Abstract
Opioid conjugate vaccines have shown promise in attenuating the behavioral effects of heroin or morphine in animals. The goal of this study was to extend this approach to oxycodone (OXY), a commonly abused prescription opioid. Haptens were generated by adding tetraglycine (Gly)4 or hemisuccinate (HS) linkers at the 6-position of OXY. Immunization of rats with OXY(Gly)4 conjugated to the carrier proteins bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) produced high-titer antibodies to OXY and its metabolite oxymorphone with substantially lower affinities for other structurally related opioid agonists and antagonists. There was no measurable binding of antibody by the (Gly)4 linker alone or off-target opioids methadone and buprenorphine. OXY(HS) conjugates were less immunogenic despite achieving protein haptenation ratios comparable to OXY(Gly)4-BSA. In rats given a single intravenous dose of OXY, immunization with OXY(Gly)4-KLH increased OXY protein binding and retention in serum while decreasing its unbound (free) concentration in plasma and distribution to brain. Vaccine efficacy correlated with serum antibody titers, and it was greatest in rats given the lowest OXY dose (0.05 mg/kg) but was significant even after a larger OXY dose (0.5 mg/kg), equivalent to the high end of the therapeutic range in humans. These effects of OXY(Gly)4-KLH on drug disposition were comparable to those of nicotine or cocaine vaccines that are in clinical trials as addiction treatments. Immunization with OXY(Gly)4-KLH also reduced OXY analgesia in a thermal nociception test. These data support further study of vaccination with the OXY(Gly)4-KLH immunogen as a potential treatment option for OXY abuse or addiction.
Introduction
There are an estimated 15 million users of illicit opioids worldwide (http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lo-res.pdf) and 1.2 million heroin users in the United States (http://oas.samhsa.gov/NSDUH/2k10NSDUH/2k10Results.htm). Until recently heroin use predominated in the United States, but over the past 10 years the abuse of prescription opioids has increased dramatically and is now more common than heroin abuse. The rise in prescription opioid abuse has been accompanied by a substantial increase in the incidence of emergency-department visits and fatal opioid overdoses. Oxycodone (OXY) is the most commonly abused prescription opioid (Compton and Volkow, 2006; Lopez et al., 2009). Treatment options have been developed for heroin addiction, but fewer options have been studied for abuse of OXY or other prescription opioids. Agonist therapies for heroin addiction such as methadone and buprenorphine can be very effective, but their own abuse potential and risk of side effects obligate careful and frequent monitoring, and their therapeutic use is legally restricted to those regularly using substantial quantities of opioid over a sustained period of time (Fareed et al., 2011). Many prescription opioid abusers do not fit this profile because their opioid use is oral rather than intravenous and may be sporadic, yet they still run the risk of overdose, social disruption, and transition to intravenous drug use and addiction. Additional treatment options for prescription opioid abuse are needed (Stotts et al., 2009; Dodrill et al., 2011; Maxwell, 2011).
Vaccines are being studied as a potential adjunct to drug abuse or addiction treatment. They are of interest because they target the drug rather than the brain and therefore lack central nervous system side effects. Addictive drugs are too small to stimulate an immune response but can be rendered immunogenic by conjugation to a foreign carrier protein through a linker arm (Chi, 2011). Such conjugate vaccines stimulate the production of drug-specific antibodies that can bind their target drug in serum and extracellular fluid and reduce or slow its distribution to brain. Efficacy in blocking a wide range of addiction-like behaviors has been shown in animals for vaccines directed against nicotine, cocaine, methamphetamine, and heroin (Chi, 2011). Nicotine and cocaine conjugate vaccines have entered clinical trials with some early evidence of efficacy and no important side effects (Martell et al., 2009; Hatsukami et al., 2011). A number of morphine vaccines have been developed that produce antibodies that cross-react with heroin and its active metabolites and block or attenuate the behavioral effects of heroin or morphine in rodents. A desirable feature for such vaccines is that they not bind or block the actions of certain off-target opioids such as methadone or buprenorphine so that these can still be used therapeutically for treating opioid addiction or for analgesia (Wainer et al., 1973; Bonese et al., 1974; Anton and Leff, 2006; Anton et al., 2009; Stowe et al., 2011). Although the immunological and behavioral effects of heroin/morphine vaccines have been studied in animals, their effects on opioid pharmacokinetics, which mediate their behavioral actions, have not been reported.
The goal of the current study was to synthesize and evaluate the immunologic and pharmacokinetic effects of candidate OXY conjugate vaccines in rats. Several linkers and carrier proteins were used to assess their immunogenicity and the influence of the degree of protein haptenation on vaccine efficacy. Effects of the lead candidate OXY(Gly)4-keyhole limpet hemocyanin (KLH) vaccine on OXY protein binding in serum, OXY distribution to brain, and OXY-induced analgesia were evaluated to provide mechanistic information and anticipate whether additional study of this vaccine is warranted. The effects of OXY(Gly)4-KLH on OXY-induced hot-plate analgesia were studied to measure the ability of this vaccine to block a centrally mediated opioid effect. The large observed effects of OXY(Gly)4-KLH on OXY pharmacokinetics, and its ability to block OXY analgesia, support its further development as a potential adjunct to addiction treatment.
Materials and Methods
Overview of Hapten Synthesis.
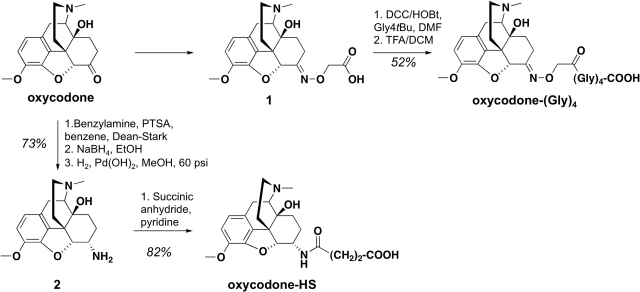
The 6-position of OXY was selected for linker attachment because it has been used previously to generate effective antibodies to heroin and its active metabolites (Bonese et al., 1974; Anton and Leff, 2006). Two linkers were chosen for evaluation: hemisuccinate, because it has been used successfully with heroin conjugate vaccines, and (Gly)4, because preliminary studies using oligoglycine as a convenient means of studying homologous linkers of different lengths suggested that (Gly)4 was the most promising of this series (data not shown).
