Abstract
Resveratrol is a plant-derived polyphenol that can attenuate the cardiotoxic effects of doxorubicin (DOX), a powerful antibiotic widely used in cancer chemotherapy. However, the underlying protective mechanisms of resveratrol remain elusive. Here, we show that resveratrol inhibited DOX-induced autophagy and cardiomyocyte death, and autophagy suppression is an important mechanism that mediates the ability of resveratrol to protect against DOX cardiotoxicity. Indeed, resveratrol, 3-methyladenine (3-MA), and a short hairpin RNA directed against autophagy gene beclin 1 (shBCN1) each was able to attenuate DOX-induced autophagy and cardiomyocyte death, but resveratrol did not provide additional protection in the presence of 3-MA or shBCN1. In contrast, up-regulation of autophagy by beclin 1 overexpression not only exacerbated DOX cardiotoxicity but also abolished the protective effects of resveratrol. Intriguingly, p70 S6 kinase 1 (S6K1) was activated by DOX, which was prevented by resveratrol. Knocking down S6K1 with small interfering RNA diminished DOX-induced autophagy and cardiotoxicity, but resveratrol failed to exert an additive effect. In addition, S6K1 overexpression impaired the ability of resveratrol to antagonize DOX-induced autophagy and cardiomyocyte death. Taken together, our data indicate that the protective effect of resveratrol against DOX cardiotoxicity largely depends on its ability to suppress DOX-induced autophagy via the inhibition of S6K1.
Introduction
Doxorubicin (DOX) is a potent anthracycline antibiotic that has been used in anticancer therapy for decades. However, DOX is also well known to exert toxic effects on normal tissues. Especially in the heart, DOX can induce a dose-dependent cardiomyopathy that ultimately leads to congestive heart failure (Minotti et al., 2004). Despite its severe cardiotoxicity, DOX remains a major component of most chemotherapeutic regimens because of its efficacy and broad-spectrum antitumor activity. As a result, sustained research effort has been devoted to identifying effective drugs or strategies that can reduce DOX cardiotoxicity without compromising its antitumor efficacy.
Resveratrol (RV) is a plant-derived polyphenol reported to extend lifespan in lower organisms through mimicking caloric restriction (Wood et al., 2004). As such, resveratrol has been shown to reduce a variety of age-related diseases in rodents, including obesity, diabetes, cancer, cardiovascular diseases, and neurodegenerative diseases (Baur and Sinclair, 2006). Consistently, resveratrol is able to inhibit DOX-induced cardiotoxicity as shown by reduced oxidative stress and improved cardiac function (Tatlidede et al., 2009). It is noteworthy that the cardioprotective effect of resveratrol is associated with enhanced anticancer efficacy of DOX in both in vitro and in vivo studies (Aggarwal et al., 2004; Rezk et al., 2006). This raises the possibility that the combined use of DOX with resveratrol may be a viable chemotherapeutic modality that can selectively destroy tumors while concurrently limiting cardiac damage. However, how resveratrol could achieve these beneficial effects in the setting of DOX chemotherapy remains poorly understood.
DOX-induced oxidative stress has been proposed as the major mechanism responsible for cardiac damage (Minotti et al., 2004), and antioxidant therapies are able to attenuate DOX cardiotoxicity in diverse animal models (Yen et al., 1996; Minotti et al., 2004). Meanwhile, resveratrol has been shown to protect against DOX-induced cardiac dysfunction, mitochondrial depolarization, and cardiomyocyte death, which are accompanied by enhanced antioxidant activity and inhibited production of reactive oxygen species (Tatlidede et al., 2009). These observations suggest that the protective effects of resveratrol against DOX cardiotoxicity may be mediated by its ability to inhibit oxidative stress. Nevertheless, clinical trials demonstrate very limited efficacy of antioxidant supplements in reducing DOX-triggered cardiac injury (Gianni et al., 2008), suggesting that mechanisms other than oxidative stress might also contribute to DOX cardiotoxicity. It is thus possible that resveratrol may exert its cardioprotective effects independent of its inhibitory effects on DOX-induced oxidative stress.
Autophagy is a degradation system for eukaryotic cells to turn over organelles and long-lived proteins, thereby maintaining cellular homeostasis. Thus, reduced autophagic activity impairs basal cardiac function and structure (Taneike et al., 2010), making animals more sensitive to stress-induced heart failure (Nakai et al., 2007). However, the activation of autophagy could be either beneficial or detrimental to the heart depending on the context. On one hand, autophagy is induced to offset energy deficit promoting myocardial survival in response to starvation (Kuma et al., 2004) or ischemia (Matsui et al., 2007). On the other hand, elevated autophagy can cause cardiac injury under certain conditions. For example, high-level autophagy during reperfusion is harmful (Matsui et al., 2007), and diphtheria toxin triggers autophagy, leading to heart failure in mice (Akazawa et al., 2004). Likewise, DOX can induce autophagy in cardiomyocytes, which is detrimental in nature because inhibiting autophagy with chemical or genetic approaches dramatically attenuates DOX-induced cardiomyocyte death (Lu et al., 2009; Kobayashi et al., 2010; Chen et al., 2011). Thus, a potential therapeutic strategy to reducing DOX cardiotoxicity is to suppress DOX-induced autophagy.
In the present study, we demonstrated that resveratrol markedly reduced DOX-induced cardiomyocyte death, which largely depended on its ability to inhibit autophagy. It is noteworthy that our results also suggested that the inhibition of p70 S6 kinase 1 (S6K1) is essential for resveratrol to suppress DOX-induced autophagy and cytotoxic effects.
Materials and Methods
Cardiomyocyte Cultures.
Neonatal rat ventricular cardiomyocytes (NRCs) were cultured as described previously (Kobayashi et al., 2010). In brief, hearts from 0- to 2-day-old Harlan Sprague-Dawley rat neonates (Harlan Laboratories, Indianapolis, IN) were dissected and isolated in cold phosphate-buffered saline, then trypsin was used to dissociate the heart tissues. To prevent cell clumping, DNase was used to digest sticky DNA released from lysed cells. Cells were preplated for 1 h after digestion to remove nonmyocytes. Cells were then seeded on gelatinized cell culture dishes overnight in Dulbecco's modified Eagle's medium (DMEM) with 15% bovine serum. Media were changed to DMEM with 2% bovine serum the next day. After being cultured for another 24 h, cells were treated with drugs as indicated. All media contained penicillin and streptomycin (100 units/ml) as well as 100 μM 5-bromo-2′-deoxyuridine (Sigma, St. Louis, MO) unless otherwise indicated. 5-Bromo-2′-deoxyuridine was used to inhibit the proliferation of nonmyocytes, including fibroblasts.
Drug Treatments.
