Abstract
Unilateral absence of a pulmonary artery (UAPA) is a rare condition with an estimated prevalence of 1 in 200,000 young adults. Most commonly, UAPA occurs in conjunction with cardiovascular abnormalities such as tetralogy of Fallot or cardiac septal defects, but it can also occur in an isolated manner. Patients with isolated UAPA can remain asymptomatic into late adulthood but usually report symptoms such as dyspnea or chest pain or suffer from hemoptysis or recurrent infections. Diagnosis can be difficult due to the rarity of the condition and its nonspecific presentation. We present a case of a 61-year-old man who presented for lung transplant evaluation and was found to have UAPA. Typical findings on chest radiograph, strategies for diagnosis, and available treatments are discussed.
CASE PRESENTATION
An obese 61-year-old man with a body mass index of 38 kg/m2 was referred to Baylor University Medical Center at Dallas with a diagnosis of a restrictive lung disease of unknown etiology. The patient reported a long history of exercise intolerance, recently complicated by dyspnea at rest. He also had hypertension, hypercholesterolemia, and diabetes mellitus type 2, but denied past or present use of tobacco products.
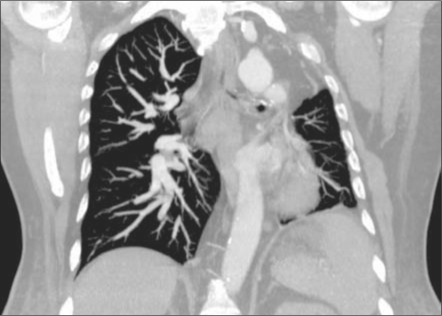
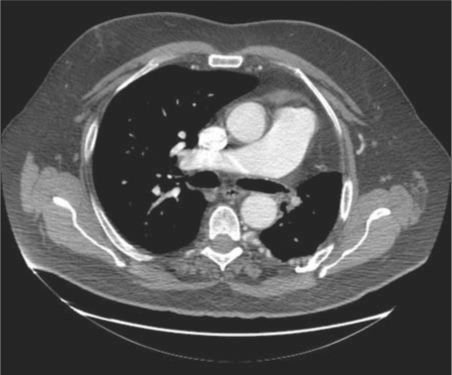
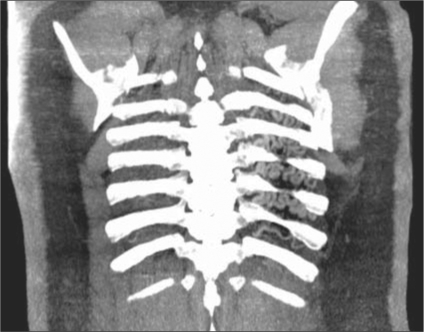
A computed tomography (CT) pulmonary angiogram showed asymmetric lung fields, with a left hemithorax that was smaller than the right. The left lung also appeared hyperlucent, while the right lung appeared plethoric. The left hemidiaphragm was elevated. Cross-sectional CT images demonstrated a hypoplastic left lung and an absent left main pulmonary artery. No evidence of embolic occlusion or surgical changes was present. Distal intrapulmonary branches of the left pulmonary artery were visible, and some of these vessels could be seen communicating across the pleura with bronchial and intercostal vessels arising from the distal thoracic aorta (Figures 1–4).
Figure 1.
Coronal CT of the chest with intravenous contrast in a lung window shows a hypovascular and hypoplastic left lung. The left hemidiaphragm is elevated.
Figure 4.
Coronal CT of the chest with intravenous contrast in a soft tissue window shows large, tortuous intercostal arteries in the patient's left chest wall.