Compound 1 was obtained as reported previously (Lamont et al., 2003) by the condensation of OXY with O-carboxymethoxylamine hemihydrochloride (2-(aminooxy)acetic acid) in refluxing methanol using pyridine as a base (see Scheme 1). This intermediate was coupled to tetraglycine tertbutyl ester (Gly4tBu) (Bieniarz et al., 1988), using a N,N′-dicyclohexylcarbodiimide/hydroxybenzotriazole procedure followed by acid hydrolysis to afford OXY(Gly)4 in moderate yield (52% overall). Compound 2 was prepared stereoselectively by using a catalytic reduction of the imine formed from the oxycodone and benzylamine, followed by debenzylation (Sayre and Portoghese, 1980). Condensation of the intermediate 2 in a manner similar to that described previously for morphine 3-succinyl (Wainer et al., 1972) with succinic anhydride in refluxing pyridine gave OXY(HS) with good yield (82%). Compounds were characterized by 1H NMR and mass spectrometry after purification. Purity of target haptens was determined by elemental analysis.
Scheme 1.
OXY hapten synthesis: chemical structures and reaction details. Intermediates are numbered in bold type.
Reagents.
All commercial reagents and anhydrous solvents were used without further purification or distillation, unless otherwise stated. Analytical thin-layer chromatography was performed on plates coated with EM Science (Gibbstown, NJ) silica gel 60 F254 (0.25 mm). Compounds were visualized by UV light and/or stained with potassium permanganate solution followed by heating. Flash column chromatography was performed on EM Science silica gel 60 (230–400 mesh). NMR (1H) spectra were recorded on a Bruker Avance 400 MHz spectrometer (Bruker Daltonics, Billerica, MA) and calibrated by using an internal reference. Chemical shifts are expressed in ppm, and coupling constants (J) are in hertz (Hz). Peak multiplicities are abbreviated as: broad, br; singlet, s; doublet, d; triplet, t; and multiplet, m. ESI mode mass spectra were recorded on a BrukerBioTOF II mass spectrometer. Elemental analyses were performed by M-H-W Laboratories (Phoenix, AZ).
OXY(Gly)4.
N,N′-dicyclohexylcarbodiimide (1.3 equivalent), carboxylic acid 1 (0.15–0.4 mmol scale, 1 equivalent), and hydroxybenzotriazole (1.2 equivalent) were dissolved in 5 ml of anhydrous dimethylformamide. The solution was cooled to 0°C and placed under a nitrogen atmosphere. After 15 min at 0°C, tetraglycine (0.15–0.4 mmol, 1 equivalent) was added. The solution was sealed under a nitrogen atmosphere and allowed to warm and stir at room temperature overnight. The reaction mixture was filtered to remove dicyclohexylurea into water (10× initial volume of dimethylformamide) and extracted with ethyl acetate. The combined organic layers were dried on magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by silicon dioxide (SiO2) chromatography (DCM/MeOH/NH4OH: 94:5:1) to afford the desired compound as a pale yellow solid (63% yield). 1H NMR (CD3OD) δ: 1.39 (m, 1H); 1.49 (s, 9H); 1.62–1.88 (m, 2H); 2.42–2.81 (m, 7H with s, 3H, NCH3 at 2.68); 3.03–3.19 (m, 2H); 3.37–3.42 (m, 8H); 3.53 (d, 1H, J = 9.4 Hz); 3.61 (m, 1H); 3.84 (s, 3H); 4.67 (s, 2H); 5.07 (s, 1H); 6.83 (d, 1H, JH1-H2 = 8.1 Hz); 6.89 (d, 1H, JH2-H1 = 8.1 Hz); ESI-TOF MS m/z: 673.789 (MH+).
Trifluoroacetic acid (TFA, 20% volume) was added to a solution of this ester (0.15–0.4 mmol scale) in DCM (5 ml). The resultant solution was stirred at room temperature. Upon complete disappearance of starting material, the solvent was removed under vacuum. The crude reaction mixture was subjected to azeotropic drying by using toluene. The residue was purified on a reverse-phase high-performance liquid chromatography column (Haisil 100-C18, 5 μm, 10 mm × 250 mm), which was eluted with acetonitrile/H2O/TFA (70:30:0.1) at a flow rate of 2 ml/min) to provide OXY(Gly)4 as a white solid (82% yield). The hapten was converted to the TFA salt to facilitate purification and recovery, and TFA was then removed by ion exchange before conjugation. 1H NMR (CD3OD) δ: 1.42 (m, 1H); 1.69–1.91 (m, 2H); 2.48–2.82 (m, 7H with s, 3H, NCH3 at 2.67); 3.05–3.23 (m, 2H); 3.34–3.38 (m, 8H); 3.48 (d, 1H, J = 9.4 Hz); 3.61 (m, 1H); 3.85 (s, 3H); 4.63 (s, 2H); 5.04 (s, 1H); 6.81 (d, 1H, JH1-H2 = 8.1 Hz); 6.88 (d, 1H, JH2-H1 = 8.1 Hz); ESI-TOF MS m/z: 617.233 (MH+); analytical calculation for C28H36N6O10: C, 54.54; H, 5.88; N, 13.63. Found: C, 54.25; H, 6.03; N, 12.72.
6α-Amino-14-Hydroxydesocodeine (2).
A benzene solution containing oxycodone base (2 g, 6.3 mmol), benzylamine (770 mg, 7.15 mmol), and a catalytic amount of p-toluenesulfonic acid (5% molar) was refluxed overnight, using a Dean-Stark Trap (Sigma-Aldrich, St. Louis, MO). The mixture was concentrated, and a solution of NaBH4 (43 mg, 1.1 mmol) in absolute ethanol was added (Sayre and Portoghese, 1980). After being stirred under N2 for 8 h, the resulting solution was diluted with H2O and concentrated to remove most of the ethanol. Additional diluted NH4OH was added, and the aqueous layer was extracted (3× DCM). Organic layers were then dried on magnesium sulfate and concentrated under reduced pressure. This residue was purified by flash chromatography using a DCM/MeOH/NH4OH mixture (96:3:1) to give the benzylated intermediate as a white solid (78%). 1H NMR (CDCl3): 1.31–1.40 (m, 2H); 1.52–1.58 (m, 2H); 2.10–2.13 (m, 2H); 2.26–2.29 (m, 2H); 2.49 (s, 3H, NCH3); 2.54 (m, 1H); 2.91–3.02 (m, 2H); 3.30 (s, 3H, OCH3); 3.81 (s, 2H); 4.60 (d, 1H, JH5-H6 = 3.7 Hz); 4.73 (m, 1H); 6.42 (d, 1H, JH1-H2 = 8.1 Hz); 6.54 (d, 1H, JH2-H1 = 8.1 Hz); 7.19–7.36 (m, 5H); ESI-TOF MS m/z: 407.538 (MH+).
