Abstract
Previous studies in vivo have shown that salsolinol, the condensation product of acetaldehyde and dopamine, has properties that may contribute to alcohol abuse. Although opioid receptors, especially the μ-opioid receptors (MORs), may be involved, the cellular mechanisms mediating the effects of salsolinol have not been fully explored. In the current study, we used whole-cell patch-clamp recordings to examine the effects of salsolinol on dopamine neurons of the ventral tegmental area (VTA) in acute brain slices from Sprague-Dawley rats. Salsolinol (0.01–1 μM) dose-dependently and reversibly increased the ongoing firing of dopamine neurons; this effect was blocked by naltrexone, an antagonist of MORs, and gabazine, an antagonist of GABAA receptors. We further showed that salsolinol reduced the frequency without altering the amplitude of spontaneous GABAA receptor-mediated inhibitory postsynaptic currents in dopamine neurons. The salsolinol-induced reduction was blocked by both naltrexone and [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin, an agonist of MORs. Thus, salsolinol excites VTA-dopamine neurons indirectly by activating MORs, which inhibit GABA neurons in the VTA. This form of disinhibition seems to be a novel mechanism underlying the effects of salsolinol.
Introduction
Ethanol is strongly addictive. Accumulating evidence suggests that some of the acute rewarding effects of ethanol are produced by its metabolites, such as salsolinol (Deng and Deitrich, 2008). In the brains of mammals racemic (R and S) salsolinol is formed by nonenzymatic Pictet-Spengler condensation of dopamine with acetaldehyde (Rommelspacher et al., 1995; Haber et al., 1996), the main metabolite of ethanol. It has been suggested that salsolinol has a role in the etiology of alcoholism (Davis and Walsh, 1970). Except for the work of Haber et al. (1996), previous studies on humans found that after acute (Faraj et al., 1989; Rommelspacher et al., 1995) or chronic (Faraj et al., 1989) alcohol drinking salsolinol levels in plasma (and presumably brain) may increase. Indeed, forced chronic ethanol consumption raised salsolinol levels in various brain regions of animals as confirmed by Starkey et al. (2006) and Rojkovicova et al. (2008), who showed that the pattern of ethanol intake determines how much salsolinol levels in the brain are enhanced. By contrast, it was found that salsolinol levels were elevated in the adrenals but not in the striatum of alcohol-preferring rats after 4 weeks of free-choice alcohol drinking (Haber et al., 1999). Other contradictory findings after ethanol consumption of alcohol-preferring rats are the research of Lee et al. (2010) and Rojkovicova et al. (2008). Rojkovicova et al. observed an increase in salsolinol levels in both the putamen and midbrain; whereas Lee et al. saw an increase in neither the striatum nor the nucleus accumbens (NAcc). Results from different experiments may vary extensively because of different experimental protocols, sampling methods, and/or drinking procedures (Hipólito et al., 2012).
Early animal studies revealed that salsolinol promotes alcohol drinking (Duncan and Deitrich, 1980; Myers et al., 1982). More recently, several groups have explored the effects of salsolinol on addiction-related behaviors. Direct injections of only 30 pM into the posterior ventral tegmental area (p-VTA) induced strong motor activity in rats, which was prevented by local pretreatment with antagonists of μ-opioids (MORs); moreover, repeated intra-VTA injections of salsolinol resulted in behavioral sensitization, like that produced by MOR agonists (Hipólito et al., 2010). Rodd et al. (2003, 2008) found that rats self-administer into the p-VTA as little as 30 pM of salsolinol; and bilateral intra-p-VTA salsolinol injections induce conditioned place preference, also in rats (Hipólito et al., 2011). Such behaviors seem to depend on the activation of dopamine neurons (Rodd et al., 2008). Indeed, intra-p-VTA salsolinol injections enhance dopamine levels in the ipsilateral NAcc shell; this increase in dopamine does not occur after pretreatment with a MOR antagonist (Hipólito et al., 2012).
We hypothesized that salsolinol may activate VTA dopamine neurons indirectly, via MORs located on VTA GABA neurons on the basis of the following observations: 1) the structure of salsolinol is similar to that of morphine (Davis and Walsh, 1970); 2) salsolinol can bind to opioid receptors and produces opioid-like effects (Fertel et al., 1980; Lucchi et al., 1982); 3) like MOR agonists, salsolinol can increase NAcc dopamine; and 4) MOR antagonists block locomotor stimulation induced by intra-VTA injection of salsolinol (Hipólito et al., 2011) and attenuate place preference for salsolinol (Matsuzawa et al., 2000). To test this hypothesis, we combined electrophysiological and pharmacological tests on VTA neurons in rat brain slices.
Materials and Methods
Experimental Procedures.
