Abstract
Background and Aims
Horsetails (Equisetopsida) diverged from other extant eusporangiate monilophytes in the Upper Palaeozoic. They are the only monilophytes known to contain the hemicellulose mixed-linkage (1 → 3, 1 → 4)-β-d-glucan (MLG), whereas all land plants possess xyloglucan. It has been reported that changes in cell-wall chemistry often accompanied major evolutionary steps. We explored changes in hemicelluloses occurring during Equisetum evolution.
Methods
Hemicellulose from numerous monilophytes was treated with lichenase and xyloglucan endoglucanase. Lichenase digests MLG to di-, tri- and tetrasaccharide repeat-units, resolvable by thin-layer chromatography.
Key Results
Among monilophytes, MLG was confined to horsetails. Our analyses support a basal trichotomy of extant horsetails: MLG was more abundant in subgenus Equisetum than in subgenus Hippochaete, and uniquely the sister group E. bogotense yielded almost solely the tetrasaccharide repeat-unit (G4G4G3G). Other species also gave the disaccharide, whereas the trisaccharide was consistently very scarce. Tetrasaccharide : disaccharide ratios varied interspecifically, but with no consistent difference between subgenera. Xyloglucan was scarce in Psilotum and subgenus Equisetum, but abundant in subgenus Hippochaete and in the eusporangiate ferns Marattia and Angiopteris; leptosporangiate ferns varied widely. All monilophytes shared a core pattern of xyloglucan repeat-units, major XEG products co-chromatographing on thin-layer chromatography with non-fucosylated hepta-, octa- and nonasaccharides and fucose-containing nona- and decasaccharides.
Conclusions
G4G4G3G is the ancestral repeat-unit of horsetail MLG. Horsetail evolution was accompanied by quantitative and qualitative modification of MLG; variation within subgenus Hippochaete suggests that the structure and biosynthesis of MLG is evolutionarily plastic. Xyloglucan quantity correlates negatively with abundance of other hemicelluloses; but qualitatively, all monilophyte xyloglucans conform to a core pattern of repeat-unit sizes.
Keywords: Equisetum, Hippochaete, Equisetum bogotense, ferns, eusporangiate monilophytes, leptosporangiate monilophytes, evolution, cell wall (primary), hemicellulose, mixed-linkage beta-glucan, xyloglucan
INTRODUCTION
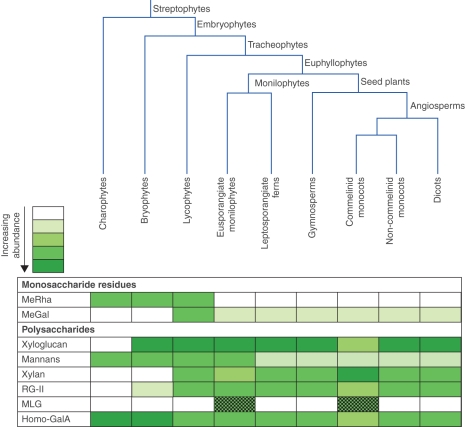
Major changes in primary cell wall composition often accompanied landmark steps of plant evolution, especially during colonization of the land and vascularization (Stace, 1981; Kenrick and Crane, 1997a; Popper and Fry, 2003). Accordingly, a potential use of primary cell-wall composition in understanding phylogenetic relationships has been considered, e.g. in algae (Popper and Fry, 2003), bryophytes (Ligrone et al., 2002; Popper and Fry, 2003, 2004), lycophytes and monilophytes (Buckeridge et al., 1999; Matsunaga et al., 2004; Popper and Fry, 2004) and angiosperms (Nothnagel and Nothnagel, 2007). Cell-wall components differ between plant taxa in parallel with their evolution and diversification (Popper, 2008) at both the monosaccharide and polysaccharide levels (Fig. 1). For instance, the earliest diverging extant vascular plants, lycopodiophytes, are unique among land plants in containing high levels of 3-O-methyl-d-galactose (Popper et al., 2001). To date, differences in cell-wall composition are commonly used as taxonomic markers in algal classification but have not been applied to land-plant classification (Stebbins, 1992; Buckeridge et al., 1999; Graham and Wilcox, 1999).
Fig. 1.
Scheme of streptophyte phylogeny, shown together with the relative abundance of selected primary cell wall components across major plant groups. Wall composition differs at both the monosaccharide and the polysaccharide levels. MeGal, 3-O-methyl-d-galactose; MeRha, 3-O-methylrhamnose; MLG, (1 → 3, 1 → 4)-β-d-glucan; RG-II, rhamnogalacturonan-II; Homo-GalA, homogalacturonan. Intensity of the shades indicates relative abundance. MLG (dotted shading) is present in only one genus (Equisetum) of eusporangiate ferns and one order (Poales) of the commelinid monocots.
The plant primary cell wall is a strong and cohesive network of cellulose microfibrils, probably tethered by hemicelluloses. Usually the principal tethers are xyloglucan (in dicots and non-commelinid monocots) or glucuronoarabinoxylan [in commelinid monocots, e.g. the Poaceae (grasses and cereals); Smith and Harris (1999)]. Structures and proportions of the hemicelluloses are variable between species and organs, and even within tissues (Harris, 2005; Fry, 2011). A third major hemicellulose, mixed-linkage (1 → 3, 1 → 4)-β-d-glucan (MLG), occurs in certain algae (Ford and Percival, 1965; Nevo and Sharon, 1969) including at least one charophytic species (Micrasterias denticulata; Eder et al., 2008), and in a narrow range of land plants (Stone and Clarke, 1992; Buckeridge et al., 2004; Trethewey et al., 2005; Fry et al., 2008a; Sørensen et al., 2008). Within land plants, MLG has been found in only two, distantly related, groups: the angiosperm order Poales (Smith and Harris, 1999; Buckeridge et al., 2004; Trethewey et al., 2005) and the ‘fern ally’ genus Equisetum (Fry et al., 2008a; Sørensen et al., 2008). Since MLG occurs in two evolutionarily very distant land-plant taxa, it appears that its biosynthesis is either deeply conserved or has arisen twice through convergent evolution (Sørensen et al., 2010).
Although the polysaccharide MLG is found in both the Poales and the Equisetales, only the latter possess MLG : xyloglucan endotransglucosylase (MXE), an enzyme capable of grafting MLG to xyloglucan chains by transglycosylation (Fry et al., 2008b). This observation may indicate that MLG serves different biological roles in the cell walls of these two plant orders. A difference in role is also suggested by the fact that poalean MLG is principally a feature of young, rapidly expanding tissues, whereas the MLG of Equisetum persists and may even increase during ageing (Fry et al., 2008a). MXE activity reaches its peak in old, tough Equisetum stems, suggesting a role in tissue strengthening (Fry et al., 2008a).
MLG is a long, unbranched, coiling β-d-glucan chain with (1 → 4) linkages plus a minority of (1 → 3) linkages. In cereals, MLG is synthesized in the Golgi apparatus (Carpita and McCann, 2010) and typically has a structure of the type
… G4G4G3G4G4G3G4G4G4G3G4G4G3G4G4G3 …
where G represents a β-d-glucose residue, and 3 and 4 represent (1 → 3) and (1 → 4) bonds, respectively. The underlined segments are effectively cello-oligosaccharides interconnected by ‘hinges’. The (1 → 4) linkages give rigidity, whereas (1 → 3) linkages confer flexibility and water solubility (Woodward et al., 1983). Luttenegger and Nevins (1985) found a marked variation in the MLG content (1–14 %) of Zea mays coleoptile primary cell walls during changes in the rate of cell expansion, suggesting a role of MLG in rapid growth in the Poales.