Catalytic hydrogenolysis of the protected intermediate (1g, 2.46 mmol) was conducted at 60 psi in MeOH using 5% molar of Pd(OH)2 for 7 h. The catalyst was filtered, and the filtrate was taken to dryness. This residue was purified by flash chromatography using a DCM/MeOH/NH4OH mixture (97/2/1) to afford compound 2 as a white solid (93% yield). 1H NMR (CDCl3): 1.31–1.40 (m, 2H); 1.52–1.58 (m, 2H); 2.10–2.13 (m, 2H); 2.26–2.29 (m, 2H); 2.49 (s, 3H, NCH3); 2.54 (m, 1H); 2.91–3.02 (m, 2H); 3.30 (s, 3H, OCH3); 4.60 (d, 1H, JH5-H6 = 3.7 Hz); 4.73 (m, 1H); 6.42 (d, 1H, JH1-H2 = 8.1 Hz; 6.54 (d, 1H, JH2-H1 = 8.1 Hz); ESI-TOF MS m/z: 317.653 (MH+).
OXY(HS).
The amine 2 (80 mg, 0.25 mmol) was dissolved in pyridine, and succinic anhydride (30 mg, 0.3 mmol) was added dropwise. The mixture was refluxed for 7 h. After the disappearance of starting material, the solvent was concentrated to dryness, and the residue was taken up with toluene (three times) and concentrated. This residue was purified by flash chromatography using a DCM/MeOH/NH4OH mixture (88:10:2) to obtain OXY(HS) as a white solid (82% yield), which was then converted to salt for bioconjugation. 1H NMR (CDCl3): 1.68–1.77 (m, 2H); 1.79–1.91 (m, 2H); 2.17–2.23 (m, 2H); 2.29 (s, 3H, NCH3); 2.34–2.83 (m, 7H); 3.04 (m, 2H); 3.83 (s, 3H, OMe); 4.09 (m, 1H); 4.63 (d, 1H, J = 4.7 Hz); 6.33 (d, 1H, JH1-H2 = 8.2 Hz); 6.37 (d, 1H, JH2-H1 = 8.2 Hz); 8.03 (d, 1H, J = 8.9 Hz, NH); ESI-TOF MS m/z: 417.328 (MH+); analytical calculation for C22H28N2O6: C, 63.45; H, 6.78; N, 6.73. Found: C, 63.36; H, 6.88; N, 6.72.
Hapten Conjugation.
Haptens were first conjugated to bovine serum albumin (BSA) to optimize reaction efficiency. The difference in molecular weight between the conjugated and unconjugated carrier protein was analyzed by matrix-assisted laser desorption ionization/TOF mass spectrometry and used to calculate the molecular haptenation ratio (number of moles of hapten attached per mole of carrier protein). Haptens were then conjugated to chicken ovalbumin (OVA) for use as coating antigen for ELISA and to KLH for immunization of rats because this protein is acceptable for administration to humans, whereas BSA is not. Hapten (5 mM) and N-ethyl-N′-(3 dimethylaminopropyl)carbodiimide hydrochloride cross-linker (50 mM) were dissolved in 0.1 M MES buffer at pH 4.5. After 10 min of stirring BSA, OVA, or KLH (Thermo Fisher Scientific, Waltham, MA) was added at final concentrations of 2.8, 1.9, or 2.8 mg/ml, respectively. These reactant concentrations provided a hapten/protein molar ratio of 120:1 in the reaction mixture for BSA or OVA and produced conjugates with the highest haptenation ratios. Reactions were stirred for 3 h at room temperature and terminated by dialysis in 0.05 M phosphate-buffered saline for 6 h at 4°C. The dialyzed conjugates were sterile-filtered and stored at 4°C. Determination of the haptenation ratio by mass spectrometry was not possible for KLH conjugates because of its large size (∼5 to 8 million Da).
Vaccination.
All protocols were approved by the Minneapolis Medical Research Foundation Animal Care and Use Committee. Male Holtzman rats weighing 350 g (Harlan Teklad, Madison, WI) were housed with a 12-h standard light/dark cycle. In immunogenicity, distribution, and behavioral studies, conjugates were injected intraperitoneally in a volume of 0.4 ml, at doses of 25 to 100 μg by using complete Freund's adjuvant for the first injection and incomplete Freund's adjuvant for two subsequent booster injections at 3 and 6 weeks (Pravetoni et al., 2011). A total of three vaccinations were administered, at 0, 21, and 42 days. Immunogenicity, distribution, and behavioral studies were conducted 7 to 10 days after the third immunization. Blood was obtained 7 to 10 days after the last immunization, corresponding to the time of expected peak antibody response, and serum was stored at −20°C.
ELISA.
ELISA plates were coated with 5 ng/well of OVA conjugate or unconjugated protein control in carbonate buffer at pH 9.6 and blocked with 1% gelatin. OXY(Gly)4-OVA was used as coating antigen. Primary antibodies were incubated with goat anti-IgG antibodies conjugated to horseradish peroxidase. Antibody specificity was characterized by competitive ELISA, and IC50 values were calculated for those inhibitors that produced distinct plateaus in the maximum percentage of inhibition. Because plateau values differed, IC50 values for each inhibitor were calculated based on the percentage of inhibition of the optical density of primary antibodies incubated with saline. Finally, to establish the relative affinity of anti-OXY antibodies to other structurally related compounds, cross-reactivity percentage was reported.
Effect of Vaccination on OXY Distribution.
Rats were anesthetized with ketamine/xylazine 1 week after the final vaccine dose and an indwelling catheter was placed in their right external jugular vein. Blood was withdrawn for ELISA, and OXY was administered as a 10-s infusion. Rats were decapitated 5 min later, and trunk blood and brain were collected.
