogenesis in Sphagnum: the role of growth regulators and the cytoskeleton. Advances in Bryology. 1993;5:129–151. [Google Scholar]
- Graham LE, Wilcox LW. The origin of alternation of generations in land plants: a focus on matrotrophy and hexose transport. Transactions of the Royal Society London. 2000;B 355:757–767. doi: 10.1098/rstb.2000.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb PJ. Observations on the structure and biology of Haplomitrium and Takakia, hepatics with roots. New Phytologist. 1970;69:303–326. [Google Scholar]
- Harrison CJ, Rezvani M, Langdale JA. Growth from two transient apical initials in the meristem of Selaginella kraussiana. Development. 2007;134:881–889. doi: 10.1242/dev.001008. [DOI] [PubMed] [Google Scholar]
- Hartung W, Weiler EW, Volk OH. Immunochemical evidence that abscisic acid is produced by several species of Anthocerotae and Marchantiales. Bryologist. 1987;90:393–400. [Google Scholar]
- Hébant C. The conducting tissues of bryophytes. Lehre: J. Cramer; 1977. [Google Scholar]
- Heinrichs J, Gradstein SR, Wilson R, Schneider H. Towards a natural classification of liverworts (Marchantiophyta) based on the chloroplast gene rbcL. Cryptogamie, Bryologie. 2005;26:131–150. [Google Scholar]
- Hemsley AR. The origin of the land plant sporophyte: an interpolation scenario. Biological Reviews. 1994;69:263–273. [Google Scholar]
- Hernick LV, Landing E, Bartowski KE. Earth's oldest liverworts – Metzgeriothallus sharonae sp. nov from the Middle Devonian (Givetian) of eastern New York, USA. Review of Palaeobotany and Palinology. 2008;148:154–162. [Google Scholar]
- Jeffares DC, Phillips MJ, Moore S, Veit B. A description of the Mei2-like protein family; structure, phylogenetic distribution and biological context. Development Genes and Evolution. 2004;214:149–158. doi: 10.1007/s00427-004-0384-6. [DOI] [PubMed] [Google Scholar]
- Jeffree CE. The fine structure of the plant cuticle. In: Riederer M, Muller C, editors. Biology of the plant cuticle. Oxford: Blackwell Publishing; 2006. pp. 11–125. [Google Scholar]
- Jeffree CE, Dale JE, Fry SC. The genesis of intercellular spaces in developing leaves of Phaseolus vulgaris L. Protoplasma. 1984;132:90–98. [Google Scholar]
- Johnson GP, Renzaglia KS. Evaluating the diversity of pteridophyte embryology in the light of recent phylogenetic analyses leads to new inferences on character evolution. Plant Systematics and Evolution. 2009;283:149–164. [Google Scholar]
- Kato M, Akiyama H. Interpolation hypothesis for origin of the vegetative sporophyte of land plants. Taxon. 2005;54:443–450. [Google Scholar]
- Kaur J, Sebastian J, Siddiqi I. The Arabidopsis-mei2-like genes play a role in meiosis and vegetative growth in Arabidopsis. Plant Cell. 2006;18:545–559. doi: 10.1105/tpc.105.039156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P. The relationships of vascular plants. Philosophical Transactions of the Royal Society London. 2000;B 355:847–855. doi: 10.1098/rstb.2000.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. Water-conducting cells in early fossil land plants: implications for the early evolution of tracheophytes. Botanical Gazette. 1991;152:335–356. [Google Scholar]
- Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature. 1997a;389:33–39. [Google Scholar]
- Kenrick P, Crane PR. The origin and early diversification of land plants. A cladistic study. Washington, DC: Smithsonian Institution Press; 1997b. [Google Scholar]
- Kerp H, Trewin NH, Hass H. New gametophytes from the Early Devonian Rhynie chert. Transactions of the Royal Society Edinburgh. 2004;94:411–428. [Google Scholar]