MLG can be structurally characterized by analysis of the oligosaccharides produced on digestion with Bacillus subtilis lichenase. This commercial endo-glucanase cleaves the (1 → 4) bond following a (1 → 3) bond (Meikle et al., 1994). From poalean MLG, lichenase thus generates the trisaccharide G4G3G plus a minority of the tetrasaccharide G4G4G3G (Stone and Clarke, 1992) (these sequences are always quoted from non-reducing to reducing end).
While poalean MLG is composed of G4G3G > G4G4G3G, Equisetum MLG when digested with lichenase releases G4G4G3G and the disaccharide G3G (laminaribiose) as the predominant products (Fry et al., 2008a; Sørensen et al., 2008). The trisaccharide : tetrasaccharide ratio in poalean MLG ranges between 1·5 in Zea mays and 4·5 in wheat (Triticum aestivum) flour (Buckeridge et al., 1999; Li et al., 2006). In addition to G4G3G and G4G4G3G, oligosaccharides with degree of polymerization (DP) up to 14 have been reported in barley MLG (Lazaridou and Biliaderis, 2007); and Equisetum MLG yielded a lichenase product tentatively identified as DP9 (Fry et al., 2008a).
Functionally, the trisaccharide : tetrasaccharide ratio affects the strength of an MLG gel, as demonstrated by studies using the large-deformation mechanical test (Lazaridou et al., 2004), suggesting a significance of this ratio in governing the strength of the primary cell wall.
In the primary cell walls of many land plants, except the commelinid monocots, xyloglucan is the major hemicellulose. Composed of long and slightly flexible chains, xyloglucans can hydrogen-bond strongly to cellulose, and probably tether adjacent microfibrils, contributing to wall architecture. Xyloglucans have a backbone of (1 → 4)- β-d-glucan (qualitatively identical to cellulose), many of the glucose residues (typically approx. 75 %) bearing an α-d-xylopyranose (Xylp) residue at position 6. A (1 → 4)-linked glucose is abbreviated G; one with xylose attached (forming a disaccharide called isoprimeverose) is abbreviated X (Fry et al., 1993). Additional sugar residues are attached to some of the isoprimeverose groups, generating structures such as β-d-Galp-(1 → 2)-α-d-Xylp-(1 → 6)-β-d-Glc (abbreviated L) and α-l-Fucp-(1 → 2)-β-d-Galp-(1 → 2)-α-d-Xylp-(1 → 6)-β-d-Glc (abbreviated F).
Xyloglucan can be broken down for analysis by digestion with xyloglucan endoglucanase (XEG; Pauly et al., 1999), which yields oligosaccharides such as the heptasaccharide XXXG, the two octasaccharides XXLG and XLXG, the two nonasaccharides XXFG and XLLG, and the decasaccharide XLFG (Hoffman et al., 2005).
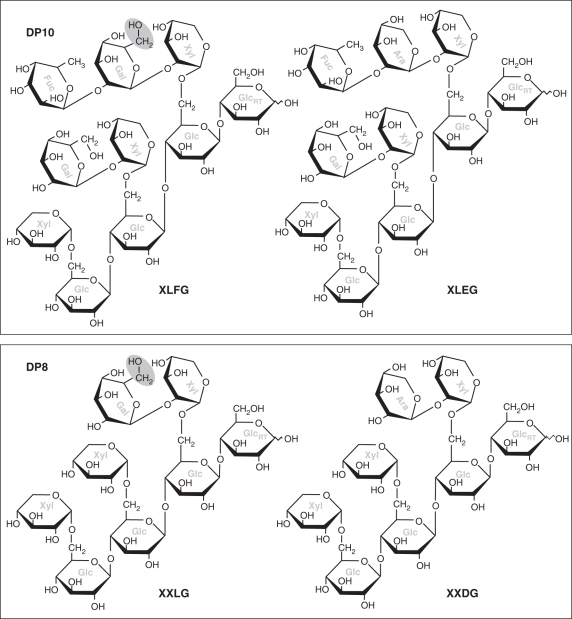
The monilophyte Equisetum hyemale and the lycophyte Selaginella kraussiana have been reported to possess additional xyloglucan repeat-units not yet detected in other plants. These are D and E, which are identical to L and F, respectively, except that the β-d-Galp residue is replaced by α-l-Arap (Peña et al., 2008). This is a highly conservative replacement: despite the α/β and d/l differences in the nomenclature, β-d-Galp and α-l-Arap differ from each other only in the fact that the former has a -CH2OH group in place of an -H. Equisetum hyemale xyloglucan includes the sequences XLEG and XLDG, which very closely resemble XLFG and XLLG, respectively (Fig. 2).
Fig. 2.
Representative xyloglucan oligosaccharides. Top, decasaccharides; bottom, octasaccharides; left, structures found in most vascular plants; right, structures detected by Peña et al. (2008) in Equisetum hyemale and Selaginella kraussiana. Note the minor chemical difference (grey ovals) between the left-hand and right-hand structures. The TLC system shown in Fig. 7 would be expected to group the two similar decasaccharides together and the two similar octasaccharides together. Sugar residues are labelled in grey: Ara, α-l-arabinopyranose; Fuc, α-l-fucopyranose; Gal, β-d-galactopyranose; Glc, β-d-glucopyranose; Xyl, α-d-xylopyranose. GlcRT indicates the reducing terminal glucose group.
Extant vascular plants are divided into two major clades: lycophytes and euphyllophytes, the latter (plants with ‘true’ leaves) being divided into spermatophytes and monilophytes (Kenrick and Crane, 1997b; Pryer et al., 2001). The horsetails (genus Equisetum), the psilophytes, and the eusporangiate and leptosporangiate ferns, are all monilophytes (Pryer et al., 2001, 2004). The class Equisetopsida (syn. Sphenopsida) emerged in the Upper Devonian, and became diverse and abundant in the Palaeozoic swamp forest (Delevoryas, 1962). It includes both the extant genus Equisetum and extinct herbaceous and arborescent horsetails such as the Calamitaceae (Bateman, 1991). The extant herbaceous horsetails are considered ‘living fossils’ as Equisetum is the only surviving genus, forming a crown group (i.e. a clade including all the extant members from their most recent common ancestor) of the Equisetopsida total group (a clade containing all descendants of a common ancestor, whether extant or extinct). Equisetum might even be the oldest surviving genus of all vascular plants owing to the ancient history of this lineage (Hauke, 1978).
Both molecular- and fossil-based estimates of age suggest that extant Equisetum had diverged from the fossil genus Equisetites in the Tertiary of the Cenozoic (approx. 49 Ma) (Des Marais et al., 2003; Pryer et al., 2004). However, recent comparisons of several anatomically preserved Equisetum fossils from the Jurassic and Cretaceous period indicate a much earlier Mesozoic origin of the crown group (approx. 100–200 Ma) (Stanich et al., 2009; Channing et al., 2011). This argument is based on the distinct morphological features found in Mesozoic Equisetum (from sinter and other volcanic deposits), which closely resemble but differ from both the Triassic Equisetites fossil compressions and the extant horsetails (Baron, 1889; Channing et al., 2011).
Leaves of Equisetum have the combined characteristics of apparent microphylls (leaves with a single vascular bundle) plus the megaphyll feature of having a gap in the stem vasculature where the leaf bundle diverges. The fossil evidence is clear that the apparent microphylls of Equisetum actually represent reduced megaphylls, based on observations from the genus Sphenophyllum in the sister group Sphenophyllales, which have more complex megaphylls from which type the leaves of Equisetum are apparently reduced (Kenrick and Crane, 1997b).
Recent phylogenetic analysis of the 15 recognized species of the genus Equisetum shows a basal trichotomy: there are two monophyletic subgenera [Hippochaete (including the neotropical E. giganteum and E. myriochaetum) and Equisetum (all of which are of temperate distribution in the northern hemisphere)] plus the diminutive neotropical species E. bogotense as a sister group (Hauke, 1978; Des Marais et al., 2003). [Note: ‘Equisetum’ in this manuscript implies the genus unless ‘subgenus’ is specified.] Species in the subgenus Equisetum typically have superficial stomata with highly branched vegetative shoots, whereas most species in Hippochaete have sunken stomata with generally unbranched vegetative shoots (Hauke, 1959).