DOX, RV, and 3-methyadenine (3-MA) were purchased from Sigma. Bafilomycin A1 (BFA) was obtained from LC Laboratories (Woburn, MA). DOX was dissolved in saline to make 1 mM stock solution and then diluted 1000 times to make a final concentration of 1 μM upon use. Resveratrol was dissolved in ethanol to achieve 10 mM stock solution and was then diluted 1000 times upon use to make a final concentration of 10 μM except for dose-response experiments, in which it was diluted to a series of concentrations. BFA can specifically inhibit vacuolar proton ATPase and efficiently blocks fusion between autophagosomes and lysosomes to prevent the maturation of autophagic vacuoles. BFA was dissolved in dimethyl sulfoxide to make a 50 μM stock solution. BFA was added to culture medium 6 h before harvesting cells for measuring autophagic flux.
Western Blot Analysis.
Protein extraction from cardiomyocytes were performed by following a protocol described previously (Kobayashi et al., 2007). In brief, cultured NRCs were washed twice in cold phosphate-buffered saline and lysed with extraction buffer (20 mM NaPO4, 150 mM NaCl, 2 mM MgCl2, 0.1% Nonidet P-40, 10% glycerol, 10 mM sodium fluoride, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 μM phenylasine oxide, 10 nM okadaic acid, 1 mM dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml pepstatin, 10 μg/ml tosyl-l-phenylalanine chloromethyl ketone, and 10 μg/ml N-tosyl-l-lysine chloromethyl ketone). After centrifugation for 30 min at 13,000g, supernatants were collected and protein concentration was quantified. Equal amounts of protein were loaded to polyacrylamide gel for electrophoresis, and then transferred to polyvinylidene difluoride membranes (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). After being blocked with 5% milk dissolved in Tris-buffered saline containing 1% Tween 20 for 1 h, the blots were incubated with primary antibodies overnight at 4°C. Afterward, the blots were incubated in 2.5% milk with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature and then processed for chemiluminescent detection using an ECL Advanced Western blotting kit (GE Healthcare). Protein abundance on Western blots was quantified by densitometry with the Quantity One program from Bio-Rad Laboratories (Hercules, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin, and extracellular signal-regulated kinase 2 (ERK2) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antiphosphoserine antibody was purchased from Abcam Inc. (Cambridge, MA). The following primary antibodies were purchased from Cell Signaling Technology (Danvers, MA): cleaved caspase 3 (c-Casp3), poly(ADP-ribose) polymerase (PARP), beclin 1, sequestosome 1/P62 (p62), p70 S6 kinase (p70S6K), phospho-p70S6K (Thr-389), S6 ribosomal protein (S6), phospho-S6 (Ser-240), PRAS40, phospho-PRAS40 (Thr-264), tuberin/TSC2, phospho-tuberin/TSC2 (Thr-1462), microtubule-associated protein light chain 3 (LC3), autophagy-related 5 (Atg 5), and Atg12.
Construction and Utilization of Replication-Deficient Adenoviruses.
Adenovirus encoding GFP-LC3 was kindly provided by Dr. Aviva Tolkovsky (University of Cambridge, Cambridge, UK). Adenoviruses expressing β-galactosidase (Adβgal), human beclin 1 (AdBCN1), the short hairpin RNA (shRNA) targeting rat BCN1 mRNA, and control shRNA were created as described previously (Kobayashi et al., 2010). To generate adenoviral vector expressing S6K1, an insert containing S6K1 cDNA sequence was amplified by PCR from GFP-tagged ORF clone of Mus musculus ribosomal protein S6 kinase (OriGene, Rockville, MD) and integrated into the TA cloning vector (Invitrogen, Carlsbad, CA). The insert was then released from TA vector and subcloned into pShuttle-CMV vector via the BamHI-BglII (compatible ligation) and SalI restriction enzyme sites. Recombinant adenoviruses expressing S6K1 were then generated and amplified by using the AdEasy Adenoviral Vector System (Agilent Technologies, Santa Clara, CA). Unless otherwise indicated, a multiplicity of infection (MOI) of 20 plaque-forming units of each virus was used to infect cardiomyocytes. Two hours after incubation, infection medium was replaced by regular DMEM containing 1% bovine serum for an additional 24 h.
siRNA Gene Silencing.
Preselected siRNAs targeting rat S6 kinase 1 mRNA and a Silencer Negative Control siRNA were obtained from Ambion (Austin, TX). The transfection was performed following the protocol provided by Ambion with minor modifications. A total of 0.7 × 106 cardiomyocytes were used for transfection in each 6-mm dish. The culture media were replaced 24 h before transfection with fresh serum and antibiotic-free DMEM. Transfections were performed with 10 nM siRNA oligos, an optimized concentration to knock down S6K1. siRNA oligos in 200 μl of Opti-MEM (Invitrogen) were mixed with Lipofectamine RNAiMAX (5 μl in 200 μl of Opti-MEM) and incubated for 15 min. The mixture was then added dropwise to the culture dish with 2 ml of media. The transfected cells were cultured for 12 h, and fresh media were added. After another 36 h, the cells were treated with DOX and/or resveratrol for the indicated time, and then harvested. The drug effects and gene silencing efficiency were evaluated with Western blot analysis and the other assays as indicated in each experiment.
Cell Death Assay.
Propidium iodide (PI; Roche Applied Science, Indianapolis, IN) was used to measure cardiomyocyte death. PI can enter dead cells through disrupted membranes to bind DNA that display as red dots under fluorescent microscopy. A PI stock solution (0.5 μg/ml) was added directly to the culture medium with 1:1000 dilutions. After 10 min, the cells were examined with a fluorescent microscope and photographed under phase contrast or fluorescent condition. The PI-positive cells (stained red) were counted and expressed as a percentage against the total number of cells examined (200–250 counted under phase contrast).
Apoptosis Assays.
Apoptosis analysis was determined by both DNA laddering assay and analysis of apoptotic proteins including the cleaved caspase 3 and PARP. DNA laddering assay was performed by using a DNA laddering kit from Maxim Biotech, Inc. (San Francisco, CA). In brief, the cells were harvested, and genomic DNA was extracted and quantified. Equal amounts of genomic DNA were ligated with specific adaptors for 14 h and then subjected to PCR amplification. The PCR products were loaded to a 1.5% agrose gel, run for 40 min with a constant voltage of 250 V/15 cm, and then examined under UV light. Cleaved caspase 3 and PARP were determined by Western blot analysis as described above.
S6K1 Kinase Activity Assay.
S6K1 activity was measured by an immune-complex kinase assay using myelin basic protein (MBP) as the substrate. In brief, cardiomyocytes were lysed and sonicated in lysis buffer composed of 40 mM Tris-HCl, pH 7.4, 137 mM NaCl, 25 mM sodium β-glycerophosphate, 2 mM sodium pyrophosphate, 2 mM EDTA, 1 mM sodium vanadate, 10% glycerol, 1% Triton X-100, leupeptin (5 μg/ml), aprotinin (5 μg/ml), and 1 mM phenylmethylsulfonyl fluoride. Homogenates were cleared by centrifugation for 5 min at 15,000g at 4°C. Supernatants containing 100 μg of proteins were then incubated with anti-S6K1 antibodies (Cell Signaling Technology) for 16 h followed by incubation with Protein A/G-agarose beads (Santa Cruz Biotechnology, Inc.) for 1 h. Beads were washed with lysis buffer two times and kinase reaction buffer (20 mM MOPS, pH 7.2, 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM sodium vanadate, and 1 mM dithiothreitol) two times. An aliquot of precipitates was subjected to immunoblotting using anti-S6K1 antibody. The rest of the precipitates were then added with 10 μg of dephosphorylated MBP (Millipore, Billerica, MA) and 10 μl of Mg/ATP cocktail (500 μM ATP, 75 mM MgCl2) in kinase reaction buffer. After incubation at 30°C for 30 min, the precipitates containing phosphorylated MBP were subjected to Western blot analysis using antiphosphoserine antibody. ERK2 antibodies and regular IgG were used as positive and negative controls, respectively.