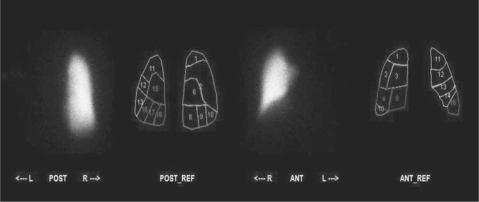
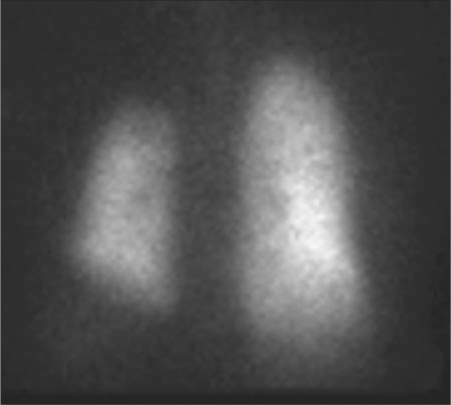
Ventilation perfusion scanning demonstrated absent perfusion of the left lung (Figure 5). Perfusion to the right lung was normal. The Xenon-133 wash-in images revealed decreased left lung volume and homogenous filling of the right lung (Figure 6). There was no abnormal Xenon-133 retention during the washout phase. Based on the Xenon-133 early wash-in phase, the right lung contributed 68% to ventilation, while the left lung contributed 32%. Based on the geometric mean analysis of anterior and posterior images, the right lung received 100% of pulmonary perfusion.
Figure 5.
Anterior and posterior technetium 99m macroaggregate albumin perfusion images with a segmental reference show the complete absence of perfusion to the left lung. Perfusion to the right lung is normal without evidence of segmental defect.
Figure 6.
Xenon-133 wash-in images show decreased left lung volume and homogenous filling of the right lung. There was no abnormal Xenon-133 retention during the washout phase (not shown).
The patient's pulmonologist was informed of the diagnosis of unilateral absence of a pulmonary artery (UAPA), and subsequent examinations were scheduled. Unfortunately, the patient failed to report for testing and was ultimately lost to follow-up.
DISCUSSION
UAPA is a rare condition, with an estimated prevalence of 1 in 200,000 young adults (1). Most commonly, UAPA occurs in conjunction with cardiovascular abnormalities such as tetralogy of Fallot or cardiac septal defects, but it can also occur in an isolated manner (1, 2). Isolated UAPA involves the right lung in about two thirds of cases (3). Due to embryologic relationships, UAPA commonly occurs on the side of the chest opposite the aortic arch (although that was not the case in our patient) (4). The exact embryologic cause of UAPA is a matter of debate and is likely different in left- vs. right-sided UAPA. In both cases, however, altered development of a sixth aortic arch segment is thought to result in a ductal origin to a pulmonary artery that leads to the proximal interruption of that vessel when the ductal tissue regresses at the time of birth (4). Distal intrapulmonary branches of the affected artery usually remain intact and can be supplied by collateral vessels from bronchial, intercostal, internal mammary, subdiaphragmatic, subclavian, or even coronary arteries (5, 6).
Patients with isolated UAPA can present in a variety of ways. A 2002 review of 108 cases of UAPA revealed a median age of presentation of 14 years (3). The combination of chest pain, pleural effusion, and recurrent infections was present in 37% of patients, while dyspnea or exercise intolerance was present in 40% of patients. Pulmonary hypertension was found in 44% of patients that were tested for the disorder. Hemoptysis occurred in about 20% of patients, and high-altitude pulmonary edema was seen in approximately 10% of patients (3). Seven deaths were noted in the case series and included mortality from massive pulmonary hemorrhage, right heart failure, respiratory failure, pulmonary hypertension, and high-altitude pulmonary edema. Only 14 of 108 patients with isolated UAPA were asymptomatic at the time of their diagnosis and throughout variable follow-up (3).
At the time of our patient's presentation, he reported dyspnea at rest and exercise intolerance. UAPA could definitely be a cause of his dyspnea, and it should be noted that his left lung received 32% of total ventilation on his ventilation perfusion scan but no detectable perfusion, resulting in a large amount of dead space. Pulmonary hypertension could also be a cause for the patient's dyspnea, and indeed, the patient's main pulmonary artery was enlarged, measuring 3.7 cm on his CT pulmonary angiogram (Figure 2). However, that study showed no signs of secondary right heart strain. An echocardiogram was ordered but not performed before the patient was lost to follow-up. Other possible causes of dyspnea and exercise intolerance include obesity and deconditioning.
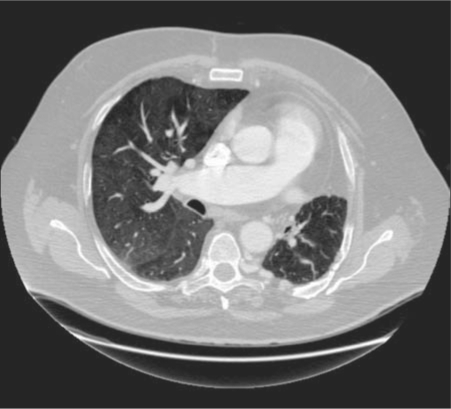
Figure 2.