Phylogenetic study of Equisetum is difficult owing to the long history of isolation and frequent inter-specific hybridization within but not between each subgenus. In addition, horsetails are capable of reproducing vegetatively, further complicating phylogenies as sterile hybrids can persist. While the phylogenetic relationships among species of Hippochaete have shown a fair degree of consistency across studies (Des Marais et al., 2003; Guillon, 2004, 2007), the phylogeny of subgenus Equisetum is still disputable, despite there being more distinguishing morphological features within the group than in Hippochaete. One suggested reason for the lack of reconciliation between molecular and morphological classification is the homoplasic characters (such as stem dimorphism), arising through convergent evolution as analogous features, not being differentiated in classical taxonomic treatments (Guillon, 2004, 2007). The systematic difficulty of Equisetum is not unique in the early-divergent lineages of euphyllophytes: there is a significant morphological gap between ferns and seed plants as a result of age, extinction and lack of fossil record (Pryer et al., 2001, 2004).
The objectives of the present work were to explore the occurrence and abundance of MLG in diverse monilophytes, and to define MLG structures from both horsetail subgenera and E. bogotense to test whether differences arose during the course of evolution in the genus Equisetum. We also aimed to develop a simple visual method for surveying the quantitative and qualitative variation occurring in xyloglucan throughout the monilophytes.
MATERIALS AND METHODS
Plant materials
Plant sources are listed in Table 1. Most fresh plants were obtained from the Royal Botanic Garden Edinburgh (RBGE) living collection with accession numbers indicated, with the exception of E. arvense and E. fluviatile which were collected from the wild in Edinburgh. Vouchers of E. bogotense were courtesy of New York Botanic Garden; four independent herbarium specimens were combined to make an adequate sample. In the genus Equisetum (except for the herbarium specimen), young tender tissues were taken from the base of each internode wrapped inside the leaf sheath; mature tissues were the tougher parts of internodes. In other genera, young (tender) and mature (tough) tissues were selected empirically.
Table 1.
Classification, sources, AIR yields, and relative abundance of MLG subunits and xyloglucan of all species analysed
| Classification | Species | Source/accession number* | Age† | Polymer content (mg g−1)‡ | Relative oligosaccharide yields§ |
|||
|---|---|---|---|---|---|---|---|---|
| MLG4 | MLG3 | MLG2 | XGOs | |||||
| HETEROSPOROUS LYCOPHYTE | ||||||||
| Selaginellaceae | Selaginella willdenowii (Desv. ex Poir.) Baker | 19762969(E) | Y | 117 | – | – | – | + |
| EUSPORANGIATE MONILOPHYTES | ||||||||
| Ophioglossaceae | Ophioglossum vulgatum L. | 19695522(E) | Y | 67 | – | – | – | + |
| Psilotaceae | Psilotum nudum (L.) P. Beauv. | 20040835(E) | Y | 45 | – | – | – | + |
| M | 109 | – | – | – | + | |||
| Equisetaceae¶ | Equisetum bogotense Kunth. | NYBG herbarium | (H) | nd | ++ + | – | – | [ ] |
| subgenus Equisetum | Equisetum arvense L. | RBGE | Y | 91 | ++ | ± | + | + |
| M | 74 | ++ + | ± | ++ | + | |||
| Equisetum fluviatile L. | KB pond | Y | 32 | ++ | ± | + | + | |
| M | nd | ++ + | ± | + | – | |||
| Equisetum pratense Ehrh. | 19821716(E) | Y | 95 | ++ | ± | ± | + | |
| M | 129 | ++ + | ± | ± | + | |||
| Equisetum sylvaticum L. | 19821736(E) | Y | 90 | ++ | ± | + | – | |
| M | 164 | ++ + | ± | + | [ ] | |||
| Equisetum × bowmanii C.N. Page (E. telmateia × E. sylvaticum) | 20060765A(E) | Y | 129 | ++ + | – | ++ | – | |
| M | 98 | ++ + | ± | + | [ ] | |||
| Equisetum × litorale Kühlewein ex Rupr. (E. arvense × E. fluviatile) | 19821740(E) | Y | 58 | ++ + | ± | + | + | |
| M | 97 | ++ + | ± | + | + | |||
| Equisetum × robertsii Dines (E. arvense × E. telmateia) | 20060766A(E) | Y | 81 | ++ + | + | ++ | ++ | |
| M | 74 | ++ | ± | ± | ++ | |||
| subgenus Hippochaete | Equisetum hyemale var. affine Engelm. | 19734597B(E) | Y | 35 | + | – | – | ++ + |
| M | 104 | + | – | ± | + | |||
| Equisetum myriochaetum Schltdl. & Cham. | 19734598(E) | Y | 35 | + | ± | + | ++ | |
| M | 100 | ++ | ± | ± | + | |||
| Equisetum ramosissimum ssp. debile Hauke | 19731694(E) | M | nd | + | – | ++ + | [ ] | |
| Marattiaceae | Marattia fraxinea Sm. | 19697184(E) | Y | 68 | – | – | – | ++ + |
| M | 65 | – | – | – | ++ + | |||
| Angiopteris evecta Hoffm. | 20060955(E) | Y | 82 | – | – | – | ++ + | |
| M | 58 | – | – | – | ++ | |||
| Angiopteris lygodiifolia Rosenstock | 19763705A(E) | Y | 29 | – | – | – | ++ + | |
| M | 57 | – | – | – | ++ + | |||
| LEPTOSPORANGIATE MONILOPHYTES | ||||||||
| Osmundaceae | Osmunda regalis L. | 19913878A(E) | Y | 108 | – | – | – | ++ + |
| M | 203 | – | – | – | + | |||
| Todea barbara (L.) T. Moore | 19652792(E) | Y | 98 | – | – | – | ++ + | |
| M | 134 | – | – | – | + | |||
| Hymenophyllaceae | Trichomanes speciosum Willd. | 19992144(E) | Y | 191 | – | – | – | ++ + |
| M | 224 | – | – | – | ++ | |||
| Dipteridaceae | Dipteris conjugata Reinw. | 20021886A(E) | Y | 145 | – | – | – | + |
| M | 249 | – | – | – | ± | |||
| Lygodiaceae | Lygodium japonicum (Thunb.) Sw. | 19734355(E) | Y | nd | – | – | – | ++ |
| M | nd | – | – | – | + | |||
| Anemiaceae | Anemia sp. | 19933657(E) | Y | 116 | – | – | – | ++ + |
| M | 154 | – | – | – | ++ | |||
| Marsileaceae | Marsilea drummondii A. Braun | 19933710(E) | Y | 100 | – | – | – | + |
| M | 111 | – | – | – | + | |||
| Thyrsopteridaceae | Thyrsopteris elegans Kunze | 20031267A(E) | Y | 108 | – | – | – | ++ + |
| M | 70 | – | – | – | + | |||
| Cyatheaceae | Cyathea spinulosa Wall. | 19941397A(E) | Y | 87 | – | – | – | ++ + |
| M | 54 | – | – | – | ++ | |||
| Woodsiaceae | Woodsia obtusa (Spr.) Torrey | 20061094A(E) | Y | 99 | – | – | – | ++ + |
| M | 73 | – | – | – | ++ | |||
| SPERMATOPHYTES | ||||||||
| Gymnosperm | Chamaecyparis lawsoniana Parl. | KB | Y | 216 | – | – | – | ++ |
| M | 255 | – | – | – | ++ | |||
| Angiosperm | Nymphaea lotus L. | 20040381(E) | Y | 26 | – | – | – | ++ + |
| M | 278 | – | – | – | ++ + | |||
* Sources/accession numbers: (E), Royal Botanic Garden, Edinburgh (RBGE) accession no.; KB, King's Buildings campus, Edinburgh; NYBG, New York Botanic Garden.