Statistical Analysis.
Data were presented as the mean ± S.E. One-way or two-way analysis of variance (ANOVA) was used to analyze the differences between experimental groups followed by Tukey post-test using Prism software (GraphPad Software Inc., San Diego, CA). Student's t tests for paired data were also used in some cases as indicated. p values <0.05 were considered statistically significant.
Results
Resveratrol Protected against DOX-Induced Cardiomyocyte Death.
The effects of resveratrol on cardiomyocytes are strictly dose-dependent (Gurusamy et al., 2010). Therefore, we first determined whether different doses of resveratrol would differentially affect the viability of NRCs. NRCs were exposed to a series of doses of resveratrol for 24 h, and the viability of NRCs was examined by PI staining that marks dead cells regardless of the cause of death and the cleavage of caspase 3 or PARP that indicates apoptosis. When used at relatively low doses (<15 μM), RV did not have any effect on the percentage of PI-positive cells (Fig. 1A; control, 10.0 ± 1.5 versus RV 10 μM, 10.7 ± 2.1; p > 0.05; n = 4). However, at higher doses (≥15 μM), resveratrol induced cardiomyocyte death as indicated by increased PI-positive cells (Fig. 1A; 10 μM, 10.7 ± 2.1 versus 15 μM, 21.1 ± 1.3; p < 0.05; n = 4) and elevated cleavage of caspase 3 and PARP (Fig. 1B), suggesting that high-dose resveratrol is toxic to cardiomyocytes. Because resveratrol has been reported to reduce DOX cardiotoxicity (Danz et al., 2009; Tatlidede et al., 2009), we then tested whether resveratrol at 10 μM could protect against DOX-induced cardiomyocyte death. As expected, pretreatment of cells with resveratrol markedly diminished DOX-induced cardiomyocyte death as shown by PI-positive cells (Fig. 1C; control, 10.2 ± 0.5 versus DOX, 43.7 ± 4.2, p < 0.01; DOX, 43.7 ± 4.2 versus DOX+RV, 19.8 ± 0.35, p < 0.01; n = 4), DNA laddering (Fig. 1D), and cleaved caspase 3 and PARP (Fig. 1E). Our results indicated that resveratrol is sufficient to attenuate DOX-induced cardiomyocyte death.
Fig. 1.
Resveratrol protected against DOX-induced cardiomyocyte death. A and B, dose-response study was performed. NRCs were cultured in DMEM with 2% bovine serum and treated with different doses of RV (1–20 μM) for 24 h. Cell death was determined by PI staining (A) and Western blot analysis of c-Casp3 and cleaved PARP (c-PARP) (B). c-Casp3 and c-PARP were sequentially normalized to GAPDH and the value at 0 μM RV. Data are expressed as mean ± S.E. and were analyzed by one-way ANOVA (n = 4). *, p < 0.05 and **, p < 0.01 versus 0 to 10 μM; #, p < 0.05 versus 15 μM. Ten micromoles of RV was selected for further study. C to E, NRCs were pretreated with RV or vehicle for 12 h, and then exposed to either DOX (1 μM) or saline. Cardiomyocyte death was determined by PI staining (C), DNA laddering (D), and cleavage of caspase 3 and PARP (E). Data are expressed as mean ± S.E. and were analyzed by one-way ANOVA (n = 4). The scale bar in C is 1 mm. **, p < 0.01 versus control (CON); #, p < 0.05 versus DOX.
Resveratrol Diminished DOX-Induced Autophagy in Cardiomyocytes.
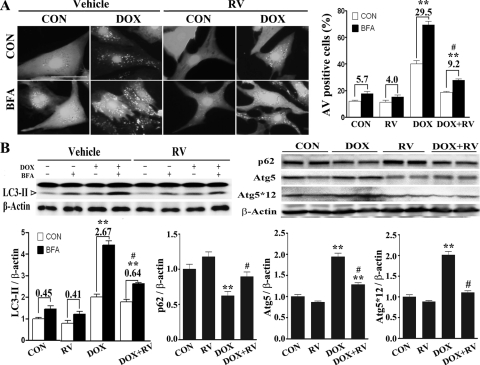
DOX can induce autophagy in cardiomyocytes, which is a major mechanism of DOX cardiotoxicity (Lu et al., 2009; Kobayashi et al., 2010; Chen et al., 2011). Therefore, we tested whether resveratrol could inhibit DOX-induced autophagy. When autophagy is induced, LC3 form I is converted to LC3-II and conjugated to the autophagosome membrane. LC3-II remains associated with autophagic vacuoles (AVs) until being degraded by lysosomal proteases (Kabeya et al., 2000). AV formation can be visualized by increased punctate structures (dots) of the GFP-LC3 fusion protein that is introduced into cells (Mizushima et al., 2010). Thus, autophagy can be measured by the amount of LC3-II protein or the number of GFP-LC3 dots formed. To differentiate whether the accumulation of LC3-II protein or GFP-LC3 dots is caused by an increased AV formation or a decreased degradation it is necessary to measure the difference in LC3-II protein levels or GFP-LC3 dots in the absence and presence of a lysosomal inhibitor. This difference is referred to as an autophagic flux that reflects the number of AVs that are delivered to and degraded in the lysosome (Mizushima and Yoshimori, 2007; Mizushima et al., 2010). To this end, we infected NRCs with an adenovirus encoding GFP-LC3. NRCs were pretreated with resveratrol (10 μM) for 12 h and then exposed to DOX (1 μM) for another 18 h. The lysosomal inhibitor BFA (50 nM) was added 6 h before the cells were examined for GFP-LC3 dots (AVs) under a confocal microscope. In line with previous reports (Lu et al., 2009; Kobayashi et al., 2010; Chen et al., 2011), DOX triggered the formation of numerous AVs as indicated by the GFP-LC3 dots, which was further increased by BFA treatment (Fig. 2A), suggesting that DOX accelerated autophagic flux in cardiomyocytes. However, the number of GFP-LC3 dots was dramatically reduced by resveratrol pretreatment, demonstrating the ability of resveratrol to inhibit DOX-induced autophagic flux. We counted cells with more than 25 GFP-LC3 dots, an arbitrary cutoff number that defines a cell as positive for increased AVs. Again, DOX increased the percentage of AV-positive cells with and without BFA treatment, which was largely inhibited by resveratrol (Fig. 2A, right; control, 5.7 ± 0.35 versus DOX, 29.5 ± 2.9, p < 0.01; DOX, 29.5 ± 2.9 versus DOX+RV, 9.2 ± 0.8, p < 0.01; n = 3).