Transaxial CT of the chest with intravenous contrast in a soft tissue window shows an absent left pulmonary artery. The main pulmonary artery measures 3.7 cm, compatible with pulmonary hypertension. The left lung is hypoplastic, and the mediastinum is shifted to the left. There is no evidence of chronic pulmonary embolism, obstructing malignancy, or surgical changes. There are large extrapleural collateral vessels in the left posterior hemithorax.
Mechanisms have been proposed for many of the common sequelae of UAPA. Pulmonary hypertension may result from blood flow directed away from the absent pulmonary artery to the remaining pulmonary artery. Increased blood flow in the contralateral pulmonary artery leads to shear stress on the endothelium, which results in the release of vasoconstrictive compounds such as endothelin (7). Chronic vasoconstriction of the pulmonary arterioles may lead to remodeling that will cause increased resistance in the pulmonary vasculature and pulmonary hypertension (7). Patients who have developed pulmonary hypertension as a result of UAPA may present with symptoms such as shortness of breath, weight gain, and poor exercise tolerance. Signs of pulmonary hypertension detected on exam may include a loud P2, tricuspid insufficiency, or a parasternal heave. If the pulmonary hypertension has led to right heart failure, jugular venous distension, peripheral edema, hepatojugular reflux, and ascites may also be present.
The etiology of recurrent infections observed in patients with UAPA is likely multifactorial. Lack of arterial blood flow to the affected lung may result in poor delivery of inflammatory cells to sites of inflammation and impair ciliary function (5). In addition, poor blood flow to the affected lung may result in alveolar hypocapnia, leading to secondary bronchoconstriction and mucous trapping (5). Chronic infection can lead to bronchiectasis in some patients (5, 8).
Hemoptysis is a potentially serious complication of UAPA. Hemoptysis appears to be caused by large collateral circulations that subject venous systems to unusually high pressures. While hemoptysis can be chronic and self-limited, cases of massive hemoptysis have been reported in the literature (5, 9, 10).
Diagnosing UAPA can be difficult, but important clues are present in chest radiographs. The chest radiograph of patients with UAPA typically shows asymmetric lung fields, with an ipsilateral small hemithorax holding a hyperlucent lung (1, 8). The mediastinum will be shifted towards the affected side, and the hilar vasculature on that side will be absent or greatly diminished. The ipsilateral hemidiaphragm may be elevated. Extensive transpleural collateral circulation in the apices of the lung may mimic tuberculosis by producing an appearance known as pulmonary pseudofibrosis (1). The contralateral lung may be hyperinflated beyond the midline and appear plethoric due to increased blood flow.
When suspicious findings are noted on a chest radiograph, the diagnosis of UAPA can be definitively made by CT, magnetic resonance imaging (MRI), or transthoracic echocardiogram. On cross-sectional imaging, the absent pulmonary artery will typically terminate within 1 cm of its expected origin from the main pulmonary artery (1). Other findings that may be noted on CT or MRI include intact peripheral branches of the pulmonary artery, variable collateral circulation, mosaic parenchymal changes, and bronchiectasis secondary to recurrent infections (1, 5, 8).
Transthoracic echocardiogram can also be used to diagnose UAPA and is advantageous because the examiner can look for coexisting cardiac malformations at the same time. Angiography is considered the gold standard for the diagnosis of UAPA but is invasive and typically unnecessary, unless it is being used as a preoperative test for a patient who has developed hemoptysis or severe infection (8). Ventilation perfusion scanning is not necessary for the diagnosis of UAPA, but if done will show normal or diffusely diminished Xenon-127 uptake during the wash-in and equilibrium phase, coupled with absent or greatly diminished perfusion in the affected lung (1). Xenon washout typically shows no delay (1).
There is currently no consensus concerning treatment of patients with UAPA. Some authors have recommended using serial echocardiography to monitor asymptomatic adults for the development of pulmonary hypertension (11). Patients who develop pulmonary hypertension can be treated medically with vasodilator therapy (3, 7). Alternatively, revascularization of peripheral branches of the affected pulmonary artery to the pulmonary hilum can be attempted, and there are reports of successful revascularization procedures, mostly in the pediatric population (2, 12, 13). Hemoptysis may be treated with embolization, lobectomy, or pneumonectomy (9, 10). Embolization is a relatively safe procedure with few side effects and is a viable alternative to pneumonectomy in patients experiencing hemoptysis (9). Severe infections may require lobectomy or pneumonectomy, and any pulmonary surgery in a patient with UAPA may be complicated by the presence of systemic collaterals (5).