- Kieffer M, Neve J, Kepinski S. Defining auxin response contexts in plant development. Current Opinion in Plant Biology. 2010;13:12–20. doi: 10.1016/j.pbi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Krings M, Taylor TN, Hass H, Kerp H, Dotzler N, Hermsen EJ. Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytologist. 2007;174:648–657. doi: 10.1111/j.1469-8137.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Korn RW. Analysis of shoot apical organization in six species of the Cupressaceae based on chimeric behavior. American Journal of Botany. 2001;88:1945–1952. [PubMed] [Google Scholar]
- Lewis AM. A test of the air-seeding hypothesis using Sphagnum hyalocysts. Plant Physiology. 1988;87:577–582. doi: 10.1104/pp.87.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG. Thallus differentiation in the marchantialean liverwort Asterella wilmsii (Steph.) with particular reference to longitudinal arrays of endoplasmic microtubules in the inner cells. Annals of Botany. 1994a;73:577–586. [Google Scholar]
- Ligrone R, Duckett JG. Cytoplasmic polarity and endoplasmic microtubules associated with the nucleus and organelles are ubiquitous features of food conducting cells in bryalean mosses (Bryophyta) New Phytologist. 1994b;127:601–614. [Google Scholar]
- Ligrone R, Duckett JG. Development of water-conducting cells in the antipodal liverwort Symphyogyna brasiliensis Nees (Metzgeriales) New Phytologist. 1996a;132:603–615. doi: 10.1111/j.1469-8137.1996.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG. Polarity and endoplasmic microtubules in food-conducting cells of mosses: an experimental study. New Phytologist. 1996b;134:503–516. [Google Scholar]
- Ligrone R, Duckett JG. The leafy stems of Sphagnum (Bryophyta) contain highly differentiated polarized cells with axial arrays of endoplasmic microtubules. New Phytologist. 1998;140:567–579. doi: 10.1111/j.1469-8137.1998.00298.x. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG. Morphology versus molecules in moss phylogeny: new insights (or controversies) from placental and vascular anatomy in Oedipodium griffithianum. Plant Systematics and Evolution. 2011;296:275–282. [Google Scholar]
- Ligrone R, Gambardella R. The sporophyte-gametophyte junction in bryophytes. Advances in Bryology. 1988;3:225–274. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. The gametophyte-sporophyte junction in land plants. Advances in Botanical Research. 1993;19:231–317. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. Conducting tissues and phyletic relationships of bryophytes. Philosophical Transactions of the Royal Society London. 2000;B 355:795–813. doi: 10.1098/rstb.2000.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Vaughn KC, Renzaglia KS, Knox JP, Duckett JG. Diversity in the distribution of polysaccharide and glycoprotein epitopes in the cell walls of bryophytes: new evidence for multiple evolution of water-conducting cells. New Phytologist. 2002;156:491–508. doi: 10.1046/j.1469-8137.2002.00538.x. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Carafa A, Lumini E, Bianciotto V, Bonfante P, Duckett JG. Glomeromycotean associations in liverworts: a molecular, cellular and taxonomic analysis. American Journal of Botany. 2007;94:1756–1777. doi: 10.3732/ajb.94.11.1756. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Carafa A, Duckett JG, Renzaglia KS, Ruel K. Immunocytochemical detection of lignin-related epitopes in cell walls in bryophytes and the charalean alga Nitella. Plant Systematics and Evolution. 2008;270:257–272. [Google Scholar]
- Lyndon RF. The shoot apical meristem: its growth and development. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- McAdam SAM, Brodribb TJ. Stomatal innovation and the rise of seed plants. Ecology Letters. 2012;15:1–8. doi: 10.1111/j.1461-0248.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- Menand B, Keke Y, Jouannic S, et al. An ancient mechanism controls the development of cells with a rooting function in land plants. Science. 2007;316:1477–1480. doi: 10.1126/science.1142618. [DOI] [PubMed] [Google Scholar]
- Mishler BD, Churchill SP. A cladistic approach to the phylogeny of the ‘bryophytes. Brittonia. 1984;36:406–424. [Google Scholar]