Fig. 2.
Resveratrol attenuated DOX-induced autophagy in cardiomyocytes. NRCs were infected with a GFP-LC3-encoding adenovirus and treated with RV (10 μM) and DOX alone or together in the presence or absence of the lysosome inhibitor BFA. A, left, DOX increased the number of AVs as shown by GFP-LC3 dots, which was prevented by RV. A, right, and B, left, autophagic flux was calculated as the percentage increase (the numbers above bars) of AV-positive cells (A, right) or the fold increase of endogenous LC3-II protein levels with and without BFA (B, upper left, Western blots; B lower left, bar graph). B, right, P62-and autophagy-related genes were also analyzed (top, Western blots; bottom, bar graphs). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA followed by paired Student's t test (n = 3). The scale bar in A is 50 μm. **, p < 0.01 versus control; #, p < 0.05 versus DOX.
Alternatively, the autophagic flux was quantified by the difference of endogenous LC3-II protein levels in the absence and presence of BFA. In this case, autophagic flux is indicated by the fold increase (the numbers above bars in Fig. 2) of LC3-II protein levels after BFA treatment. Similar to the results obtained with GFP-LC3 dots, autophagic flux was increased by DOX treatment, which was prevented by resveratrol (Fig. 2B; control, 0.45 ± 0.11 versus DOX, 2.67 ± 0.15, p < 0.01; DOX, 2.67 ± 0.15 versus DOX+RV, 0.64 ± 0.09, p < 0.01; n = 3). We also determined p62 protein levels to assess autophagic flux. p62/SQSTM1 is a polyubiquitin-binding protein, which is degraded by autophagy, and its protein levels are inversely related to autophagy activity (Komatsu et al., 2007; Mizushima and Yoshimori, 2007; Nakai et al., 2007). As shown in Fig. 2B, right, DOX decreased p62 levels, which were attenuated by resveratrol. Likewise, DOX increased the levels of Atg5 and Atg5-Atg12 complex. However, resveratrol reversed this effect. Taken together, these results demonstrated that resveratrol is able to inhibit DOX-induced autophagy. Because low-dose resveratrol was shown to induce autophagy in H9c2 cardiac myoblast cells (Gurusamy et al., 2010), we tested whether this holds true in primary cardiomyocyte culture. We found that resveratrol at lower doses (∼0.1–1 μM) did not affect autophagic flux (data not shown).
The Ability of Resveratrol to Antagonize DOX-Induced Cardiomyocyte Death Is Mediated through Autophagy Inhibition.
Induction of autophagy could be an adaptive response to protect cells from environmental stresses. However, enhanced autophagy can also trigger cell death under certain conditions (Matsui et al., 2007; Kang and Avery, 2008). Indeed, DOX induced-autophagy contributes to cardiomyocyte death, which is a major mechanism of DOX cardiotoxicity (Lu et al., 2009; Kobayashi et al., 2010; Chen et al., 2011). Given the ability of resveratrol to concurrently suppress DOX-induced autophagy and cardiomyocyte death, it is possible that the protective effects of resveratrol against DOX toxicity are mediated through autophagy inhibition. To test this possibility, we infected cardiomyocytes with adenoviruses encoding either autophagy-related protein beclin1 or β-gal and 36 h later treated cells with DOX in the absence or presence of resveratrol. Overexpression of beclin 1 accelerated autophagic flux as evidenced by increased LC3-II levels with or without BFA treatment (Fig. 3A; Adβgal, 0.62 ± 0.07 versus AdBCN1, 1.05 ± 0.13; p < 0.01; n = 3), which enhanced DOX-induced cardiomyocyte death as shown by PI staining (Fig. 3, B and C; Adβgal+DOX, 44 ± 4.4 versus AdBCN1+DOX, 73 ± 9.3; p < 0.01; n = 3), DNA laddering (Fig. 3D), and the cleavage of caspase 3 and PARP (Fig. 3E). Resveratrol blocked DOX-induced cardiomyocyte death in Adβgal-infected cells, but this ability was abrogated in AdBCN1-infected cells (Fig. 3, B and C; PI-positive cells; AdBCN1+DOX, 73 ± 9.3 versus AdBCN1+DOX+RV, 69 ± 8.1; p > 0.05; n = 3). These results demonstrate that up-regulation of autophagy by beclin 1 overexpression enhanced DOX-induced cardiotoxicity and abolished the protective effects of resveratrol, suggesting that autophagy suppression is critical for resveratrol to antagonize DOX-induced cardiomyocyte death.
Fig. 3.
Up-regulation of autophagy by beclin 1 overexpression impaired the ability of resveratrol to antagonize DOX-induced cardiomyocyte death. NRCs were infected with Adβgal or AdBCN1 for 24 h. A, autophagic flux was examined by Western blot analysis of LC3-II in the presence or absence of BFA. B to E, cardiomyocytes were further treated with DOX (1 μM) alone or plus RV (10 μM), and cell death was determined by PI staining (B and C), DNA laddering (D), and cleavage of caspase 3 and PARP (E). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA followed by paired Student's t test (n = 3–4). The scale bar in B is 500 μm. *, p < 0.05; **, p < 0.01. n.s., not significant.
To further corroborate the role of autophagy inhibition in resveratrol-mediated cytoprotection, we determined the cardioprotective effects of resveratrol in the presence of the autophagy inhibitor 3-MA. Cardiomyocytes were treated with DOX, resveratrol, and 3-MA (2.5 mM), either alone or in combination for 18 h. PI staining showed that DOX-induced cell death was attenuated by 3-MA, but it was not further reduced by resveratrol (Fig. 4A; DOX, 43.4 ± 6.01 versus DOX + 3-MA, 24.9 ± 3.4, p < 0.01; DOX + 3-MA, 24.9 ± 3.4 versus DOX + 3-MA+RV, 28.1 ± 2.6, p > 0.05; n = 4). Likewise, DOX-induced DNA laddering was mitigated by either 3-MA or resveratrol alone, but both drugs together did not produce additive effects (Fig. 4B). To rule out the potential nonspecific effects of 3-MA, we down-regulated autophagy in cardiomyocytes with an adenovirus encoding a shBCN1. A scrambled shRNA (shCON) was used as control. Autophagic flux was significantly reduced after beclin 1 knockdown as indicated by the decreased LC3-II protein levels (Fig. 4C; shCON, 0.68 ± 0.4 versus shBCN1, 0.38 ± 0.3; p < 0.05; n = 3). Similar to 3-MA, beclin 1 knockdown attenuated DOX-induced cardiomyocyte death as shown by PI staining (Fig. 4D; DOX, 45.7 ± 5.11 versus DOX+shBCN1, 26.4 ± 2.4; p < 0.01; n = 4), which was not further reduced by resveratrol (DOX+shBCN1, 26.4 ± 2.4 versus DOX+shBCN1+RV, 22.4 ± 2.3; p > 0.05; n = 4). In addition, resveratrol did not further enhance the effects of beclin 1 knockdown on DOX-induced DNA laddering (Fig. 4E) and cleavage of caspase 3 and PARP (Fig. 4F). Together, these results indicate that down-regulation of autophagy by either 3-MA or beclin 1 knockdown alleviated DOX-induced cardiotoxicity, but resveratrol failed to exert an additive effect. Thus, our data from studies with either up- or down-regulation of autophagy strongly suggest that the protective effects of resveratrol against DOX cardiotoxicity probably are mediated through its ability to inhibit autophagy.