† Age of tissues tested: Y, young tender tissues; M, mature tough tissues; H, herbarium tissue.
‡ Polymer contents: yield of alcohol-insoluble residue (mg AIR g−1 fresh weight); nd, not determined.
§ Oligosaccharides indicative of MLG and xyloglucan: MLG4, MLG3, MLG2, tetra-, tri- and disaccharide of MLG released by lichenase; XGOs, xyloglucan oligosaccharides released by XEG. Yields of the oligosaccharides are scored as: –, undetectable; ±, inconsistently or barely detectable; +, ++ , + ++ , low, moderate and high abundance; [ ], not determined.
¶ E. bogotense cannot be placed clearly in either subgenus.
Preparation of alcohol-insoluble residue (AIR) and hemicellulose B
Methods followed previous protocol (Fry, 2000). AIR was obtained by homogenizing plant materials in 96 % ethanol, filtering on Miracloth (Calbiochem, EMD, USA), then repeatedly rinsing with 96 % ethanol until all the chlorophyll had been removed and the filtrate was colourless. After drying at room temperature, AIR (100 mg) was shaken at 37 °C for 17 h with 5 mL 6 m NaOH containing 0·1 % NaBH4. The supernatant was neutralized with acetic acid and the suspension was dialysed in 12–14-kDa cut-off tubing (Medicell Int. Ltd, London, UK) against running tap-water for 3 d. The suspension from inside the sac was centrifuged at 6000 g for 20 min. Both the pellet (hemicellulose A) and the supernatant (hemicellulose B) were retained for analysis.
Lichenase digestion
Hemicellulose was digested with lichenase (from Bacillus subtilis; Megazyme), yielding MLG repeat-units, as described by Popper and Fry (2003). Hemicellulose solution [200 µL, 0·5 % w/v in pyridine/acetic acid/chlorobutanol/water (1 : 1 : 0·5 : 98, v/v/w/v; PyAW)] was mixed with 200 µL lichenase solution (1·43 unit mL−1) and incubated at 20 °C with gentle shaking for 16 h. Digestion was then stopped with 200 µL formic acid. The products were dried, re-dissolved in 150 µL PyAW, and analysed by thin-layer chromatography (TLC).
Xyloglucan endoglucanase (XEG) digestion
XEG (a gift from Novo Nordisk, Bagsværd) from Aspergillus aculeatus contained traces of pectinase and β-galactosidase. Conditions for XEG digestion, with minimal side reactions, were devised in preliminary studies, with citrus pectin and tamarind seed xyloglucan as substrate, monitoring galacturonic acid and galactose production, respectively.
For the XEG digestion, 200 µL of sample (0·5 % w/v; suspension of hemicellulose A or solution of hemicellulose B) in PyAW was mixed with 200 µL of 0·05 % (w/v) XEG in PyAW and incubated at 25 °C for 64 min. The reaction was stopped with 200 µL formic acid. Products were dried and re-dissolved in 150 µL water; 3 µL was analysed by TLC.
Thin-layer chromatography
Samples were loaded on to a TLC plate (Merck, silica gel 60, 20 × 20 cm; Sigma-Aldrich, UK) and developed in either butanol/acetic acid/water (2 : 1 : 1, v/v/v) or butanol/ethanol/water (2 : 1 : 1, v/v/v), as specified in the figure legends. Sugars were stained with thymol (Franková and Fry, 2011).
RESULTS
Preparation of alcohol-insoluble residue
Samples of young and mature tissues from 27 species, mainly monilophytes, were converted to alcohol-insoluble residue (AIR; i.e. total polymers, a high proportion of which will be cell wall material). The species investigated are listed in Table 1, and most are illustrated in Fig. 3. There was considerable variability between species in total polymer content per gram fresh weight (Table 1), reflecting wide variation in the species' adaptations to diverse environments (wet and dry, exposed and sheltered, insolated and shaded, etc.). However, data for all 21 monilophyte species in which both mature and young tissues were quantified show a mature : young ratio (of mg AIR obtained per g fresh weight) of 1·42 ± 0·15 (mean ± s.e., N = 21), supporting our subjective description of these samples as ‘tough’ and ‘tender’, respectively. The mature tissues are likely to be richer in secondary cell wall material, deposited after the cells had lost the ability to expand.
Fig. 3.
Plant specimens analysed in the present work. (A) Selaginella willdenowii; (B) Equisetum × bowmanii; (C) Equisetum × robertsii; (D) Equisetum arvense; (E, E′) Equisetum fluviatile; (F) Equisetum sylvaticum; (G) Equisetum pratense; (H) Equisetum hyemale var. affine; (I) Equisetum myriochaetum; (J) Ophioglossum vulgatum; (K) Psilotum nudum; (L) Marattia fraxinea; (M, M′, M″) Angiopteris evecta; (N) Angiopteris lygodiifolia; (O) Osmunda regalis; (P) Todea barbara; (Q) Dipteris conjugata; (R) Trichomanes speciosum; (S, S′) Lygodium japonicum; (T) Anemia sp.; (U) Marsilea drummondii; (V, V′) Thyrsopteris elegans; (W) Cyathea spinulosa; (X) Woodsia obtusa; (Y) Chamaecyparis lawsoniana; (Z) Nymphaea lotus. Extraneous species, e.g. the pink-flowering Geranium seen in (D), were removed prior to analysis of the plants of interest. Letters A–Z are colour-coded to agree taxonomically with the labelling on Figs 4–7.
Mixed-linkage (1 → 3, 1 → 4)-β-d-glucan (MLG)
Hemicelluloses from AIR of numerous species of monilophyte plus a representative lycophyte and two spermatophytes were tested for MLG by TLC analysis of lichenase digests. Hemicellulose A (i.e. the alkali-extracted material that precipitates when neutralized) gave almost no MLG oligosaccharides (chromatograms not shown). On the other hand, the hemicellulose B (i.e. that which remains in solution after neutralization) from certain species gave a series of oligosaccharides diagnostic of MLG. Production of these oligosaccharides was dependent on the presence of lichenase (Fig. 4), confirming that they were not due to artifactual degradation of polysaccharides. A summary of all the following MLG oligosaccharide data is given in Table 1.
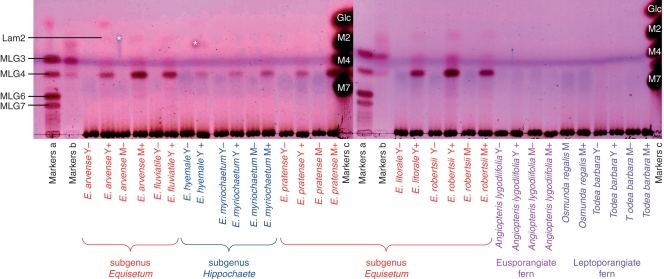
Fig. 4.