- Mishler BD, Churchill SP. Transition to a land flora: phylogenetic relationships of the green algae and bryophytes. Cladistics. 1985;1:305–328. doi: 10.1111/j.1096-0031.1985.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Mishler BD, Lewis LA, Buchheim MA, et al. Phylogenetic relationships of the ‘green algae’ and ‘bryophytes. Annals of the Missouri Botanical Gardens. 1985;81:451–483. [Google Scholar]
- Niklas KJ. The evolution of plant body plans – a biomechanical perspective. Annals of Botany. 2000;85:411–438. [Google Scholar]
- Niklas KJ, Kutschera U. The evolution of the land plant life cycle. New Phytologist. 2010;185:27–41. doi: 10.1111/j.1469-8137.2009.03054.x. [DOI] [PubMed] [Google Scholar]
- Nip M, Tegelaar EW, De Leeuw JW, Schenck PA, Holloway PJ. A new non-saponifiable highly aliphatic and resistant biopolymer in plant cuticles Evidence from pyrolysis and 13C-NMR analysis of present-day and fossil plants. Naturwissenshaften. 1986;73:579–585. [Google Scholar]
- Oliver MJ, Velten J, Mishler BD. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats. Integrative and Comparative Biology. 2005;45:788–799. doi: 10.1093/icb/45.5.788. [DOI] [PubMed] [Google Scholar]
- Paton JA, Pearce JV. The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society. 1957;3:228–259. [Google Scholar]
- Philipson WR. The significance of apical meristems in the phylogeny of land plants. Plant Systematics and Evolution. 1990;173:17–38. [Google Scholar]
- Poli DB, Jacobs M, Cook TJ. Auxin regulation of axial growth in bryophyte sporophytes: its potential significance for the evolution of early land plants. American Journal of Botany. 2003;90:1405–1415. doi: 10.3732/ajb.90.10.1405. [DOI] [PubMed] [Google Scholar]
- Pressel S, Duckett JG. Bryophyte surfaces; new functional perspectives from cryo-scanning electron microscopy. Field Bryology. 2011;104:50–53. [Google Scholar]
- Pressel S, Ligrone R, Duckett JG. Cellular differentiation in moss protonemata; a morphological and experimental study. Annals of Botany. 2008;102:227–245. doi: 10.1093/aob/mcn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressel S, Ligrone R, Proctor MCF, Duckett JG. The cell biology and physiology of de- and rehydration in the desiccation-tolerant liverwort Southbya nigrella. International Journal of Plant Science. 2009;170:182–199. [Google Scholar]
- Pressel S, Bidartondo MJ, Ligrone R, Duckett JG. Fungal symbioses in bryophytes: new insights in the twenty first century. Phytotaxa. 2010;9:238–253. [Google Scholar]
- Proctor MCF. Structure and eco-physiological adaptations in bryophytes. In: Clarke GCS, Duckett JG, editors. Bryophyte systematics. London: Academic Press; 1979. pp. 479–509. [Google Scholar]
- Proctor MCF. Ferns, evolution, scale and intellectual impedimenta. New Phytologist. 2007;176:504–506. doi: 10.1111/j.1469-8137.2007.02232.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. Poikilohydry and homoiohydry: anthithesis or spectrum of possibilities? New Phytologist. 2002;156:327–349. doi: 10.1046/j.1469-8137.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. Desiccation-tolerance in bryophytes: a review. Bryologist. 2007;110:591–621. [Google Scholar]
- Qiu Y-L. Phylogeny and evolution of charophytic algae and land plants. Journal of Systematics and Evolution. 2008;46:287–306. [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. The deepest divergences in land plants inferred from phylogenomic evidence. Proceedings of the National Academy of Sciences, USA. 2006;103:15511–15516. doi: 10.1073/pnas.0603335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. International Journal of Plant Sciences. 2007;168:691–708. [Google Scholar]
- Quodt V, Faigl W, Saedler H, Münster T. The MADS-domain protein PPM2 preferentially occurs in gametangia and sporophytes of the moss Physcomitrella patens. Gene. 2007;400:25–34. doi: 10.1016/j.gene.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Raven JA. The evolution of vascular plants in relation to quantitative functioning of dead water-conducting cells and stomata. Biological Reviews. 1993;68:337–363. [Google Scholar]