Fig. 4.
Down-regulation of autophagy by 3-MA or beclin 1 knockdown alleviated DOX-induced cardiomyocyte death, and resveratrol failed to exert an additive effect. A and B, NRCs were treated with DOX, resveratrol, and 3-MA (2.5 mM), either alone or in combination for 18 h, and cell death was determined by PI staining (A) and DNA laddering (B). C, alternatively, NRCs were infected with adenoviruses encoding either a shCON or a shBCN1 for 36 h. shBCN1-mediated down-regulation of autophagy was confirmed by the inhibited autophagic flux as measured by the LC3-II protein levels in the presence or absence of BFA. D to F, cardiomyocytes were then treated with vehicle, DOX (1 μM), or DOX plus RV (10 μM), and cell death was determined by PI staining (D), DNA laddering (E), and c-Casp3 and c-PARP (F). Data in A, C, D, and F are expressed as mean ± S.E. and were analyzed by two-way ANOVA (n = 3–4). The scale bars in A and D are 500 μm. *, p < 0.05; **, p < 0.01.
DOX Activated S6K1, which Was Prevented by Resveratrol.
To explore the underlying molecular mechanisms that mediate the ability of resveratrol to inhibit DOX-triggered autophagy in cardiomyocytes, we examined several signaling pathways known to either positively or negatively regulate autophagy. The intracellular energy sensor AMP-activated protein kinase (AMPK) is a positive regulator of autophagy. DOX and resveratrol, either alone or in combination, did not have appreciable effects on AMPK activity as indicated by Western blot analysis of phosphorylated AMPKα, AMPKβ, and its downstream target acetyl-CoA carboxylase (data not shown), suggesting that AMPK might not be involved in autophagy induction by DOX or its subsequent inhibition by resveratrol. In addition, we found that DOX robustly induced AKT signaling, as shown by the increased phosphorylation of ATK at Thr-308 and Ser-473 and its downstream effecter glycogen synthase kinase 3β at Ser-9. This DOX effect was completely reversed by resveratrol (data not shown). However, because AKT is a negative regulator of autophagy, the above specific changes in AKT signaling are unlikely responsible for autophagy induction by DOX and its inhibition by resveratrol. We then examined the effects of DOX and/or resveratrol on the mammalian target of rapamycin (mTOR). DOX enhanced mTOR signaling as shown by the increased phosphorylation of mTOR downstream targets S6K and S6 (Fig. 5A). Given that mTOR is a kinase that in general negatively regulates autophagy, this result did not seem to support a role for mTOR in DOX-induced autophagy. Moreover, resveratrol largely inhibited mTOR signaling either in the absence or presence of DOX, as indicated by the reduced phosphorylation of TSC2, PRAS40, S6K, and S6 (Fig. 5A). Apparently, the general inhibitory effects of resveratrol on mTOR signaling did not seem to be compatible with its ability to antagonize DOX-induced autophagy. Intriguingly, however, S6K1 has been shown to positively regulate autophagy independent of mTOR signaling in some contexts (Scott et al., 2004) and has been proposed to mediate the inhibitory effect of resveratrol on autophagy in human NIH3T3 and human embryonic kidney 293 cells (Armour et al., 2009). In this respect, DOX treatment led to a 6.77-fold increase in the phosphorylation of S6K1 on Thr-389, which was drastically reduced by resveratrol (Fig. 5A; DOX, 6.77 ± 1.36 versus DOX+RV, 2.69 ± 0.69; p < 0.05; n = 4). We further measured the kinase activity of S6K1 under these conditions by an immuno-complex kinase assay using MBP as the substrate. As shown in Fig. 5B, resveratrol did not affect S6K1 activity at baseline, but it inhibited DOX-induced S6K1 activation, consistent with the phosphorylation levels of S6K1 and its downstream target S6. Thus, these results raise the possibility that DOX may induce autophagy in cardiomyocytes through S6K1 activation, and resveratrol may prevent autophagy induction by inhibiting S6K1 signaling.
Fig. 5.
DOX enhanced S6K1 signaling, whereas resveratrol attenuated it. A, cardiomyocytes were treated with resveratrol and DOX either alone or in combination for 18 h before being subjected to Western blot analysis (left). The protein levels of phosphorylated S6K1 and S6 were normalized to total S6K1 and total S6 levels, respectively, and quantified by densitometry (right). B, the kinase activity of S6K1 was measured by an immuno-complex kinase assay using MBP as the substrate. The immunoprecipitation (IP) was performed by using a S6K1 antibody. An ERK2 antibody and regular IgG served as positive and negative controls, respectively. The α1-adrenergic receptor agonist phenylephrine (PE; 25 μM) was used to stimulate ERK activity. Phosphorylated MBP on the serine site was detected by immunoblot (IB) using an antiphosphoserine antibody and quantified by densitometry assay. Data are expressed as mean ± S.E. and were analyzed by one-way ANOVA (n = 3–4). *, p < 0.05, **, p < 0.01 versus vehicle control; #, p < 0.05 versus DOX.
S6K1 Knockdown Attenuated DOX-Induced Autophagy and Cardiomyocyte Death, but Resveratrol Failed to Exert an Additive Effect.
To characterize the role of S6K1 in DOX-induced autophagy and cardiomyocyte death, we knocked down S6K1 expression with siRNA. Cardiomyocytes were transfected with a control siRNA (siCON) or a siRNA against S6K1 (siS6K1), and then treated with DOX at 36 h after transfection. Western blot analysis showed that siS6K dramatically reduced S6K1 protein levels and its phosphorylation on Thr-389 (Fig. 6A), indicating the effectiveness of the siRNA-mediated S6K1 gene silencing. We then performed autophagic flux assays as described earlier. DOX accelerated autophagic flux, which was blocked by S6K1 knockdown, as quantified by the difference of endogenous LC3-II protein levels in the absence and presence of BFA (Fig. 6, B and D; DOX, 2.40 ± 0.21 versus DOX+siS6K1, 0.58 ± 0.08; p < 0.01; n = 4). In addition, the autophagic flux was determined with GFP-LC3 reporter. DOX induced the formation of numerous AVs indicated by the GFP-LC3 dots, whereas S6K1 knockdown markedly attenuated AV formation (Fig. 6C). Quantitatively, DOX increased the percentage of AV-positive cells with or without BFA intervention, which was largely inhibited by S6K1 knockdown (Fig. 6E; DOX, 30.7 ± 5.2 versus DOX+siS6K1, 6.1 ± 3.1; p < 0.01; n = 4). These results confirmed the ability of S6K1 knockdown to block DOX-induced autophagic flux, suggesting an essential role for S6K in autophagy induction. It is noteworthy that after S6K1 knockdown DOX-induced autophagic flux was not further reduced by the addition of resveratrol as evidenced by LC3-II levels (Fig. 6D; DOX+siS6K1, 0.58 ± 0.08 versus DOX+siS6K1+RV, 0.66 ± 0.13; p > 0.05; n = 4) and AV-positive cells (Fig. 6E; DOX+siS6K1, 6.1 ± 3.1 versus DOX+siS6K1+RV, 8.3 ± 2.2; p > 0.05; n = 4). These data support the notion that resveratrol may inhibit DOX-induced autophagy through its suppressing effect on S6K1.