The effects of immunization with OXY(Gly)4-BSA or OXY(Gly)4-KLH doses of 25 and 100 μg were compared in groups of five rats and an OXY dose of 0.5 mg/kg administered intravenously. Controls were vaccinated with unconjugated protein in place of conjugate. The effects of vaccination on the distribution of OXY doses of 0.05, 0.1, and 0.5 mg/kg were compared by using groups of five rats vaccinated with 100 μg of the OXY(Gly)4-BSA conjugate, 7 to 10 days after the third immunization. The BSA conjugate was used for this experiment because of the ability to verify the haptenation ratio by mass spectrometry. These OXY doses were chosen as representative of the therapeutic single dose range in humans (Leow et al., 1992; Pöyhiä et al., 1992).
OXY Assay.
Drug doses and concentrations are expressed as weight of the base. OXY concentrations were measured by gas chromatography-MS and represent the total drug (protein or antibody-bound as well as free) in each sample. Extraction and quantitation of OXY in brain and serum samples was based on a previously described method (Lewis et al., 2005) using d6-OXY as internal standard (Cerilliant Corporation, Round Rock, TX), solid-phase extraction, derivatization with N,O-bis(trimethylsilyl)trifluoroacetamide in 1% trimethylchlorosilane, and analysis on an Agilent 6890 gas chromatograph with methyl siloxane capillary column and 5973 quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA). Adaptations to the method included omission of NaF, analysis of 0.5-ml samples rather than 3-ml samples, and an initial 1:4 rather than 1:2 dilution of brain into 0.1 M phosphate buffer pH 6.0, and solid-phase extraction of brain homogenate supernatant rather than whole homogenate. The limit of quantitation was 5 ng/ml OXY in serum and 50 ng/ml in brain.
Protein Binding.
Equilibrium dialysis was carried out in Sorenson's buffer at pH 7.35 for 4 h at 37°C by using 1 ml of Teflon cells (Hieda et al., 1997). The free (unbound) oxycodone concentration was calculated as the product of the percentage of bound and the total serum oxycodone concentration before dialysis.
Thermal Nociception Test.
A dose-effect curve was obtained by using groups of six unvaccinated (naive) rats to select an appropriate OXY dose for further study. In addition, serum and brain were obtained from the same naive rats to determine oxycodone concentrations 30 min after subcutaneous dosing. Based on these data, the effects of immunization were tested in rats before and after receiving OXY (2.25 mg/kg s.c.). Groups of 10 rats were vaccinated with 100 μg of OXY(Gly)4-KLH or unconjugated KLH (controls), and thermal nociception testing was carried out on a hot-plate (Columbus Instruments, Columbus, OH) set at 54°C, 7 to 10 days after the third immunization. Rats were habituated to the testing environment for 1 h, and then pretested on the hot-plate to obtain their baseline latency. Two hours later, rats were injected subcutaneously with OXY, and their postdrug latency was obtained 30 min after that. A cutoff value of 60 s was used to prevent tissue damage, and the maximum possible effect (MPE%) was calculated as: (postdrug latency − baseline latency)/(maximal cutoff − baseline latency) × 100. Licking the hind paw or jumping were considered endpoints for thermal nociception (Lemberg et al., 2006).
Data Analysis.
Vaccinated groups were compared with unconjugated protein controls by using unpaired two-tailed t tests or, for multiple groups, one-way analysis of variance followed by Bonferroni's post-test. One subject from the OXY(Gly)4-BSA group and one subject from the KLH group were removed from the analysis of equilibrium dialysis data because the unbound OXY concentrations were <5 ng/ml. The relationship of log titer to brain OXY concentrations was analyzed by linear regression.
Results
Conjugate Vaccine Synthesis.
Conjugation using a molar hapten/protein ratio of 120:1 in the reaction mixture proved optimal and produced BSA conjugates of OXY(Gly)4 or OXY(HS) with mean haptenation ratios of 16 or 17:1, respectively (Table 1). Hapten was also conjugated to KLH, because KLH is suitable for administration to humans, whereas BSA is not. Conjugation of KLH could not be quantitated by matrix-assisted laser desorption ionization/TOF because of its large molecular weight but was confirmed qualitatively by ELISA in which the conjugate was used as the coating antigen. Conjugates of OXY(Gly)4-OVA and OXY(HS)-OVA used as the coating ELISA had haptenation ratios of ≥17:1.
TABLE 1.
Conjugate haptenation ratios and serum antibody titers
| Linker | Carrier | Haptenation Ratio | Immunogen Dose | Serum Titer |
|---|---|---|---|---|
| × 103 | ||||
| (Gly)4 | BSA | 16 | 25 | 80 ± 70 |
| (Gly)4 | BSA | 16 | 100 | 130 ± 90 |
| (Gly)4 | KLH | 25 | 170 ± 110 | |
| (Gly)4 | KLH | 100 | 110 ± 60 | |
| (HS) | BSA | 17 | 100 | 11 ± 11 |
| (HS) | KLH | 100 | 20 ± 14 |
Serum Antibody Titers and Affinity.
The OXY(Gly)4-BSA and OXY(HS)-BSA conjugates had nearly identical haptenation ratios, yet the conjugate containing the (Gly)4 linker elicited substantially higher titers regardless of the immunogen dose (Table 1). The OXY(Gly)4-OVA conjugate had a similar haptenation ratio but was not further characterized because it was used only as a coating antigen for ELISA. Competitive ELISA using serum from animals immunized with OXY(Gly)4-KLH, with OXY(Gly)4-OVA as the coating antigen (same linker), showed that the resulting antibodies had high affinities (low IC50 values) for both the OXY(Gly)4 hapten and OXY without linker (Table 2). The (Gly)4 linker alone showed no measurable competition or binding. Anti-OXY antibodies exhibited 63% cross-reactivity with oxymorphone, a minor but active metabolite of OXY in rat and human (Lalovic et al., 2006; Chan et al., 2008). Affinities for morphine, hydrocodone, hydromorphone, naloxone, and naltrexone were substantially lower, and there was no measurable binding of the off-target opioids methadone or buprenorphine.
TABLE 2.