- Raven JA. Into the voids: the distribution, function, development and maintenance of gas spaces in plants. Annals of Botany. 1996;78:137–142. [Google Scholar]
- Raven JA. Selection pressures on stomatal evolution. New Phytologist. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Raven JA, Edwards D. Roots: evolutionary origins and biogeochemical evidence. Journal of Experimental Botany. 2001;52:381–401. doi: 10.1093/jexbot/52.suppl_1.381. [DOI] [PubMed] [Google Scholar]
- Read DJ, Duckett JG, Francis R, Ligrone R, Russell A. Symbiotic fungal associations in ‘lower’ land plants. Philosophical Transactions of the Royal Society London. 2000;B 355:795–813. doi: 10.1098/rstb.2000.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy W, Taylor TN, Hass H, Kerp H. Four-hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- Renzaglia KS. A comparative morphology and developmental anatomy of the Anthocerotophyta. Journal of the Hattori Botanical Laboratory. 1978;44:31–90. [Google Scholar]
- Renzaglia KS. A comparative developmental investigation of the gametophyte generation in the Metzgeriales (Hepatophyta) Bryophytorum Bibliotheca. 1982;24:1–253. [Google Scholar]
- Renzaglia KS, Brown RC, Lemmon BE, Duckett JG, Ligrone R. Occurrence and phylogenetic significance of monoplastidic meiosis in liverworts. Canadian Journal of Botany. 1993;72:65–72. [Google Scholar]
- Renzaglia KS, McFarlard KD, Smith DK. Anatomy and ultrastructure of the sporophyte of Takakia ceratophylla (Bryophyta) American Journal of Botany. 1997;84:1337–1350. [PubMed] [Google Scholar]
- Renzaglia KS, Duff RJ, Nickrent DL, Garbary DJ. Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philosophical Transactions of the Royal Society London B. 2000;355:769–793. doi: 10.1098/rstb.2000.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Schuette S, Duff RJ, et al. Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist. 2007;110:179–213. [Google Scholar]
- Renzaglia KS, Villareal JC, Duff RJ. New insights into morphology, anatomy, and systematics of hornworts. In: Goffinet B, Shaw J, editors. Bryophyte biology. Cambridge: Cambridge University Press; 2009. pp. 139–171. [Google Scholar]
- Roland JC. Cell wall differentiation and stages involved with intercellular gas space opening. Journal of Cell Science. 1978;32:325–336. doi: 10.1242/jcs.32.1.325. [DOI] [PubMed] [Google Scholar]
- Rothwell GW. The fossil history of branching: implications for the phylogeny of land plants. In: Hoch PC, Stephenson AG, editors. Experimental and molecular approaches to plant biosystematics. St. Louis, MO: Missouri Botanical Garden; 1995. pp. 71–86. [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, et al. Land plants acquired stomatal control early in their evolutionary history. Current Biology. 2011;21:1030–1035. doi: 10.1016/j.cub.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Deguchi H, Hasebe M. Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella but do function in sporophyte development. Evolution and Development. 2008;10:555–566. doi: 10.1111/j.1525-142X.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- Sano R, Juàrez CM, Hass B, et al. Knox homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evolution and Development. 2005;7:69–78. doi: 10.1111/j.1525-142X.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- Schertler MM. Development of archegonium and embryo in Lophocolea heterophylla. Bryologist. 1979;82:576–582. [Google Scholar]
- Schreiber L. Transport barriers made of cutin, suberin and associated waxes. Trends in Plant Science. 2010;15:546–553. doi: 10.1016/j.tplants.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Schuster RM. Studies on the Hepaticae XV. Calobryales. Nova Hedwigia. 1966;13:1–63. [Google Scholar]