Fig. 6.
S6K1 knockdown attenuated DOX-induced autophagy, and resveratrol failed to further reduce it. NRCs were transfected with control siRNA (siCON) or siRNA against S6K1 (siS6K1) for 36 h, and then treated with DOX and resveratrol in the presence or absence of BFA. A, phosphorylated and total S6K1 were analyzed after siRNA transfection. B and C, autophagic activity was assessed by the protein levels of LC3-II (B) and the accumulation of AVs was indicated by GFP-LC3 dots (C) in the absence and presence of BFA. The scale bar in C is 50 μm. D and E, autophagic flux was quantified by LC3-II levels (D) or percentage of AV-positive cells (E). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA followed by paired Student's t test (n = 4). **, p < 0.01.
We next examined cell death after S6K1 knockdown. DOX-induced cell death was significantly inhibited by S6K1 knockdown, and the addition of resveratrol failed to exert an additive effect, as indicated by PI-positive cells (Fig. 7, A and B; DOX, 45.0 ± 4.21 versus DOX+siS6K, 31 ± 3.88, p < 0.05; DOX+siS6K, 31 ± 3.8 versus DOX+siS6K+RV, 32 ± 2.06, p > 0.05; n = 3), DNA laddering (Fig. 7C), and the cleavage of caspase 3 and PARP (Fig. 7D). Again, these results raise the possibility that the cardioprotective effects of resveratrol may be mediated through S6K1 inhibition.
Fig. 7.
S6K1 knockdown attenuated DOX-induced cardiomyocyte death, but resveratrol did not provide further protection. NRCs were transfected with siCON or siS6K1 for 36 h, and then treated with resveratrol (10 μM) and DOX (1 μM), alone or together. Cardiomyocyte death was determined by PI staining (A and B), DNA laddering (C), and c-Casp3 and c-PARP (D). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA (n = 3–4). The scale bar in A is 500 μm. *, p < 0.05; **, p < 0.01.
Overexpression of S6K1 Did Not Enhance DOX-Induced Autophagy and Cardiomyocyte Death, but It Abolished the Protective Effects of Resveratrol.
The results from the above loss-of-function experiments demonstrate an essential role for S6K1 in DOX-induced autophagy and cardiomyocyte death. Resveratrol per se is sufficient to reduce DOX cardiotoxicity, but it cannot further enhance the protective effect of S6K1 knockdown, suggesting S6K1 inhibition as a major mechanism that mediates the ability of resveratrol to antagonize DOX cardiotoxicity. If this is true, overexpression of S6K1 should attenuate or abolish the protective effects of resveratrol. To test this possibility, we infected cardiomyocytes with adenoviruses encoding either β-gal or S6K1. As shown in Fig. 8A, adenovirus-mediated gene transfer increased the protein levels of S6K in a MOI-dependent manner, leading to elevated phosphorylation levels of S6, a downstream target of S6K1 (Fig. 8A). We then determined whether S6K1 overexpression could affect autophagy in cardiomyocytes with or without DOX treatment. To our surprise, increasing S6K1 expression did not induce autophagy, nor did it further accelerate DOX-triggered autophagic flux, as shown by the LC3-II protein levels (Fig. 8, B and D; DOX, 2.1 ± 0.31 versus DOX+AdS6K1, 2.2 ± 0.28; p > 0.05; n = 3) and the percentages of AV-positive cells before and after BFA treatment (Fig. 8, C and E). Strikingly, when S6K1 was overexpressed, resveratrol could no longer inhibit DOX-induced autophagy as indicated by either LC3-II (Fig. 8, B and D; DOX+AdS6K1, 2.2 ± 0.28 versus DOX+AdS6K1+RV, 2.0 ± 0.30; p > 0.05; n = 3) or AV-positive cells (Fig. 8, C and E; DOX+AdS6K1, 38 ± 7.28 versus DOX+AdS6K1+RV, 32 ± 8.50; p > 0.05; n = 3). These data strongly suggest that S6K1 inhibition is important for resveratrol to suppress autophagic activity in cardiomyocytes treated with DOX.
Fig. 8.
Overexpression of S6K1 abolished the ability of resveratrol to inhibit DOX-induced autophagy. A, NRCs were infected with Adβgal or AdS6K1 at the indicated MOI for 36 h, and P70S6K and the phosphorylation of its downstream target ribosome S6 were analyzed with Western blotting. After adenoviral infection, cardiomyocytes were treated with resveratrol for 12 h before exposed to DOX for another 18 h. B and C, autophagic activity was assessed by either LC3-II protein levels (B) or GFP-LC3 dots (C) in the presence or absence of BFA. The scale bar in C is 50 μm. D and E, autophagic flux was quantified by relative LC3-II levels (D) or AV-positive cells (E). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA followed by paired Student's t test (n = 3). **, p < 0.01.
In addition, we measured cardiomyocyte death under this condition. Although S6K1 overexpression did not affect DOX-induced cell death, it markedly compromised the ability of resveratrol to reduce DOX cytotoxicity, as shown by PI staining (Fig. 9, A and B; DOX, 44.0 ± 3.21 versus DOX+AdS6K1, 46 ± 3.88, p > 0.05; DOX+AdS6K1, 46 ± 3.88 versus DOX+AdS6K1+RV, 42 ± 4.08, p > 0.05; n = 4), the cleavage of caspase 3 and PARP (Fig. 9C), and DNA laddering (Fig. 9D). These observations suggest the inhibition of S6K1 as an important underlying mechanism that mediates the protective effects of resveratrol against DOX cardiotoxicity.
Fig. 9.
Overexpression of S6K1 impaired the ability of resveratrol to antagonize DOX-induced cardiomyocyte death. NRCs were infected with Adβgal or AdS6K1 for 24 h and treated with 10 μM resveratrol for 12 h before being exposed to 1 μM DOX. Cell death was determined by PI staining (A and B), Western blot analysis of c-Casp3 and c-PARP (C), and DNA laddering (D). Data are expressed as mean ± S.E. and were analyzed by two-way ANOVA (n = 4). The scale bar in A is 500 μm. *, p < 0.05; **, p < 0.01.