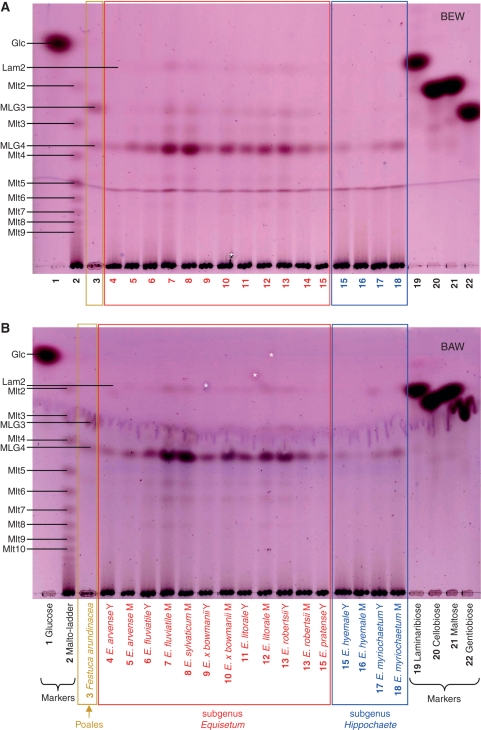
Lichenase digestion products of hemicellulose B from several monilophytes, with enzyme-free controls. Digests of: Y, young tender tissues; M mature tough tissues; ‘ + ’ lichenase-treated; ‘–’, enzyme-free. The TLCs were developed in butanol/acetic acid/water, and stained with thymol. Markers a and b: oligosaccharides of DP 3–7 obtained by partial (a) and complete (b) lichenase digestion of commercial barley MLG (MLG3, G4G3G; MLG4, G4G4G3G; MLG6, G4G3G4G4G3G; MLG7, G4G4G3G4G4G3G and/or G4G3G4G4G4G3G). Markers c: M2, maltose; M4, maltotetraose; M7, maltoheptaose. Laminaribiose (Lam2) was detectable in some digests. Spots marked ‘*’ are contamination, not seen on replicate plates.
All species of the genus Equisetum gave MLG oligosaccharides, but in varying yields (Fig. 4). The major product in most Equisetum species was the tetrasaccharide G4G4G3G, in contrast to the situation with poalean MLG (e.g. fully lichenase-digested commercial barley MLG; Fig. 4), which gave mainly the trisaccharide G4G3G. There was little consistent difference, either quantitative or qualitative, between young and mature tissues. No non-Equisetum monilophytes, either eusporangiate or leptosporangiate, gave detectable MLG oligosaccharides (Fig. 4 and all other species listed in Table 1; chromatograms not shown). Likewise, no MLG was detected in the lycophyte Selaginella willdenowii or either of the non-commelinid spermatophytes examined (results not shown).
Samples rich in starch may give traces of malto-oligosaccharides on lichenase digestion owing to slight contamination of the enzyme with amylases. Therefore, digests were analysed by TLC in two different solvent systems, with a malto-oligosaccharide marker ladder. MLG oligosaccharides were confirmed to be present in all Equisetum samples (Fig. 5). Somewhat different patterns were noted among the products generated from the nine surveyed species of horsetail. In general, there was a higher concentration of MLG in the hemicellulose of subgenus Equisetum and E. bogotense than in subgenus Hippochaete. In some samples of E. hyemale MLG was almost undetectable.
Fig. 5.
Lichenase digestion products of hemicellulose B from nine Equisetum species. The TLCs were developed either in butanol/ethanol/water (A) or in butanol/acetic acid/water (B), and stained with thymol. A lichenase digest of the hemicellulose B from an MLG-rich grass, Festuca arundinacea, was run in parallel. Abbreviations: H, herbarium specimen; M, mature tissue; Y, young tissue; Glc, glucose; Lam2, laminaribiose; MLG3-4, tri- and tetrasaccharide repeat-unit of MLG; Mlt2-10, maltose to maltodecaose (‘malto-ladder’ obtained by partial hydrolysis of amylose on long-term storage of a sterile aqueous solution). Spots marked ‘*’ are contamination, not seen on replicate plates.
The trisaccharide G4G3G (the major repeat-unit in poalean MLG) was consistently a very minor component of Equisetum MLG. The tetrasaccharide G4G4G3G and disaccharide G3G (= laminaribiose) were the major repeat-units. There was no consistent difference between the subgenera in tetrasaccharide : disaccharide ratio. In some samples from both subgenera (e.g. some samples of mature E. arvense and mature E. ramosissimum; Fig. 5), the disaccharide predominated over the tetrasaccharide; but in other samples the tetrasaccharide was predominant (Fig. 6). The very isolated (probably earliest-diverging) species E. bogotense uniquely had MLG composed almost solely of the tetrasaccharide.
Fig. 6.
Lichenase digestion products of hemicellulose B from additional Equisetum species. Other details as in Fig. 5.
Xyloglucan
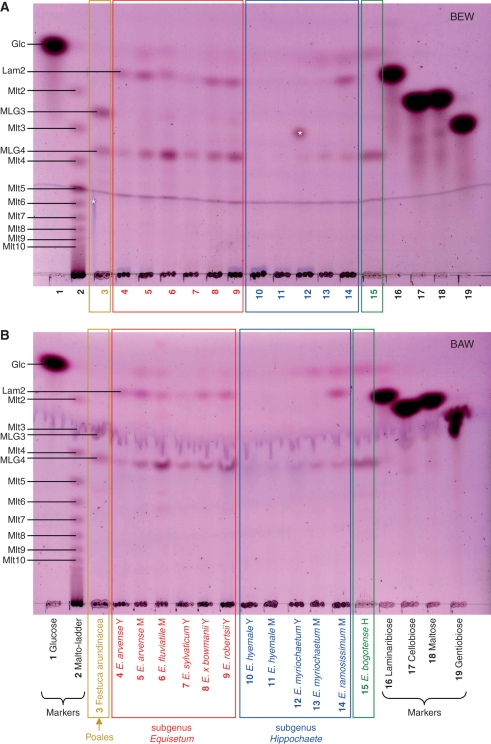
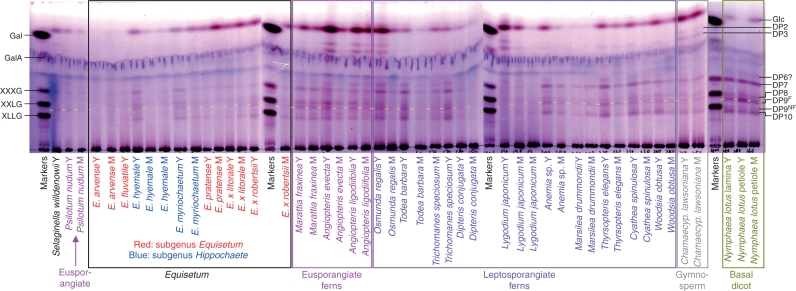
Xyloglucan-derived oligosaccharides were almost undetectable in digests of hemicellulose A (chromatograms not shown). Therefore, only hemicellulose B was analysed in detail. A summary of the yields of hemicellulose B xyloglucan-derived oligosaccharides is given in Table 1. As expected, xyloglucan tended to be more abundant in the hemicellulose B of young tissues than of mature (Fig. 7), the former being richer in primary cell walls. Among the eusporangiate monilophytes, total xyloglucan was scarce in the horsetail subgenus Equisetum and in Psilotum nudum, but abundant in the horsetail subgenus Hippochaete and in the ferns Marattia and Angiopteris. The leptosporangiate ferns varied widely in xyloglucan content: in some (Osmunda, Todea, Trichomanes, Anemia, Thyrsopteris, Cyathea and Woodsia) it was abundant, whereas in others (Dipteris, Lygodium and Marsilea) it was scarce (Fig. 7).
Fig. 7.
Xyloglucan endoglucanase (XEG) digestion products of hemicellulose B from numerous monilophytes and three other vascular plants. The TLCs were developed in butanol/acetic acid/water, and stained with thymol. Markers (labelled at left): Gal, galactose; GalA, galacturonic acid; XXXG, XXLG and XLLG, oligosaccharides of DP 7, 8 and 9, respectively, from tamarind xyloglucan. Size-classes of products (labelled at right) are indicated by their degree of polymerization (DP); e.g. DP7 is a heptasaccharide. DP9F, Nonasaccharides containing a fucose residue; DP9NF, lacking fucose. One residue of fucose (6-deoxy-l-galactose) considerably increases chromatographic mobility compared with an oligosaccharide of similar size lacking a deoxy-sugar. The DP9F band is highlighted with a yellow stripe. Abbreviations: M, mature tissue; Y, young tissue.