Competitive binding IC50 values and cross-reactivity percentage
OXY(Gly)4-OVA was used as ELISA coating antigen.
| Inhibitor | IC50 | Cross-Reactivity |
|---|---|---|
| μM | % | |
| OXY(Gly)4 | 0.003 | >100 |
| OXY | 0.017 | 100 |
| Oxymorphone | 0.03 | 63 |
| Naloxone | 1 | 1.2 |
| Hydromorphone | 4 | 0.4 |
| Naltrexone | 5 | 0.4 |
| Hydrocodone | 10 | 0.2 |
| Morphine | 20 | 0.1 |
| (Gly)4 | — | |
| Methadone | — | |
| Buprenorphine | — | |
| Nicotine | — |
—, no measurable binding.
OXY Distribution.
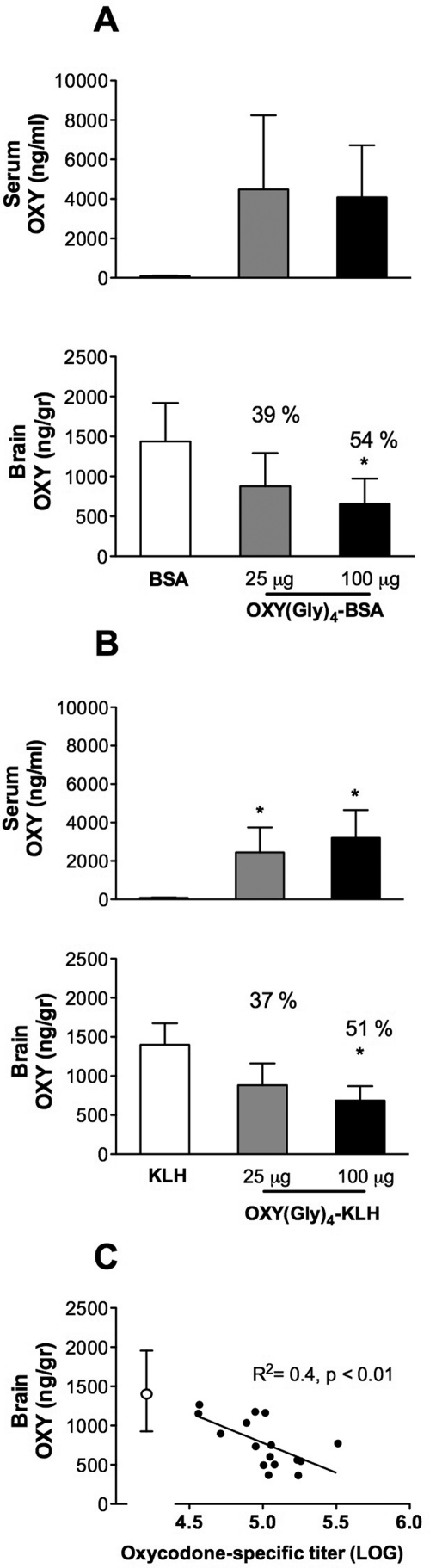
Immunization with OXY(Gly)4-KLH increased retention of OXY in serum measured 5 min after the OXY dose (Fig. 1) and reduced distribution of OXY to brain. Immunization with OXY(Gly)4-BSA showed a similar trend toward increased retention of OXY in serum (p = 0.07) and, like the KLH conjugate, significantly decreased OXY distribution to brain. There was a trend but no significant difference in efficacy for either immunogen between the 25- and 100-μg doses. However, the 100-μg doses produced reductions of more than 50% in brain OXY concentrations that differed significantly from controls, whereas the 25-μg doses did not, suggesting greater efficacy for the higher immunogen doses (Fig. 1). There was a significant correlation between the log serum antibody titers from all vaccinated rats and their respective brain OXY concentration (Fig. 1).
Fig. 1.
Effects of two vaccine doses on OXY distribution. Rats immunized with either 25 or 100 μg of conjugate (five per group) received 0.5 mg/kg i.v. OXY, and samples were collected 5 min later. A and B, data (mean ± S.D.) represent the total OXY concentration in serum or brain for animals immunized with OXY(Gly)4-BSA (A) or OXY(Gly)4-KLH (B). Numbers over the data bars are the percentage of change compared with control. Vaccination with OXY(Gly)4-KLH increased retention of OXY in serum and both immunogens decreased OXY distribution to brain, *, p < 0.05 compared with controls. C, there was a significant correlation between the log serum antibody titer and the brain OXY concentration. ●, values for all rats receiving either conjugate vaccine shown in A and B; ○, mean ± S.D. for control rats from those experiments.
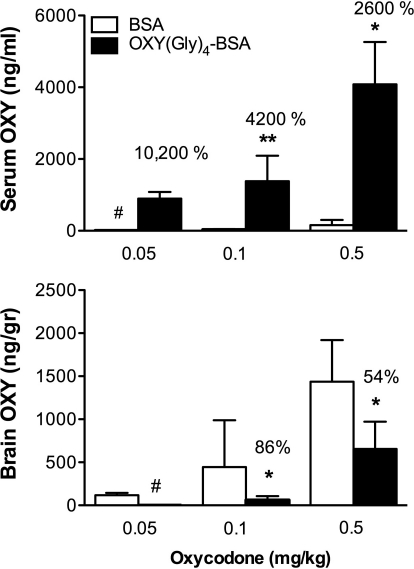
Immunization with 100 μg of OXY(Gly)4-BSA altered OXY distribution in a dose-related manner (Fig. 2) with substantially larger effects on the lower OXY doses as indicated by the greater percentage of change in serum and brain levels at the lower OXY doses. The distribution of OXY to brain was substantially reduced, by 86% in rats receiving 0.1 mg/kg OXY, and the percentage of reduction could not be calculated at the lowest OXY dose because the brain OXY concentration was below the assay's limit of quantitation.
Fig. 2.
Effect of vaccination on several OXY doses. Rats were immunized with 100 μg of OXY(Gly)4-BSA and treated as in Fig. 1 but received different OXY doses intravenously. Effects on OXY distribution were greatest at the lower OXY doses as indicated by the percentage of control concentrations. Data are represented as mean ± S.D. *, p < 0.05; **, p < 0.01 compared with control. #, OXY concentrations too low to quantitate.
Protein Binding.
The protein binding of OXY in the serum of control rats was low (10–12%) but increased markedly to 99% in rats vaccinated with either the BSA or KLH conjugate. The free (unbound) OXY concentration in serum was reduced to 19% (BSA conjugate) or 29% (KLH conjugate) that of controls in vaccinated rats (Table 3).
TABLE 3.