Discussion
DOX-induced cardiotoxicity is the major obstacle that limits its therapeutic usage in cancer chemotherapy (Minotti et al., 2004). Dexrazoxane is the only drug approved by the Food and Drug Administration to reduce DOX cardiotoxicity, yet its combined use with DOX has been limited, because of fears of its negative effect on the antitumor efficacy of DOX (Tebbi et al., 2007). However, resveratrol has been shown to enhance the anticancer activity of DOX and at the same time exert cardioprotective effects (Aggarwal et al., 2004; Rezk et al., 2006), raising the possibility that the combined use of resveratrol and DOX may be a viable chemotherapeutic modality that will find increased use in DOX antitumor therapy in the near future. The mechanisms by which resveratrol could deliver cardiac beneficial effects in the setting of DOX chemotherapy remain poorly understood. In the present study, we demonstrated that DOX-induced cardiomycyte death was dramatically reduced by resveratrol, and this protective effect largely depended on the ability of resveratrol to suppress autophagy. Our data also indicate that S6K1 is a critical molecular mediator of DOX-induced autophagy and cardiomyocyte death, and that inhibiting S6K1 is important for resveratrol to prevent autophagy induction and reduce DOX cardiotoxicity.
Resveratrol-conferred protection against DOX cardiotoxicity is associated with increased superoxide dismutase activity and reduced production of reactive oxygen species (Tatlidede et al., 2009), suggesting that the antioxidant properties of resveratrol may play a role in its cardioprotective effects. However, antioxidant therapies have failed to produce satisfactory results in clinical trials (Gianni et al., 2008), casting doubt on the notion that the inhibition of oxidative stress is the only mechanism responsible for the cardioprotective effects of resveratrol. In this respect, we found that the ability of resveratrol to attenuate DOX-induced cardiomyocyte death depends on autophagy suppression, providing another explanation for the protective effects of resveratrol. Because oxidative stress can induce autophagy, triggering autophagic cancer cell death (Chen et al., 2008), it is possible that resveratrol-elicited autophagy inhibition may result, at least in part, from its ability to antagonize DOX-induced oxidative stress. However, given the failure of antioxidant therapies in cancer patients that receive DOX, it is more likely that autophagy suppression is an independent mechanism by which resveratrol attenuates DOX cardiotoxicity.
Autophagy is a catabolic process involving the degradation of a cell's own components through the lysosomal machinery, which can be either protective or detrimental depending on the specific cellular context. DOX-induced autophagy in cardiomyocytes is detrimental because inhibiting autophagy with a chemical or genetic approach dramatically attenuates DOX-induced cardiomyocyte death as shown previously (Lu et al., 2009; Kobayashi et al., 2010; Chen et al., 2011). Thus, a potential therapeutic strategy for reducing DOX cardiotoxicity is to suppress DOX-induced autophagy. Consistently, the transcription factor GATA4 and the caloric restriction mimetic 2-deoxyglucose are able to inhibit DOX-induced autophagy, thereby reducing cardiomyocyte death (Kobayashi et al., 2010; Chen et al., 2011). To reinforce this concept, resveratrol is also capable of antagonizing DOX cardiotoxicity through autophagy inhibition (Figs. 2–4). Likewise, resveratrol attenuates starvation-induced autophagy in multiple noncardiac mammalian cell lines, although its effect on cell viability is unknown (Armour et al., 2009).
However, resveratrol has been shown to enhance hypoxia-reoxygenation-induced autophagy in H9c2 cardiac myoblast cells at low doses (0.1 and 1 μM), which correlates with improved cell survival (Gurusamy et al., 2010). The same study has also demonstrated that high-dose resveratrol (100 μM) inhibits autophagy, leading to increased cell death. Moreover, resveratrol can induce autophagy in multiple ovarian carcinoma cell lines, which seems to cause cell death (Opipari et al., 2004). Apparently, the specific effects of resveratrol on autophagy and cell fate depend on multiple factors including drug dose, cell type, and the nature, degree, and duration of the stress placed on the cell.
Intriguingly, our study not only demonstrated that resveratrol attenuated DOX cardiotoxicity by suppressing autophagy, but also suggested that the ability of resveratrol to prevent DOX-induced autophagy probably is mediated through its inhibitory effect on S6K1. The evidence is 3-fold: first, the phosphorylation of S6K1 is significantly elevated in response to DOX treatment but largely prevented by addition of resveratrol; second, S6K1 knockdown attenuated DOX-induced autophagy, which was not further reduced by resveratrol; and finally, S6K1 overexpression abolished the effect of resveratrol on DOX-induced autophagy, suggesting that suppressing S6K1 may be important for resveratrol to inhibit autophagy. Nevertheless, we cannot completely rule out other possibilities that may explain the relationship between S6K and resveratrol. For instance, resveratrol may not protect the cells through directly inhibiting S6K. Rather, it could act on a downstream effecter to provide cardioprotection. In addition, the ability of S6K overexpression to antagonize resveratrol action could be mediated by the opposing effects of S6K and resveratrol on an unidentified common target. In addition, overexpression of S6K could modify the cellular response to DOX such that DOX induces autophagy and cell death through a different pathway that is insensitive to resveratrol. However, these speculations can be confirmed only by further experimentations.
At first sight, the observation that S6K1 positively regulates autophagy upon DOX exposure seems contradictory to the fact that S6K is a downstream effecter of mTOR, a well established negative regulator of autophagy. Indeed, previous studies demonstrated that inhibition of S6K kinase can induce autophagy (Lee et al., 2007), whereas activation of ribosomal protein S6 can suppress autophagy (Blommaart et al., 1995). However, accumulating evidence has suggested that S6K can contribute to autophagy induction under many other conditions. For example, S6K1 can positively regulate 6-thioguanine-induced autophagy (Zeng and Kinsella, 2008). In Drosophila melanogaster fat body, S6K promotes rather than suppresses autophagy in response to starvation (Scott et al., 2004). Except for the gain-of-function studies, knockdown of both S6K1 and S6K2 genes decreased starvation-dependent GFP-LC3 conversion in 293A cells (Chan et al., 2007). Likewise, inhibition of S6K1 by either shRNA or dominant-negative S6K1 mutant suppresses autophagy in human embryonic kidney 293 cells (Armour et al., 2009). Collectively, these results demonstrate that whether S6K1 positively or negatively regulates autophagy depends on specific cellular contexts such as cell type, culture condition, and the nature of the treatment. Nevertheless, the underlying molecular mechanisms that confer the proautophagy or antiautophagy property of S6K remain poorly understood. One possible explanation for the proautophagy function of S6K is that S6K1 negatively regulates insulin signaling (Shah et al., 2004). The latter normally inhibits autophagy.
Our data also demonstrated that S6K1 knockdown markedly attenuated DOX-induced cardiomyocyte death, suggesting that S6K1 activation is required for DOX to induce cardiotoxicity. Although S6K1 overexpression is not sufficient to enhance DOX cardiotoxicity, it abolishes the protective effect of resveratrol. Apparently, our observation that S6K1 mediates DOX cardiotoxicity challenges a longstanding view that S6K1 acts downstream of mTOR to promote cell growth and survival. One possibility is that S6K1 may control either prosurvival or prodeath signaling in response to different environmental stimuli. When nutrients are abundant to favor cell growth, S6K1 is activated by mTOR to exert prosurvival effects. On the other hand, when dealing with excessive stress, S6K1 can activate cell death pathway, which is probably independent of mTOR signaling. It is noteworthy that, in support of a prodeath role of S6K1, several studies have demonstrated that down-regulation of S6K1 is protective. For instance, S6K1 deficiency is able to protect against hepatocyte apoptosis (González-Rodriguez et al., 2009). In yeast, deletion of Sch9, the homolog of mammalian S6K/Akt, protects against age-dependent defects and extends chronological lifespan (Wei et al., 2008). In Drosophila melanogaster, expression of a dominant negative S6K1 results in resistance to oxidative stress (Patel and Tamanoi, 2006). It is noteworthy that deletion of S6K1 is sufficient to extend mouse lifespan (Selman et al., 2009), underscoring the beneficial nature of down-regulating S6K1 signaling. Therefore, resveratrol-conferred protection against DOX cardiotoxicity could be attributed, at least in part, to its ability to inhibit S6K1 as well as autophagy.
