Abstract
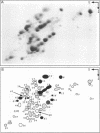
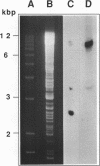
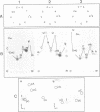
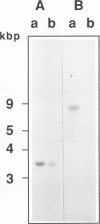
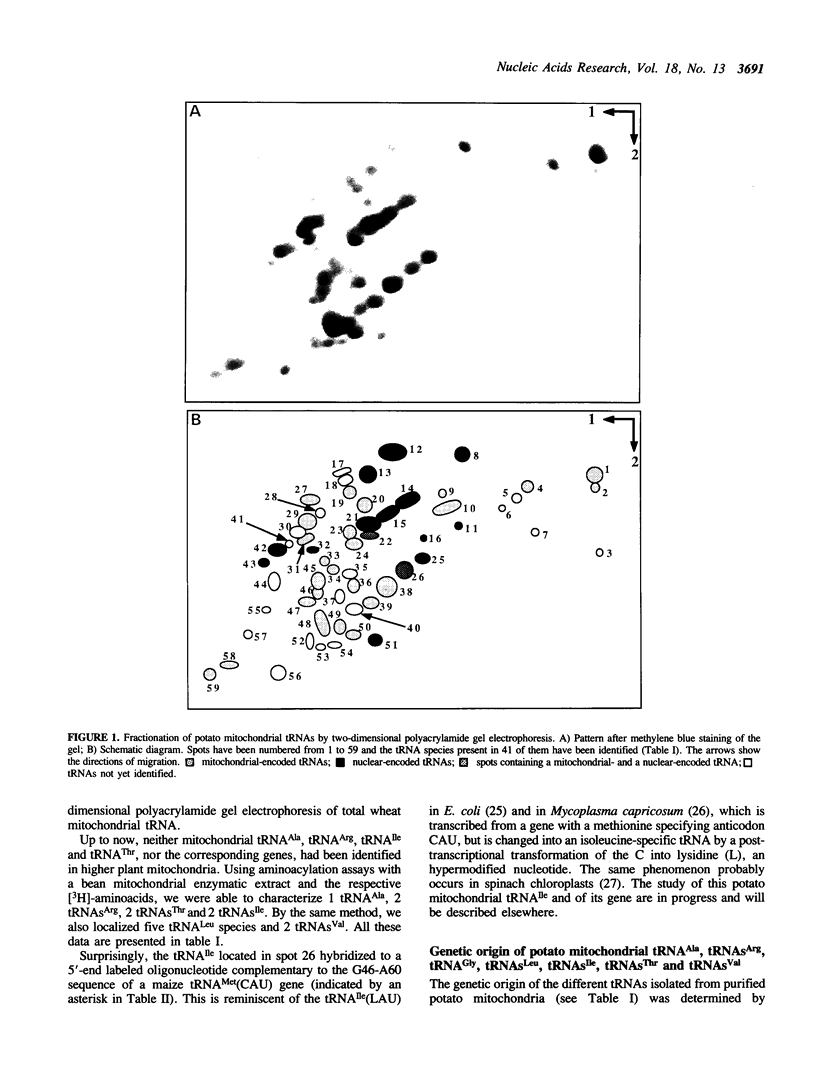
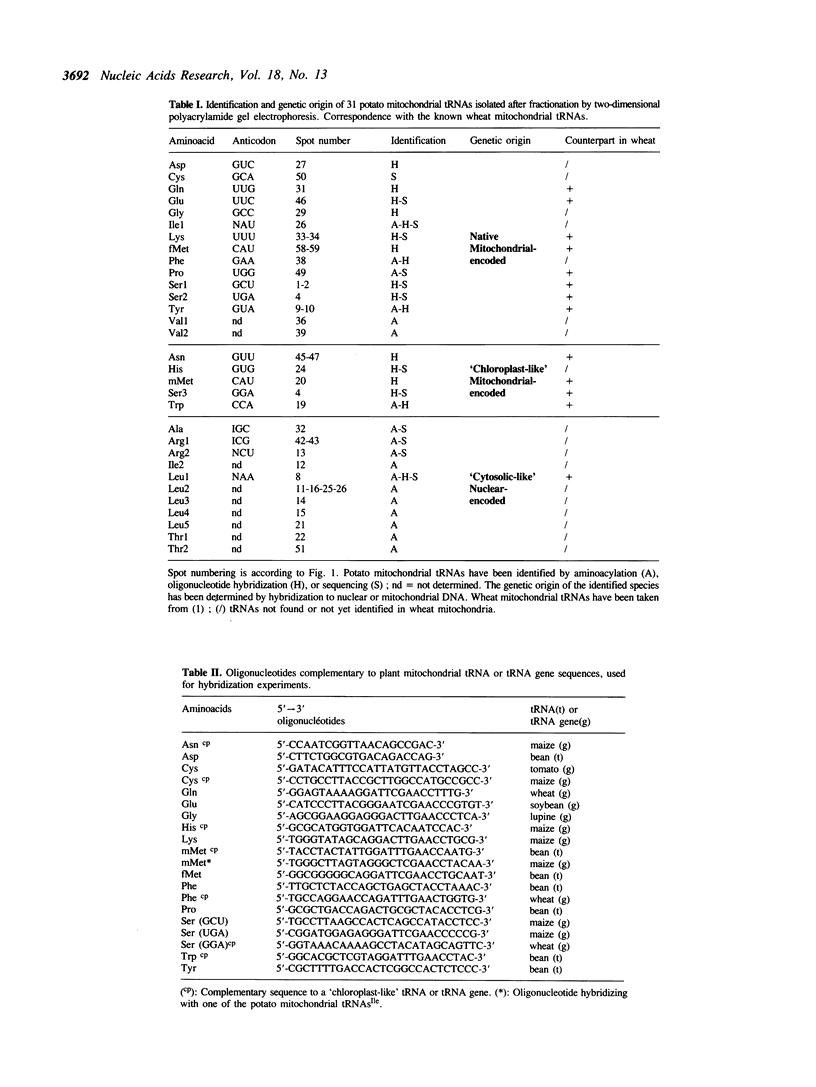
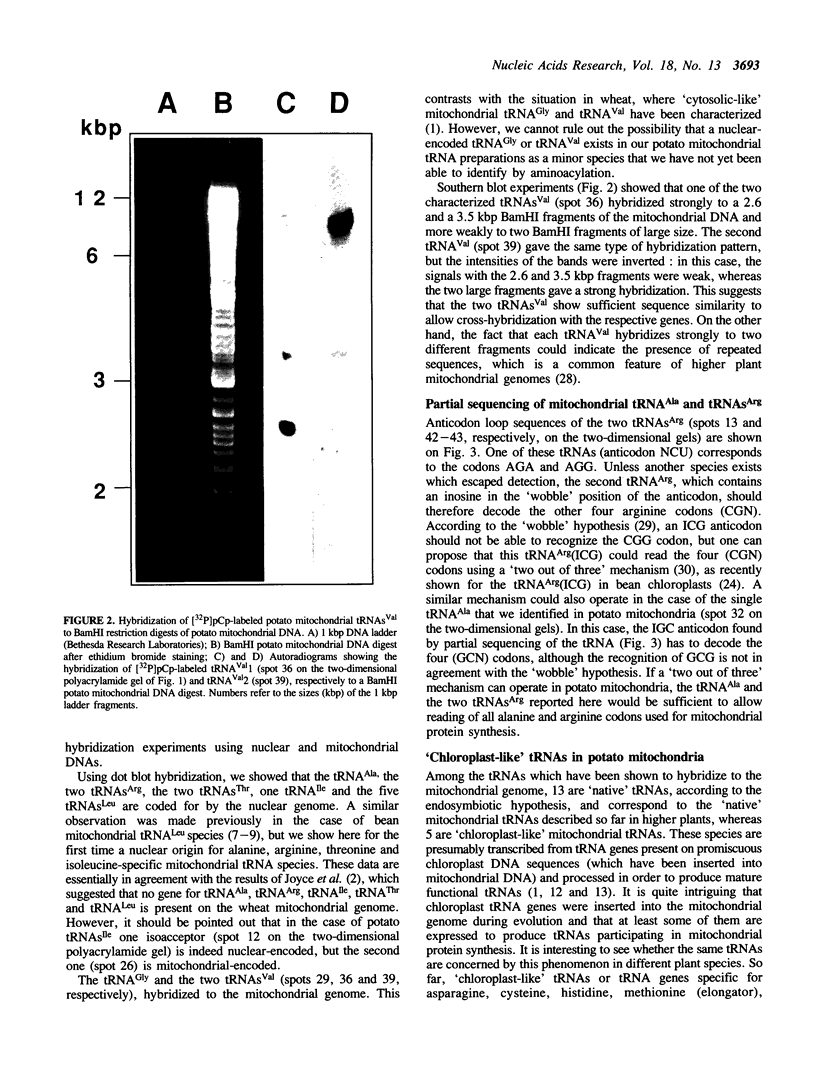
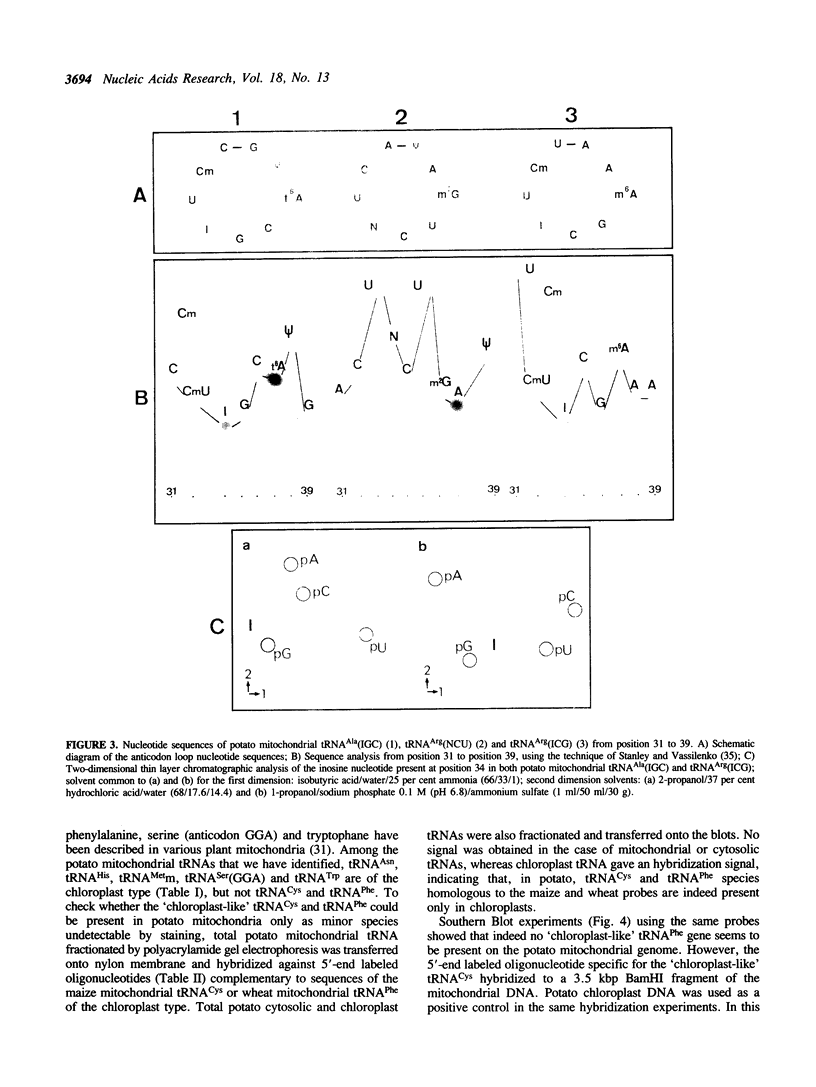
Total transfer RNAs were extracted from highly purified potato mitochondria. From quantitative measurements, the in vivo tRNA concentration in mitochondria was estimated to be in the range of 60 microM. Total potato mitochondrial tRNAs were fractionated by two-dimensional polyacrylamide gel electrophoresis. Thirty one individual tRNAs, which could read all sense codons, were identified by aminoacylation, sequencing or hybridization to specific oligonucleotides. The tRNA population that we have characterized comprises 15 typically mitochondrial, 5 'chloroplast-like' and 11 nuclear-encoded species. One tRNA(Ala), 2 tRNAs(Arg), 1 tRNA(Ile), 5 tRNAs(Leu) and 2 tRNAs(Thr) were shown to be coded for by nuclear DNA. A second, mitochondrial-encoded, tRNA(Ile) was also found. Five 'chloroplast-like' tRNAs, tRNA(Trp), tRNA(Asn), tRNA(His), tRNA(Ser)(GGA) and tRNA(Met)m, presumably transcribed from promiscuous chloroplast DNA sequences inserted in the mitochondrial genome, were identified, but, in contrast to wheat (1), potato