Oxycodone serum protein binding
Group sizes n = 4 or 5.
| Total OXY | OXY | Unbound OXY | |
|---|---|---|---|
| ng/ml | % bound | ng/ml | |
| BSA | 160 ± 150 | 12 ± 2 | 140 ± 130 |
| OXY(Gly)4-BSA | 3000 ± 1100*** | 99 ± 1*** | 30 ± 15 |
| KLH | 80 ± 20 | 10 ± 3 | 70 ± 20 |
| OXY(Gly)4-KLH | 3200 ± 1500** | 99 ± 1*** | 20 ± 15* |
, P < 0.05;
, P < 0.01;
, P < 0.001 compared with controls.
Thermal Nociception Test.
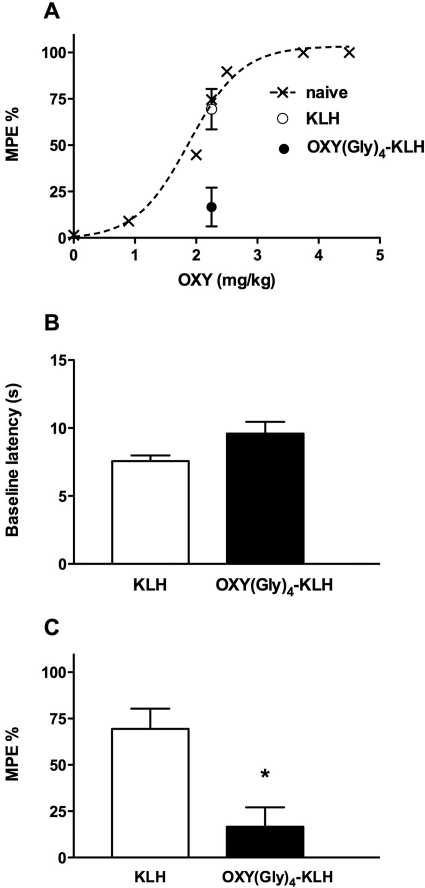
An OXY dose of 2.25 mg/kg was selected for studying effects of immunogens because it produced a nearly maximal effect on latency in unvaccinated naive rats (Fig. 3A). In the same naive rats (n = 6) an OXY dose of 2.25 mg/kg, at 30 min after subcutaneous injection, resulted into serum OXY concentrations of 340 ± 50 ng/ml and brain concentrations of 2700 ± 290 ng/ml, indicating that concentrations were higher than concentrations obtained with an intravenous dose of 0.5 mg/kg at 5 min. Baseline latencies did not differ between groups immunized with OXY(Gly)4-KLH and KLH control (Fig. 3B). The MPE% after OXY administration was significantly lower in the group vaccinated with OXY(Gly)4-KLH than in the KLH control group (17 ± 10% versus 69 ± 11%; p < 0.01) (Fig. 3C).
Fig. 3.
Effect of vaccination on OXY-induced analgesia. Rats were immunized with 100 μg of OXY(Gly)4-KLH or KLH control and tested for blockage of OXY-induced analgesia on a hot-plate thermal nociception test. A, dose-response relationship for OXY effect on MPE% at 30 min after injection. B, rats immunized with OXY(Gly)4-KLH or KLH control exhibited similar baseline latencies. C, effects of 2.25 mg/kg OXY administered subcutaneously were blunted in rats vaccinated with OXY(Gly)4-KLH compared with controls immunized with unconjugated KLH. Data are expressed as mean ± S.E.M. *, p < 0.01 compared with control.
Discussion
The OXY(Gly)4-KLH immunogen elicited high titers of OXY-specific antibodies that were highly selective for OXY and its active metabolite oxymorphone and had substantially lower affinities for a variety of off-target opioids. Vaccination of rats with this immunogen increased OXY binding and retention in serum, decreased the free OXY concentration in serum, and decreased OXY distribution to brain after administration of clinically relevant doses of OXY. Vaccination also substantially reduced OXY-induced analgesia in a test of thermal nociception, highlighting its ability to attenuate a centrally mediated behavioral effect of OXY. These data support further study of this immunogen as a potential therapeutic agent for OXY abuse.
Linker composition and length contribute to conjugate vaccine immunogenicity (Kubler-Kielb et al., 2006), but the extent of this contribution is often difficult to interpret because the corresponding haptenation ratios for the conjugates being compared are not reported. Greater conjugate immunogenicity could be caused by enhanced recognition by antigen-presenting or B cells but could also be caused by differences in carrier protein haptenation because higher haptenation ratios are generally associated with greater immunogenicity (Carroll et al., 2011). In the current study haptenation ratios for the OXY(Gly)4-BSA and OXY(HS)-BSA conjugates were nearly identical, yet OXY(Gly)4-BSA elicited higher ELISA titers. Greater immunogenicity in this case was therefore caused by differences in the linker per se rather than haptenation efficiency. Because (Gly)4 and HS differ in both composition and length, it is not clear from the available data which of these factors contributed.
Competitive ELISA showed that the full hapten OXY(Gly)4 had a somewhat higher affinity for vaccine-generated antibodies than did OXY alone. Similar data with a nicotine conjugate vaccine suggest that linkers may contribute to the epitope (part of an antigen) that is recognized by the immune system (Hieda et al., 1997). This is not surprising given the small size of nicotine or OXY. Oligoglycine was used as a linker in this study because it provided a convenient means of studying and optimizing linker length, and (Gly)4 was shown in preliminary studies to be an effective linker. A potential concern with using a peptide linker is cross-reactivity with native proteins containing (Gly)4 sequences. This is unlikely because the minimum size generally associated with peptide immunogenicity is 8 to 12 amino acids (Mayrose et al., 2007). In support of this, the (Gly)4 linker alone produced no measurable inhibition in competitive ELISA. Cross-reactivity of antibodies with native proteins would therefore not be expected.
Antibodies generated by OXY(Gly)4-KLH cross-reacted with oxymorphone, an active but minor metabolite of OXY in rat and human (Pöyhiä et al., 1991; Lalovic et al., 2006). It is unlikely that the binding of oxymorphone is necessary for an effective OXY vaccine, because it is largely undetected in human plasma; however, oxymorphone is marketed as an analgesic and its abuse has been reported (http://www.justice.gov/ndic/pubs44/44817/sw0011p.pdf). The OXY(Gly)4-KLH vaccine could therefore be of interest with regard to oxymorphone abuse. Our findings are similar to a previous report of an OXY immunogen that also used the 6-position for linker attachment and elicited antibodies with similar IC50 values for OXY and oxymorphone (Findlay et al., 1981).