In all cases where the yield of xyloglucan oligosaccharides was sufficient for a TLC profile to be clearly discerned, a ‘core pattern’ of five classes of oligosaccharide repeat-unit was similar across the eusporangiate and leptosporangiate monilophytes, resembling that in the seed-plants Chamaecyparus and Nymphaea. The major repeat-unit classes co-migrated with XLFG, XLLG, XXFG, XXLG (and/or XLXG), and XXXG (DP 10, 9NF, 9F, 8 and 7, respectively, where NF and F indicate non-fucosylated and fucosylated), giving a characteristic ‘‖-‖--|’ pattern, where ‘|’ represents a band.
There was taxonomic variation in the intensity of the five bands in the core ‘‖-‖--|’ pattern of xyloglucan oligosaccharides. For example, Marattia, Angiopteris, E. hyemale and Nymphaea exhibited relatively high intensities of the DP9NF fragment. In addition, many species also exhibited a probable DP6 band running slightly faster than XXXG.
A discordant selection of species, upon XEG digestion, also gave two major, unidentified oligosaccharides that appeared to be a di- and trisaccharide of glucose. After purification by gel-permeation chromatography, they gave no isoprimeverose on Driselase digestion, and only glucose on acid hydrolysis. On TLC, the disaccharide and trisaccharide migrated slightly slower than cellobiose and cellotriose, respectively (marker chromatograms, not shown). The disaccharide was also distinguished chromatographically from authentic maltose, isomaltose and gentiobiose, and remains an unidentified product of XEG action. The lack of xylose, and non-identity with cello-oligosaccharides, suggests that the two small oligosaccharides were not derived from any known xyloglucan structure. They were particularly abundant in XEG digests of the eusporangiate fern Angiopteris, two leptosporangiate ferns (Osmunda and Lygodium), and the gymnosperm Chamaecyparis, but were not abundant in any Equisetum species nor in Nymphaea, an early-diverging dicot nested in the basal ‘ANITA’ (Amborella, Nymphaea, Illicium, Trimenia and Austrobaileya) grade according to APGII (2003).
Hemicellulose B preparations from the tested species gave widely varying yields of glucose on XEG digestion, and there was a tendency for glucose to correlate with the yield of the small oligosaccharides mentioned in the previous paragraph. Several authentic polysaccharides (including starch, lichenan and yeast glucan) gave small amounts of glucose on digestion with XEG under the conditions used. Therefore, the glucose detected was not necessarily of xyloglucan origin.
DISCUSSION
Evolution of MLG in Equisetum
The fact that MLG is consistently abundant in the subgenus Equisetum, and scarce or very scarce in E. hyemale and E. myriochaetum but relatively high in E. ramosissimum, suggests fluctuations in MLG biosynthesis during the evolution of the horsetails. Likewise, in the Poales, levels of MLG can vary dramatically between species, subspecies and varieties and even between genetically identical plants grown under different environmental conditions (Stone and Clarke, 1992).
The phylogenetic position of E. bogotense is suggested to be either sister to Hippochaete or basal to the entire genus (Des Marais et al., 2003; Guillon, 2004, 2007). Thus the abundance in E. bogotense of an MLG built almost only of the tetrasaccharide repeat-unit, G4G4G3G, suggests that this structure is a primordial feature of the genus Equisetum. In all other Equisetum species, the disaccharide repeat-unit G3G is also an appreciable component. MLG has been retained abundantly in the subgenus Equisetum, but almost lost in some members of the subgenus Hippochaete. Its reappearance in one Hippochaete species (E. ramosissimum) was in a form with a highly unusual repeat-unit composition, predominantly based on the disaccharide G3G. This may indicate that MLG acquired a subtly different role in this species. However, E. ramosissimum is closely related to E. hyemale (Des Marais et al., 2003; Guillon, 2004, 2007), implying a very rapid evolution of MLG structure.
It has been suggested that the Equisetopsida total group diverged from the Marattiales in the period from mid-Triassic to Upper Palaeozoic (238–295 million years ago) (Des Marais et al., 2003; Pryer et al., 2004). The complete absence of MLG in the extant Marattiales and all other non-Equisetum monilophytes, and its presence in all Equisetum species, would suggest that MLG was acquired later than the Equisetopsida–Marattiales split and at least by the crown group diversification in the Tertiary or Mesozoic. Nevertheless, it cannot be excluded that MLG was originally present in all eusporangiate monilophytes but then lost except in Equisetum. The invention of MLG in the seed plant lineage appears to be an entirely separate event. The Poales date back to the Cretaceous (>65 million years ago) (Bremer, 2002) and poalean MLG is therefore assumed to have been acquired around this time.
The significance of the different tetrasaccharide : trisaccharide : disaccharide ratios in the MLGs of different species is uncertain. An MLG composed mainly of one specific repeat-unit (either trisaccharide or tetrasaccharide) will tend to self-aggregate, thus readily coming out of aqueous solution or forming a stiff gel (Lazaridou et al., 2004). Such MLGs are those of the lichen Cetraria islandica and many Equisetum species (with predominantly the trisaccharide or the tetrasaccharide repeat-unit, respectively). We have indeed noted that pure dried MLG from Equisetum is difficult to redissolve in water. Correspondingly, wheat MLG, which is mainly composed of the trisaccharide repeat-unit (trisaccharide : tetrasaccharide ratio ≈ 4·5 : 1), is less water-soluble than oat MLG, which has a less extreme ratio (trisaccharide : tetrasaccharide ≈ 2 : 1) (Lazaridou et al., 2004; Li et al., 2006).
It may be predicted that MLGs possessing a high proportion of disaccharide repeat-unit (e.g. that of E. ramosissimum) will be highly flexible molecular chains, since the (1 → 3) bond allows free rotation. Where a disaccharide is flanked by two tetrasaccharides, there is a six-glucosyl run with alternating (1 → 3) and (1 → 4) bonds ( … G4G3G4G3G4G … ); such domains would be expected neither to self-aggregate readily nor to hydrogen-bond to the surface of cellulose microfibrils. The self-aggregation and solubility of MLGs and their ability to hydrogen-bond to cellulose are all features that may be expected to influence the biological characteristics of this cell-wall polysaccharide.
Evolution of xyloglucan in the monilophytes
Although small amounts of xyloglucan may be present in mature xylem walls (Mellerowicz et al., 2008), this hemicellulose is mainly a component of primary cell walls. As therefore expected, young tissues were richer in xyloglucan than mature tissues, which possess a higher proportion of secondary walls. Xyloglucan-derived oligosaccharides were detectable in all monilophytes tested, though it was a very minor component in some. In fact, xyloglucan is thought to be present in all land-plants; however, it appears not to be essential for viability in arabidopsis (Cavalier et al., 2008), suggesting that other cell-wall components can replace it functionally when xyloglucan biosynthesis is compromised.
Among the eusporangiate monilophytes, the total xyloglucan content showed interesting taxonomic trends. It was scarce throughout the MLG-rich horsetail subgenus Equisetum, suggesting that MLG and xyloglucan may be capable of serving equivalent roles in primary cell-wall architecture. It was also very low in abundance in Psilotum nudum, which is extremely rich in a different hemicellulose, glucomannan (Popper and Fry, 2004), suggesting that the latter may be able to replace xyloglucan functionally. Indeed, mannose-rich polysaccharides are consistently abundant in the cell walls of eusporangiate monilophytes (Popper and Fry, 2004; Nothnagel and Nothnagel, 2007). On the other hand, xyloglucan was relatively abundant in the MLG-poor horsetail subgenus Hippochaete and in the (MLG-free) eusporangiate ferns Marattia and Angiopteris. Most of the (MLG-free) leptosporangiate ferns also had high xyloglucan contents in their young tissues, although Dipteris, Lygodium and Marsilea had less. It will be interesting to determine whether these last three fern genera functionally replace xyloglucan with a different hemicellulose.