In summary, our study demonstrates that resveratrol is able to attenuate DOX-induced cardiomyocyte death, which largely depends on its ability to inhibit autophagy. Our results have also suggested that the inhibition of S6K1 is a critical event that probably mediates the ability of resveratrol to suppress DOX-induced autophagy and cytotoxic effects. Therefore, therapeutic strategies that aim to reduce S6K1 signaling and autophagic activity will presumably be able to ameliorate DOX cardiotoxicity, leading to improved clinical use of DOX in cancer chemotherapy.
Acknowledgments
We thank Dr. Aviva Tolkovsky (University of Cambridge, Cambridge, UK) for providing the adenovirus expressing GFP-LC3; Andy Cypher (Cell Culture Core Facility, Sanford Research/University of South Dakota) for providing cultured cardiomyocytes; Tricia Krueger and Yuan Huang (Virus Core Facility, Sanford Research/University of South Dakota) for preparing several adenoviruses; and members of the Imaging Core Facility (Sanford Research/University of South Dakota) for assisting with confocal microscopy. These core facilities are supported by the National Institutes of Health National Center for Research Resources [Grant 2P20-RR-017662-06A1].
This work was supported by the National Institutes of Health National Center for Research Resources [Grant 2P20-RR-017662-06A1]; the Juvenile Diabetes Research Foundation [Grant 1-2007-741]; and a Career Development Grant from the American Diabetes Association [Grant 1-09-CD-09].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- DOX
- doxorubicin
- NRC
- neonatal rat ventricular cardiomyocyte
- mTOR
- mammalian target of rapamycin
- AMPK
- AMP-activated protein kinase
- DMEM
- Dulbecco's modified Eagle's medium
- RV
- resveratrol
- 3-MA
- 3-methyladenine
- BFA
- bafilomycin A1
- PARP
- poly(ADP-ribose) polymerase
- c-PARP
- cleaved PARP
- siRNA
- small interfering RNA
- siCON
- control siRNA
- siS6K1
- siRNA against S6K1
- PI
- propidium iodide
- ANOVA
- analysis of variance
- shRNA
- short hairpin RNA
- shBCN1
- shRNA directed against autophagy gene BCN1
- shCON
- control shRNA
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- AV
- atophagic vacuole
- GFP
- green fluorescent protein
- S6K1
- p70 S6 kinase 1
- LC3
- microtubule-associated protein light chain 3
- Atg
- autophagy-related
- BCN1
- beclin 1
- MOPS
- 4-morpholinepropanesulfonic acid
- c-Casp3
- cleaved caspase 3
- Adβgal
- adenovirus expressing β-galactosidase
- AdBCN1
- adenovirus expressing BCN1
- MOI
- multiplicity of infection
- PCR
- polymerase chain reaction
- ERK
- extracellular signal-regulated kinase
- MBP
- myelin basic protein
- CON
- control
- IP
- immunoprecipitation
- IB
- immunoblot
- PE
- phenylephrine
- n.s.
- not significant.
Authorship Contributions
Participated in research design: Xu and Liang.
Conducted experiments: Xu, Chen, and Kobayashi.
Contributed new reagents or analytic tools: Xu, Kobayashi, and Timm.
Performed data analysis: Xu, Chen, and Kobayashi.
Wrote or contributed to the writing of the manuscript: Xu and Liang.
References
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24:2783–2840 [PubMed] [Google Scholar]
- Akazawa H, Komazaki S, Shimomura H, Terasaki F, Zou Y, Takano H, Nagai T, Komuro I. (2004) Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem 279:41095–41103 [DOI] [PubMed] [Google Scholar]
- Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. (2009) Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 1:515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506 [DOI] [PubMed] [Google Scholar]
- Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. (1995) Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 270:2320–2326 [DOI] [PubMed] [Google Scholar]
- Chan EY, Kir S, Tooze SA. (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282:25464–25474 [DOI] [PubMed] [Google Scholar]
- Chen K, Xu X, Kobayashi S, Timm D, Jepperson T, Liang Q. (2011) Caloric restriction mimetic 2-deoxyglucose antagonizes doxorubicin-induced cardiomyocyte death by multiple mechanisms. J Biol Chem 286:21993–22006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15:171–182 [DOI] [PubMed] [Google Scholar]
- Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. (2009) Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med 46:1589–1597 [DOI] [PubMed] [Google Scholar]
- Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. (2008) Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol 26:3777–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. (2009) S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology 50:216–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK. (2010) Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res 86:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Avery L. (2008) To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy 4:82–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Mao K, Zheng H, Wang X, Patterson C, O'Connell TD, Liang Q. (2007) Diminished GATA4 protein levels contribute to hyperglycemia-induced cardiomyocyte injury. J Biol Chem 282:21945–21952 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. (2010) Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem 285:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163 [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. (2004) The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036 [DOI] [PubMed] [Google Scholar]
- Lee SB, Kim S, Lee J, Park J, Lee G, Kim Y, Kim JM, Chung J. (2007) ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep 8:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wu W, Yan J, Li X, Yu H, Yu X. (2009) Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol 134:82–90 [DOI] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922 [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. (2010) Methods in mammalian autophagy research. Cell 140:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624 [DOI] [PubMed] [Google Scholar]
- Opipari AW, Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. (2004) Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res 64:696–703 [DOI] [PubMed] [Google Scholar]
- Patel PH, Tamanoi F. (2006) Increased Rheb-TOR signaling enhances sensitivity of the whole organism to oxidative stress. J Cell Sci 119:4285–4292 [DOI] [PubMed] [Google Scholar]
- Rezk YA, Balulad SS, Keller RS, Bennett JA. (2006) Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol 194:e23–e26 [DOI] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7:167–178 [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol 14:1650–1656 [DOI] [PubMed] [Google Scholar]
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, et al. (2010) Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6:600–606 [DOI] [PubMed] [Google Scholar]
- Tatlidede E, Sehirli O, Velioğlu-Oğünc A, Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S, Sener G. (2009) Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res 43:195–205 [DOI] [PubMed] [Google Scholar]
- Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. (2007) Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 25:493–500 [DOI] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. (2008) Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430:686–689 [DOI] [PubMed] [Google Scholar]
- Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. (1996) The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98:1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Kinsella TJ. (2008) Mammalian target of rapamycin and S6 kinase 1 positively regulate 6-thioguanine-induced autophagy. Cancer Res 68:2384–2390 [DOI] [PubMed] [Google Scholar]