A desirable feature of an OXY vaccine is the production of antibodies that do not bind other opioids that might be needed for therapeutic use in vaccinated individuals. These include methadone, buprenorphine, and naltrexone, which are used for opioid addiction treatment, and naloxone, which is used to reverse excessive opioid effect. Antibodies from the OXY(Gly)4-KLH vaccine had no measurable affinity for methadone or buprenorphine and had IC50 values substantially higher for naltrexone and naloxone than for OXY. Their clinical use would not be expected to be impeded by vaccination, although this remains to be demonstrated. Cross-reactivity with other abusable opioids could be a useful feature of an OXY vaccine, but this was found only for oxymorphone. Because OXY and oxymorphone differ only in their 3-position substituent, this position does not seem to contribute importantly to the drug-binding epitope. In contrast, absence of a hydroxyl group at the 14-position (hydrocodone and hydromorphone) was sufficient to greatly reduce the affinity for these ligands. These findings help to define the structural features of OXY contributing to epitope recognition.
There are very limited data regarding the effects of opioid vaccines on opioid pharmacokinetics and none pertaining to OXY. Berkowitz and Spector (1972) reported a 7- to 30-fold increase in the serum concentration of [3H]dihydromorphine in mice immunized with a morphine-BSA conjugate vaccine compared with controls and slower radiolabel elimination. Rats or mice passively infused with immune serum from vaccinated rabbits showed reduced distribution of [3H]dihydromorphine to brain (Berkowitz et al., 1974). Hill et al. (1975) found no increase in [14C]morphine concentration in the serum of rabbits immunized with a similar vaccine but reported modestly prolonged radiolabel elimination. The use of radiolabel rather than specific drug assays is an important limitation of these studies. In the current study, using gas chromatography-mass spectrometry for OXY assay, vaccination with OXY(Gly)4-BSA greatly enhanced OXY retention in serum and reduced OXY distribution to brain by up to 84%. This magnitude of effect is similar to that of nicotine and cocaine vaccines that block addiction-related behaviors in rats and are in clinical trials (Fox et al., 1996; Pentel et al., 2000). Single oral OXY doses used in humans commonly range from 0.07 to 0.38 mg/kg of immediate release formulations. Corresponding peak or early serum OXY concentrations are typically 10 to 100 ng/ml (Leow et al., 1992; Pöyhiä et al., 1992). In the current study, OXY was administered over a comparable dose range of 0.05 to 0.5 mg/kg, but intravenously instead of orally, and produced serum OXY concentrations equaling or exceeding the peak levels from oral dosing in humans. Serum OXY concentrations after subcutaneous dosing at 2.25 mg/kg, as used in the hot-plate analgesia experiment, were even higher. Vaccination was effective in altering OXY distribution and hot-plate analgesia despite this rigorous challenge. Greater effects of vaccination on drug distribution at lower OXY doses were anticipated based on data with other addiction vaccines, because vaccine efficacy is greatest when the antibody-to-drug ratio is highest (Keyler et al., 2005).
The large effect of immunization on OXY distribution was mirrored by its behavioral efficacy. Immunization with OXY(Gly)4-KLH reduced OXY-induced analgesia in a test of thermal nociception, a centrally mediated effect of opioids. These observations suggest that further study of vaccination using models of OXY addiction are warranted.
Antibodies are too large to cross the blood-brain barrier, and drug bound to antibody is also excluded from the brain. The primary action of addiction vaccines is presumed to be the binding of drug to antibody in serum, reducing the unbound or free drug concentration and its subsequent distribution to brain (Keyler et al., 2005; Pravetoni et al., 2011). Changes in OXY protein binding and the unbound concentration of OXY in serum after vaccination with OXY(Gly)4-KLH were substantial and strongly support this mechanism of action. In addition to reducing the early distribution of drug to brain, nicotine vaccines slow nicotine distribution to brain and nicotine elimination (Keyler et al., 1999). These additional effects of vaccination on nicotine disposition may contribute to their effects on drug-related behavior. It will be of interest to examine these parameters for OXY vaccines.
The role of addiction vaccines in treatment is as yet unclear because they are in the early stages of development. It is likely that they will be used as adjunctive therapies or in combination with other medications rather than as single modalities. Antibodies generated by the OXY(Gly)4-KLH vaccine did not appreciably cross-react with methadone, buprenorphine, or naltrexone so that the therapeutic use of these medications as a treatment for addicts with sufficiently regular and severe opioid use would still be possible. The combination of vaccine and agonist therapy is of interest because a substantial minority of opioid addicts continue to use their opioid of choice even while on methadone maintenance therapy (D'Aunno and Vaughn, 1992). It is of course possible that someone abusing OXY, the opioid targeted by this vaccine, could switch to abusing a different opioid. Combining a vaccine with other adjunctive therapies would presumably help to minimize this possibility. It may also be feasible to combine multiple vaccines directed at different opioids to provide broader coverage. This strategy is widely used for infectious diseases by combining multiple unrelated vaccines, e.g., measles, mumps, and rubella for convenience. Because the immune system has the capacity to respond to multiple simultaneous challenges, the individual vaccine components retain their individual immunogenicity when combined in this manner. Early data with nicotine vaccines suggest that this strategy can be generalized to conjugate vaccines as well (Keyler et al., 2008). Whichever approach is adopted, the development of an effective OXY vaccine represents a first step toward investigating whether this is a viable therapeutic strategy.
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grant DA026300]. M.P. was supported by a Career Development Award from the Minneapolis Medical Research Foundation.
Part of this work was presented previously: Pravetoni M, Le Naour M, Portoghese PS, Harmon TM, and Pentel PR (2011) Vaccination with an oxycodone conjugate vaccine alters oxycodone pharmacokinetics and its distribution to brain in rats, at the 73rd Annual Meeting of the College on Problems of Drug Dependence; 2011 June 18–23; Hollywood, FL. College on Problems of Drug Dependence, Philadelphia, PA.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- OXY
- oxycodone
- HS
- hemisuccinate
- KLH
- keyhole limpet hemocyanin
- OVA
- ovalbumin
- BSA
- bovine serum albumin
- ELISA
- enzyme-linked immunosorbent assay
- TFA
- trifluoroacetic acid
- ESI
- electrospray ionization
- TOF
- time of flight
- DCM
- dichloromethane
- MS
- mass spectrometry
- MPE%
- maximum possible effect.