mitochondria do not seem to contain 'chloroplast-like' tRNA(Cys) and tRNA(Phe). The two identified tRNAs(Val), as well as the tRNA(Gly), were found to be coded for by the mitochondrial genome, which again contrasts with the situation in wheat, where the mitochondrial genome apparently contains no tRNA(Val) or tRNA(Gly) gene (2).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Bartnik E., Borsuk P. A glycine tRNA gene from lupine mitochondria. Nucleic Acids Res. 1986 Mar 11;14(5):2407–2407. doi: 10.1093/nar/14.5.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burkard G., Guillemaut P., Weil J. H. Comparative studies of the tRNA's and the aminoacyl-tRNA synthetases from the cytoplasm and the chloroplasts of Phaseolus vulgaris. Biochim Biophys Acta. 1970 Nov 12;224(1):184–198. doi: 10.1016/0005-2787(70)90632-5. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Francis M. A., Dudock B. S. Nucleotide sequence of a spinach chloroplast isoleucine tRNA. J Biol Chem. 1982 Oct 10;257(19):11195–11198. [PubMed] [Google Scholar]
- Gray M. W., Boer P. H. Organization and expression of algal (Chlamydomonas reinhardtii) mitochondrial DNA. Philos Trans R Soc Lond B Biol Sci. 1988 May 31;319(1193):135–147. doi: 10.1098/rstb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Cedergren R., Abel Y., Sankoff D. On the evolutionary origin of the plant mitochondrion and its genome. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2267–2271. doi: 10.1073/pnas.86.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. The evolutionary origins of organelles. Trends Genet. 1989 Sep;5(9):294–299. doi: 10.1016/0168-9525(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Izuchi S., Sugita M. Nucleotide sequence of a tomato mitochondrial tRNA(Cvs) (GCA) gene. Nucleic Acids Res. 1989 Feb 11;17(3):1248–1248. doi: 10.1093/nar/17.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Chloroplast-like transfer RNA genes expressed in wheat mitochondria. Nucleic Acids Res. 1989 Jul 25;17(14):5461–5476. doi: 10.1093/nar/17.14.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist U. "Two out of three": an alternative method for codon reading. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1759–1762. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Guillemaut P. Nucleotide sequence of bean mitochondrial tRNALeu4 and of its cytoplasmic counterpart. Re-examination of the modified nucleotide present at position 12 in bean mitochondrial and cytoplasmic tRNALeu1 sequences. Nucleic Acids Res. 1988 Dec 23;16(24):11812–11812. doi: 10.1093/nar/16.24.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Neuburger M., Guillemaut P., Douce R., Weil J. H., Dietrich A. A nuclear-encoded potato (Solanum tuberosum) mitochondrial tRNA(Leu) and its cytosolic counterpart have identical nucleotide sequences. FEBS Lett. 1990 Mar 26;262(2):170–172. doi: 10.1016/0014-5793(90)80181-h. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal L., Guillemaut P., Grienenberger J. M., Jeannin G., Weil J. H. Sequence and codon recognition of bean mitochondria and chloroplast tRNAsTrp: evidence for a high degree of homology. Nucleic Acids Res. 1985 Jun 25;13(12):4411–4416. doi: 10.1093/nar/13.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988 Nov 10;336(6195):179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Pillay D. T., Guillemaut P., Weil J. H. Nucleotide sequences of three soybean chloroplast tRNAsLeu and re-examination of bean chloroplast tRNA2Leu sequence. Nucleic Acids Res. 1984 Mar 26;12(6):2997–3001. doi: 10.1093/nar/12.6.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R. Structural variation in mitochondrial DNA. Adv Genet. 1984;22:1–108. doi: 10.1016/s0065-2660(08)60038-3. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Wintz H., Grienenberger J. M., Weil J. H., Lonsdale D. M. Location and nucleotide sequence of two tRNA genes and a tRNA pseudo-gene in the maize mitochondrial genome: evidence for the transcription of a chloroplast gene in mitochondria. Curr Genet. 1988 Mar;13(3):247–254. doi: 10.1007/BF00387771. [DOI] [PubMed] [Google Scholar]