A ‘core pattern’ of five xyloglucan repeat-units, co-migrating on TLC with XLFG, XLLG, XXFG, XXLG and XXXG, was recognizable in all monilophytes studied, resembling that in the two representative seed-plants tested (a gymnosperm and a basal dicot). This core pattern even applies to E. hyemale, which has recently been reported to possess several unusual xyloglucan repeat-units not found in most other plants (Peña et al., 2008). It is likely that TLC in a single solvent system does not separate oligosaccharides containing the L repeat-unit from those in which L is replaced by the chemically similar D, nor those containing F from those in which F is replaced by the chemically similar E (Fig. 2). Thus, the DP10 band in E. hyemale (and possibly other Equisetum spp.; Fig. 7) is expected to include all three of the very similar fucose-containing decasaccharides reported by Peña et al. (2008): XLEG, XLFG and XDEG. Likewise, the DP9F band will include both XXFG and XXEG; the DP9NF band XLDG, XLLG and XDDG; and the DP8 band XXLG, XLXG and XXDG. If the E. hyemale oligosaccharides reported by Peña et al. (2008) are grouped into the five classes that are resolvable by TLC, their data show a DP10 : DP9NF : DP9F : DP8 : DP7 ratio of 23 : 46 : 5 : 22 : 4. In contrast, our TLC patterns (which are similar for young E. hyemale and E. myriochaetum) show a ratio of roughly 24 : 7 : 17 : 23 : 29 (estimated by intensity of thymol staining; Fig. 7). Although our data are only semi-quantitative, they nevertheless show appreciable differences from the ratios found by Peña et al. (2008), especially in exhibiting a higher abundance of the heptasaccharide and the fucose-containing nonasaccharides and much less of the non-fucosylated nonasaccharides such as XLLG.
Interspersed with the five ‘core pattern’ bands are additional bands that show greater taxonomic diversity. For example, many of the species tested also possess a probable hexasaccharide. This may be XXGG, a major repeat-unit of many members of the Poales and Solanales (Hoffman et al., 2005; Fry, 2011), although it was not definitively identified. The DP6 component was found in varying abundance across the monilophytes and in the basal early-diverging dicot Nymphaea, but was least abundant in the genus Equisetum, supporting the view that hemicellulose structures show phylogenetically based variation.
CONCLUSIONS
After confirming that MLG is confined, among all monilophytes tested, to Equisetum, we also show varied MLG structures among the subgenera Equisetum and Hippochaete. The sister group E. bogotense possesses its own potentially ancestral MLG structure, very predominantly composed of the tetrasaccharide repeat-unit, G4G4G3G. Other members of the genus seem to have refined this simple structure, e.g. varying in the tetrasaccharide : disaccharide ratio. Furthermore, MLG was lost as a predominant hemicellulose of the primary cell wall (by E. hyemale and E. ramosissimum) at least once during the adaptive radiation of the genus.
Xyloglucan, in contrast to MLG, is found in all land plants tested, and a ‘core pattern’ of repeat-units (co-migrating with XLFG, XXFG, XXLG/XLXG, XXXG) appears consistently in the TLC profiles of all species tested. However, xyloglucan has become a very minor hemicellulose in some monilophytes – especially Psilotum and the horsetail subgenus Equisetum, which possess abundant alternative hemicelluloses (mannans in both; also MLG in Equisetum).
It is clear that land plants have experimented extensively with the structures and proportions of their hemicelluloses during evolution.
ACKNOWLEDGEMENTS
We thank RBGE horticultural staff Fiona Inch and Andrew Ensoll for the collection of plant materials, and New York Botanic Garden for herbarium material of E. bogotense. We thank Christopher Jeffree and Quentin Cronk for constructive comments on the manuscript. This work was supported by the BBSRC.
LITERATURE CITED
- APG II: The Angiosperm Phyogeny Group. An update of the Angiosperm Phyologeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- Baron R. Notes on the geology of Madagascar: with an appendix on the fossils. Quarterly Journal of the Geological Society. 1889;45:305–331. [Google Scholar]
- Bateman RM. Palaeobiological and phylogenetic implications of anatomically-preserved Archaeocalamites from the Dinantian of Oxroad Bay and Loch Humphrey Burn, southern Scotland. Palaeontographica. 1991;B 223:1–59. [Google Scholar]
- Bremer K. Gondwanan evolution of the grass alliance of families (Poales) Evolution. 2002;56:1374–1387. doi: 10.1111/j.0014-3820.2002.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC. The mechanism of synthesis of a mixed-linkage (1→3), (1→4)-β-d-glucan in maize: evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiology. 1999;120:1105–1116. doi: 10.1104/pp.120.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckeridge MS, Rayon C, Urbanowicz B, Tine MAS, Carpita NC. Mixed linkage (1→3), (1→4)-β-d-glucans of grasses. Cereal Chemistry. 2004;81:115–127. [Google Scholar]
- Carpita NC, McCann MC. The maize mixed-linkage (1→3), (1→4)-β-d-glucan polysaccharide is synthesized at the Golgi membrane. Plant Physiology. 2010;153:1362–1371. doi: 10.1104/pp.110.156158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, et al. Disruption of two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell. 2008;20:1519–1537. doi: 10.1105/tpc.108.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channing A, Zamuner A, Edwards D, Guido D. Equisetum thermale sp. nov. (Equisetales) from the Jurassic San Agustín hot spring deposit, Patagonia: anatomy, paleoecology, and inferred paleoecophysiology. American Journal of Botany. 2011;98:680–697. doi: 10.3732/ajb.1000211. [DOI] [PubMed] [Google Scholar]
- Delevoryas T. Morphology and evolution of fossil plants. New York, NY: Holt, Rinehart and Winston; 1962. [Google Scholar]
- Des Marais DL, Smith AR, Britton DM, Pryer KM. Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcl and trnl-f) International Journal of Plant Sciences. 2003;164:737–751. [Google Scholar]
- Eder M, Tenhaken R, Driouich A, Lütz-Meindl U. Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta) Journal of Phycology. 2008;44:1221–1234. doi: 10.1111/j.1529-8817.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- Ford CW, Percival E. The carbohydrates of Phaeodactylum tricornutum. Part i. Preliminary examination of the organism, and characterisation of low molecular weight material and of a glucan. Journal of the Chemical Society. 1965;1965:7035–7041. [Google Scholar]
- Franková L, Fry SC. Phylogenetic variation in glycosidases and glycanases acting on plant cell wall polysaccharides, and the detection of transglycosidase and trans-β-xylanase activities. The Plant Journal. 2011;67:662–681. doi: 10.1111/j.1365-313X.2011.04625.x. [DOI] [PubMed] [Google Scholar]
- Fry SC. The growing plant cell wall: chemical and metabolic analysis. Reprint edn. Caldwell, NJ: The Blackburn Press; 2000. [Google Scholar]
- Fry SC. Encyclopedia of applied plant sciences. Oxford: Academic Press; 2003. Cell walls; pp. 75–87. [Google Scholar]
- Fry SC. Cell wall polysaccharide composition and covalent crosslinking. In: Ulvskov P, editor. Annual plant reviews: plant polysaccharides, biosynthesis and bioengineering. Vol. 41. Oxford: Blackwell Publishing; 2011. [Google Scholar]