Figure 3.
Transaxial CT of the chest with intravenous contrast in a lung window shows distal branches of the left pulmonary artery that inappropriately communicate across the pleura with intercostal arteries. Once again, no proximal left pulmonary artery is noted. The left lung is hypoattenuating compared to a plethoric right lung.
References
- 1.Bouros D, Pare P, Panagou P, Tsintiris K, Siafakas N. The varied manifestation of pulmonary artery agenesis in adulthood. Chest. 1995;108(3):670–676. doi: 10.1378/chest.108.3.670. [DOI] [PubMed] [Google Scholar]
- 2.Presbitero P, Bull C, Haworth SG, de Leval MR. Absent or occult pulmonary artery. Br Heart J. 1984;52(2):178–185. doi: 10.1136/hrt.52.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ten Harkel AD, Blom NA, Ottenkamp J. Isolated unilateral absence of a pulmonary artery: a case report and review of the literature. Chest. 2002;122(4):1471–1477. doi: 10.1378/chest.122.4.1471. [DOI] [PubMed] [Google Scholar]
- 4.Pfefferkorn JR, Löser H, Pech G, Toussaint R, Hilgenberg F. Absent pulmonary artery. A hint to its embryogenesis. Pediatr Cardiol. 1982;3(4):283–286. doi: 10.1007/BF02427028. [DOI] [PubMed] [Google Scholar]
- 5.Kadir IS, Thekudan J, Dheodar A, Jones MT, Carroll KB. Congenital unilateral pulmonary artery agenesis and aspergilloma. Ann Thorac Surg. 2002;74(6):2169–2171. doi: 10.1016/s0003-4975(02)03979-6. [DOI] [PubMed] [Google Scholar]
- 6.Bockeria LA, Makhachev OA, Khiriev TK, Abramyan MA. Congenital isolated unilateral absence of pulmonary artery and variants of collateral blood supply of the ipsilateral lung. Interact Cardiovasc Thorac Surg. 2011;12(3):509–510. doi: 10.1510/icvts.2010.250795A. [DOI] [PubMed] [Google Scholar]
- 7.Shostak E, Sarwar A. A 50-year-old woman with dyspnea, lower extremity edema, and volume loss of the right hemithorax. Chest. 2009;136(2):628–632. doi: 10.1378/chest.09-0080. [DOI] [PubMed] [Google Scholar]
- 8.Griffin N, Mansfield L, Redmond KC, Dusmet M, Goldstraw P, Mittal TK, Padley S. Imaging features of isolated unilateral pulmonary artery agenesis presenting in adulthood: a review of four cases. Clin Radiol. 2007;62(3):238–244. doi: 10.1016/j.crad.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Reñé M, Sans J, Dominguez J, Sancho C, Valldeperas J. Unilateral pulmonary artery agenesis presenting with hemoptysis: treatment by embolization of systemic collaterals. Cardiovasc Intervent Radiol. 1995;18(4):251–254. doi: 10.1007/BF00239422. [DOI] [PubMed] [Google Scholar]
- 10.Bekoe S, Pellegrini RV, DiMarco RF, Jr, Grant KJ, Woelfel GF. Pneumonectomy for unremitting hemoptysis in unilateral absence of pulmonary artery. Ann Thorac Surg. 1993;55(6):1553–1554. doi: 10.1016/0003-4975(93)91108-y. [DOI] [PubMed] [Google Scholar]
- 11.Turner DR, Vincent JA, Epstein ML. Isolated right pulmonary artery discontinuity. Images Paediatr Cardiol. 2000;4:24–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Welch K, Hanley F, Johnston T, Cailes C, Shah MJ. Isolated unilateral absence of right proximal pulmonary artery: surgical repair and follow-up. Ann Thorac Surg. 2005;79(4):1399–1402. doi: 10.1016/j.athoracsur.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Toews WH, Pappas G. Surgical management of absent right pulmonary artery with associated pulmonary hypertension. Chest. 1983;84(4):497–499. doi: 10.1378/chest.84.4.497. [DOI] [PubMed] [Google Scholar]