Authorship Contributions
Participated in research design: Pravetoni, Portoghese, and Pentel.
Conducted experiments: Pravetoni, Harmon, and Tucker.
Contributed new reagents or analytic tools: Le Naour and Harmon.
Performed data analysis: Pravetoni and Pentel.
Wrote or contributed to the writing of the manuscript: Pravetoni, Tucker, and Pentel.
References
- Anton B, Leff P. (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240 [DOI] [PubMed] [Google Scholar]
- Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, Leff P. (2009) Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin 5:214–229 [DOI] [PubMed] [Google Scholar]
- Berkowitz B, Spector S. (1972) Evidence for active immunity to morphine in mice. Science 178:1290–1292 [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Ceretta KV, Spector S. (1974) Influence of active and passive immunity on the disposition of dihydromorphine-H3. Life Sci 15:1017–1028 [DOI] [PubMed] [Google Scholar]
- Bieniarz C, Barnes G, Welch CJ, Schlesinger CA. (1988) inventors; Abbott Laboratories, assignee. Preparation of poly(amino acid)-based heterobifunctional coupling agents and conjugates containing them. European patent application 314127 A2 19890503 1988 Oct 27
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252:708–710 [DOI] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Pidaparthi RR, Abraham P, Gong PK, Deng L, Huang X, Gunnell M, Lay JO, Jr, Peterson EC, et al. (2011) Synthesis of mercapto-(+)-methamphetamine haptens and their use for obtaining improved epitope density on (+)-methamphetamine conjugate vaccines. J Med Chem 54:5221–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT. (2008) Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35:295–302 [DOI] [PubMed] [Google Scholar]
- Chi KR. (2011) Vaccines move forward against a range of addictions. Nat Med 17:146. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. (2006) Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81:103–107 [DOI] [PubMed] [Google Scholar]
- D'Aunno T, Vaughn TE. (1992) Variations in methadone treatment practices. Results from a national study. JAMA 267:253–258 [PubMed] [Google Scholar]
- Dodrill CL, Helmer DA, Kosten TR. (2011) Prescription pain medication dependence. Am J Psychiatry 168:466–471 [DOI] [PubMed] [Google Scholar]
- Fareed A, Vayalapalli S, Stout S, Casarella J, Drexler K, Bailey SP. (2011) Effect of methadone maintenance treatment on heroin craving, a literature review. J Addict Dis 30:27–38 [DOI] [PubMed] [Google Scholar]
- Findlay JW, Butz RF, Jones EC. (1981) Relationships between immunogen structure and antisera specificity in the narcotic alkaloid series. Clin Chem 27:1524–1535 [PubMed] [Google Scholar]
- Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, et al. (1996) Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 2:1129–1132 [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, et al. (2011) Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, Raphael DE, Niedbalas RS, Pentel PR. (1997) Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther 283:1076–1081 [PubMed] [Google Scholar]
- Hill JH, Wainer BH, Fitch FW, Rothberg RM. (1975) Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J Immunol 114:1363–1368 [PubMed] [Google Scholar]
- Keyler DE, Hieda Y, St Peter J, Pentel PR. (1999) Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res 1:241–249 [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, Fuller S, Le CT, Pentel PR. (2005) Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos 33:1056–1061 [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. (2008) Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol 8:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler-Kielb J, Liu TY, Mocca C, Majadly F, Robbins JB, Schneerson R. (2006) Additional conjugation methods and immunogenicity of Bacillus anthracis poly-γ-D-glutamic acid-protein conjugates. Infect Immun 74:4744–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. (2006) Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 79:461–479 [DOI] [PubMed] [Google Scholar]
- Lamont JV, McConnell RI, Fitzgerald SP, Benchikh EO, Lowry AP. (2003) inventors; Randox Laboratories Ltd., assignee. Preparation of haptens, immunogens and antibodies to oxycodone and its metabolites. European Patent Application 1323718 A2 20030702 2003 Aug 21
- Lemberg K, Kontinen VK, Viljakka K, Kylänlahti I, Yli-Kauhaluoma J, Kalso E. (2006) Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg 102:1768–1774 [DOI] [PubMed] [Google Scholar]
- Leow KP, Smith MT, Watt JA, Williams BE, Cramond T. (1992) Comparative oxycodone pharmacokinetics in humans after intravenous, oral, and rectal administration. Ther Drug Monit 14:479–484 [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Johnson RD, Hattrup RA. (2005) Simultaneous analysis of thebaine, 6-MAM and six abused opiates in postmortem fluids and tissues using Zymark automated solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 822:137–145 [DOI] [PubMed] [Google Scholar]
- Lopez MF, Compton WM, Volkow ND. (2009) Changes in cigarette and illicit drug use among US teenagers. Arch Pediatr Adolesc Med 163:869–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. (2009) Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66:1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JC. (2011) The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev 30:264–270 [DOI] [PubMed] [Google Scholar]
- Mayrose I, Shlomi T, Rubinstein ND, Gershoni JM, Ruppin E, Sharan R, Pupko T. (2007) Epitope mapping using combinatorial phage-display libraries: a graph-based algorithm. Nucleic Acids Res 35:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, Milstein JR, Basham LE, Coy RT, Moon JW, et al. (2000) A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav 65:191–198 [DOI] [PubMed] [Google Scholar]
- Pöyhiä R, Olkkola KT, Seppälä T, Kalso E. (1991) The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol 32:516–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhiä R, Seppälä T, Olkkola KT, Kalso E. (1992) The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol 33:617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, Pettersson S, Pentel PR. (2011) Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol 81:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Portoghese PS. (1980) Stereospecific synthesis of the 6-α- and 6-β-amino derivatives of naltrexone and oxymorphone. J Org Chem 45:3366–3368 [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. (2009) Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother 10:1727–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. (2011) A vaccine strategy that induces protective immunity against heroin. J Med Chem 54:5195–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer BH, Fitch FW, Rothberg RM, Fried J. (1972) Morphine-3-succinyl–bovine serum albumin: an immunogenic hapten-protein conjugate. Science 176:1143–1145 [DOI] [PubMed] [Google Scholar]
- Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1973) In vitro morphine antagonism by antibodies. Nature 241:537–538 [DOI] [PubMed] [Google Scholar]