- Fry SC, York WS, Albersheim P, et al. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiologia Plantarum. 1993;89:1–3. [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BHWA, Franková L. Mixed-linkage β-glucan: xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. The Plant Journal. 2008a;55:240–252. doi: 10.1111/j.1365-313X.2008.03504.x. [DOI] [PubMed] [Google Scholar]
- Fry SC, Nesselrode BHWA, Miller JG, Mewburn BR. Mixed-linkage (1→3,1→4)-β-d-glucan is a major hemicellulose of Equisetum (horsetail) cell walls. New Phytologist. 2008b;179:104–115. doi: 10.1111/j.1469-8137.2008.02435.x. [DOI] [PubMed] [Google Scholar]
- Graham LE, Wilcox L. Algae. San Francisco, CA: Benjamin Cummings; 1999. [Google Scholar]
- Guillon JM. Phylogeny of horsetails (Equisetum) based on the chloroplast rps4 gene and adjacent noncoding sequences. Systematic Botany. 2004;29:251–259. [Google Scholar]
- Guillon JM. Molecular phylogeny of horsetails (Equisetum) including chloroplast atpb sequences. Journal of Plant Research. 2007;120:569–574. doi: 10.1007/s10265-007-0088-x. [DOI] [PubMed] [Google Scholar]
- Harris PJ. Diversity in plant cell walls. In: Henry RJ, editor. Plant diversity and evolution: genotypic and phenotypic variation in higher plants. Wallingford, UK: CAB International; 2005. pp. 201–227. [Google Scholar]
- Hauke RL. Ann Arbor, MI: PhD Thesis, University of Michigan; 1959. A taxonomic monograph of the genus Equisetum subgenus Hippochaete. [Google Scholar]
- Hauke RL. Taxonomic monograph of Equisetum subgenus Equisetum. Nova Hedwigia. 1978;30:385–455. [Google Scholar]
- Hoffman M, Jia Z, Peña MJ, et al. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydrate Research. 2005;340:1826–1840. doi: 10.1016/j.carres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature. 1997a;389:33–39. [Google Scholar]
- Kenrick P, Crane PR. The origin and early diversification of land plants: a cladistic study. Smithsonian Series in Comparative Evolutionary Biology. Washington, DC: Smithsonian Institution Press; 1997b. [Google Scholar]
- Lazaridou A, Biliaderis CG. Cryogelation phenomena in mixed skim milk powder–barley β-glucan–polyol aqueous dispersions. Food Research International. 2007;40:793–802. [Google Scholar]
- Lazaridou A, Biliaderis CG, Micha-Screttas M, Steele BR. A comparative study on structure–function relations of mixed-linkage (1→3), (1→4) linear β-d-glucans. Food Hydrocolloids. 2004;18:837–855. [Google Scholar]
- Li W, Cui SW, Kakuda Y. Extraction, fractionation, structural and physical characterization of wheat β-d-glucans. Carbohydrate Polymers. 2006;63:408–416. [Google Scholar]
- Ligrone R, Vaughn KC, Renzaglia KS, Knox JP, Duckett JG. Diversity in the distribution of polysaccharide and glycoprotein epitopes in the cell walls of bryophytes: new evidence for the multiple evolution of water-conducting cells. New Phytologist. 2002;156:491–508. doi: 10.1046/j.1469-8137.2002.00538.x. [DOI] [PubMed] [Google Scholar]
- Luttenegger DG, Nevins DJ. Transient nature of a (1→3), (1→4)-β-glucan in Zea mays coleoptile cell walls. Plant Physiology. 1985;77:175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, et al. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes: implications for the evolution of vascular plants. Plant Physiology. 2004;134:339–351. doi: 10.1104/pp.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle PJ, Hoogenraad NJ, Bonig I, Clarke AE, Stone BA. A (1→3,1→4)-β-glucan-specific monoclonal antibody and its use in the quantitation and immunocytochemical location of (1→3,1→4)-β-glucans. The Plant Journal. 1994;5:1–9. doi: 10.1046/j.1365-313x.1994.5010001.x. [DOI] [PubMed] [Google Scholar]
- Mellerowicz EJ, Immerzeel P, Hayashi T. Xyloglucan: the molecular muscle of trees. Annals of Botany. 2008;102:659–665. doi: 10.1093/aob/mcn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Z, Sharon N. Cell wall of Peridinium westii: a non cellulosic glucan. Biochimica et Biophysica Acta. 1969;173:161–175. doi: 10.1016/0005-2736(69)90099-6. [DOI] [PubMed] [Google Scholar]
- Nothnagel AL, Nothnagel EA. Primary cell wall structure in the evolution of land plants. Journal of Integrative Plant Biology. 2007;49:1271–1278. [Google Scholar]
- Pauly M, Anderson LN, Kaupinnen S, Kofod LV, Darvill AG. A xyloglucan specific endo-β-1,4-glucanase from Aspergillus aculeatus: expression in yeast, purification and characterisation of the recombinant enzyme. Glycobiology. 1999;9:93–100. doi: 10.1093/glycob/9.1.93. [DOI] [PubMed] [Google Scholar]
- Peña MJ, Darvill AG, Eberhard S, York WS, O'Neill MA. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology. 2008;18:891–904. doi: 10.1093/glycob/cwn078. [DOI] [PubMed] [Google Scholar]
- Popper ZA. Evolution and diversity of green plant cell walls. Current Opinion in Plant Biology. 2008;11:286–292. doi: 10.1016/j.pbi.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. Primary cell wall composition of bryophytes and charophytes. Annals of Botany. 2003;91:1–12. doi: 10.1093/aob/mcg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Fry SC. Primary cell wall composition of pteridophytes and spermatophytes. New Phytologist. 2004;164:165–174. doi: 10.1111/j.1469-8137.2004.01146.x. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Sadler IH, Fry SC. 3-O-Methyl-d-galactose residues in lycophyte primary cell walls. Phytochemistry. 2001;57:711–719. doi: 10.1016/s0031-9422(01)00140-6. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schneider H, Smith AR, et al. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature. 2001;409:618–622. doi: 10.1038/35054555. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany. 2004;91:1582–1598. doi: 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- Smith BG, Harris PJ. Biochemical Systematics and Ecology. Vol. 27. Poaceae cell walls are not unique; 1999. The polysaccharide composition of Poales cell walls; pp. 33–53. [Google Scholar]
- Sørensen I, Pettolino FA, Wilson SM, et al. Mixed-linkage (1→3,1→4)-β-d-glucan is not unique to the Poales and is an abundant component of Equisetum arvense cell walls. The Plant Journal. 2008;54:510–521. doi: 10.1111/j.1365-313X.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- Sørensen I, Domozych D, Willats WGT. How have plant cell walls evolved? Plant Physiology. 2010;153:366–372. doi: 10.1104/pp.110.154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stace CA. Plant taxonomy and biosystematics. 2nd edn. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Stanich NA, Rothwell GW, Stockey RA. Phylogenetic diversification of Equisetum (Equisetales) as inferred from lower cretaceous species of British Columbia, Canada. American Journal of Botany. 2009;96:1289–1299. doi: 10.3732/ajb.0800381. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Comparative aspects of plant morphogenesis: a cellular, molecular and evolutionary approach. American Journal of Botany. 1992;79:589–598. [Google Scholar]
- Stone BA, Clarke AE. Victoria, Australia: La Trobe University Press; 1992. Chemistry and biology of (1→3)-β-glucans. Bundoora. [Google Scholar]
- Trethewey JAK, Campbell LM, Harris PJ. (1→3), (1→4)-β-d-glucans in the cell walls of the Poales (sensu lato): an immunogold labeling study using a monoclonal antibody. American Journal of Botany. 2005;92:1660–1674. doi: 10.3732/ajb.92.10.1660. [DOI] [PubMed] [Google Scholar]
- Woodward JR, Fincher GB, Stone BA. Water-soluble (1→3), (1→4)-β-d-glucans from barley (Hordeum vulgare) endosperm. II. Fine structure. Carbohydrate Polymers. 1983;3:207–225. [Google Scholar]