- Shaw AJ, Renzaglia KS. Phylogeny and diversification of bryophytes. American Journal of Botany. 2004;91:1557–1581. doi: 10.3732/ajb.91.10.1557. [DOI] [PubMed] [Google Scholar]
- Shaw AJ, Szövényi P, Shaw B. Bryophyte diversity and evolution: windows into early evolution of land plants. American Journal of Botany. 2011;98:352–369. doi: 10.3732/ajb.1000316. [DOI] [PubMed] [Google Scholar]
- Singer SD, Ashton NW. Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Reports. 2007;26:2039–2054. doi: 10.1007/s00299-007-0409-5. [DOI] [PubMed] [Google Scholar]
- Smith EC, Griffiths H. A pyrenoid-based carbon-concentrating mechanism is present in terrestrial bryophytes of the class Anthocerotae. Planta. 1996;200:203–212. [Google Scholar]
- Smith SA, Read DJ. Mycorrhizal symbiosis. Amsterdam: Academic Press; 2008. [Google Scholar]
- Sugano SS, Shimada T, Okawa K, Tamai A, Mori M, Hara-Nishimura I. Stomagen positively regulates stomatal density in Arabidopsis. Nature. 2010;463:241–246. doi: 10.1038/nature08682. [DOI] [PubMed] [Google Scholar]
- Sussex IM, Kerk NM. The evolution of plant architecture. Current Opinion in Plant Biology. 2001;4:33–37. doi: 10.1016/s1369-5266(00)00132-1. [DOI] [PubMed] [Google Scholar]
- Szövényi P, Rensing SA, Lang D, Wray G, Shaw AJ. Generation-biased gene expression in a bryophyte model system. Molecular Biology and Evolution. 2011;28:803–812. doi: 10.1093/molbev/msq254. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Hasebe M, Sekimoto H, et al. Characterization of MADS-box genes in charophycean algae and its implications for the evolution of MADS-box genes. Proceedings of the National Academy of Sciences USA. 2005;102:2436–2441. doi: 10.1073/pnas.0409860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Kerp H, Hass H. Life history biology of early land plants: deciphering the gametophyte phase. Proceedings of the National Academy of Sciences USA. 2005;102:5892–5897. doi: 10.1073/pnas.0501985102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Taylor EL, Krings M. Paleobotany. The biology and evolution of fossil plants. Amsterdam: Academic Press; 2009. [Google Scholar]
- Thomas BA. A probable moss from the Lower Carboniferous of the Forest of Dean, Gloucestershire. Annals of Botany. 1972;36:155–161. [Google Scholar]
- Vaizey JR. On the absorption of water and its relation to the constitution of the cell-wall in Mosses. Annals of Botany. 1887;1:147–152. [Google Scholar]
- Vaughn KC, Ligrone R, Owen HA, et al. The anthocerote chloroplast: a review. New Phytologist. 1992;120:169–190. [Google Scholar]
- Volkmar U, Knoop V. Introducing intron locus cox1i624 for phylogenetic analyses in bryophytes: on the issue of Takakia as sister genus to all other extant mosses. Journal of Molecular Evolution. 2010;70:506–518. doi: 10.1007/s00239-010-9348-9. [DOI] [PubMed] [Google Scholar]
- Wang B, Jeun LH, Xue J-Y, Liu Y, Ané J-M, Qiu Y-L. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytologist. 2010;186:514–525. doi: 10.1111/j.1469-8137.2009.03137.x. [DOI] [PubMed] [Google Scholar]
- Watkins JE, Mack MC, Sinclair TR, Mulkey SS. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist. 2007;176:708–717. doi: 10.1111/j.1469-8137.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- Wellman CH, Gray J. The microfossil record of early land plants. Philosophical Transactions of the Royal Society London. 2000;B 355:717–732. doi: 10.1098/rstb.2000.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CH, Osterloff PL, Mohiuddin U. Fragments of the earliest land plants. Nature. 2003;425:282–285. doi: 10.1038/nature01884. [DOI] [PubMed] [Google Scholar]
- Zobell O, Faigl W, Saedler H, Münster T. MIKC* MADS-Box proteins: conserved regulators of the gametophytic generation of land plants. Molecular Biology and Evolution. 2010;27:1201–1211. doi: 10.1093/molbev/msq005. [DOI] [PubMed] [Google Scholar]