All experiments were performed according to the guidelines of the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey. All efforts were made to minimize animal suffering and reduce the number of animals. All experiments were performed on Sprague-Dawley rats (Taconic, Germantown, NY) aged 14 to 25 (19 ± 1) postnatal days.
Slice Preparation.
The midbrain slices were prepared as described previously (Xiao et al., 2007). Rats were anesthetized and then decapitated. A midbrain block containing the VTA was glued to the cutting stage of a VF-200 slicer (Precisionary Instruments, Greenville, NC). We cut 250- to 300-μm-thick coronal slices while keeping the brain in ice-cold glycerol-based artificial cerebrospinal fluid (ACSF) containing 252 mM glycerol, 1.6 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 18 mM NaHCO3, and 11 mM glucose and carbogenated with 95% O2/5% CO2, giving the ACSF a pH of 7.4. The slices (two per animal) were left to recover for at least 1 h in a holding chamber in regular ACSF in which glycerol was replaced with 126 mM NaCl.
Electrophysiological Recording in Midbrain Slices.
Cells in midbrain slices were visualized with an upright microscope (E600FN; Nikon, Tokyo, Japan) and near-infrared illumination. Signals were recorded by whole-cell patch clamp with MultiClamp 700A amplifiers (Molecular Devices, Sunnyvale, CA), a Digidata 1320A A/D converter (Molecular Devices), and pCLAMP 9.2 software (Molecular Devices). Data were filtered at 2 kHz and sampled at 5 kHz. The patch electrodes, containing 125 mM KCl, 2.8 mM NaCl, 2 mM MgCl2, 0.6 mM EGTA, 10 mM HEPES, 2 mM ATP-Na2, and 0.3 mM GTP-Na (with the pH adjusted to 7.2 with Tris buffer base and osmolarity to 300 mOsM with sucrose) had a resistance of 2 to 5 MΩ. A single slice was transferred into a 0.4-ml recording chamber, where it was held down by a platinum ring. Warm carbogenated ACSF flowed through the bath (1.5–2.0 ml/min). All recordings were at 32°C, maintained by an automatic temperature control (Warner Instruments, Hamden, CT). Whole-cell voltages were recorded with a K-gluconate-based pipette solution, in which KCl was replaced by 125 mM K-gluconate. The pH of pipette solutions was adjusted to 7.2 with Tris-base, and the osmolarity was adjusted to 280 to 300 mOsm with sucrose. We calculated changes in firing by measuring the rate of firing over 1-min intervals both before administration of drugs and during the drug effect; peak drug-induced changes were expressed as the percentage change from the baseline firing rate, thus controlling for small variations that may occur over time. All inhibitory postsynaptic currents (IPSCs) were recorded at a holding potential of −70 mV. If the steady-state inward relaxation (Ih-like) was greater than 60 pA during a step from −50 to −100 mV cells were considered to be dopamine neurons, following our standard criteria, based on these cells' physiological and pharmacological properties, as described previously (Xiao et al., 2007). Bearing in mind that the expression of Ih may not be sufficient to identify dopamine cells unequivocally, it is possible that some of the reported cells were not dopamine neurons (Margolis et al., 2006).
Data Analysis.
Spontaneous discharges, spontaneous IPSCs (sIPSCs), and miniature IPSCs (mIPSCs) were counted and analyzed with Clampfit 9.2 (Molecular Devices). sIPSCs (or mIPSCs) were screened automatically by a template with a threshold of 10 pA. Then they were visually inspected and accepted or rejected on the basis of their time course; rise times were 0.2 to 6.3 ms, and decay times were 0.2 to 20.1 ms. Cumulative probability plots of the incidence of various interevent intervals and amplitudes (for 100–300 sIPSCs), recorded under different conditions from the same neuron, were compared by the Kolmogorov-Smirnov test. For other plots, data obtained over a 2-min period at the peak of a drug response were normalized to the average values of the frequency and amplitude of sIPSCs or mIPSCs during the initial control period (2 min). Data were expressed as means (± S.E.M.). The statistical significance of drug effects was assessed by a paired two-tailed t test on normalized data. Values of p < 0.05 were considered significant.
Chemicals and Applications.
1-Methyl-6,7-dihydroxy-1,2,3, 4-tetrahydroisoquinoline hydrobromide (salsolinol), naltrexone hydrochloride, 2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)pyridazinium bromide (SR-95531; gabazine), dl-2-amino-5-phosphono-valeric acid (dl-APV), 6,7-dinitroquinoxaline-2,3-dione (DNQX), reserpine hydrochloride, tetrodotoxin (TTX), and [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin (DAMGO) were obtained from Sigma-Aldrich (St. Louis, MO). The 1000× stock solutions were aliquoted and then stored at −20°C, 4°C, or room temperature according to the manufacturer's recommendations. Drugs were added to the superfusate.
Results
Salsolinol Depolarized VTA Putative Dopamine Neurons and Increased Their Ongoing Firing.
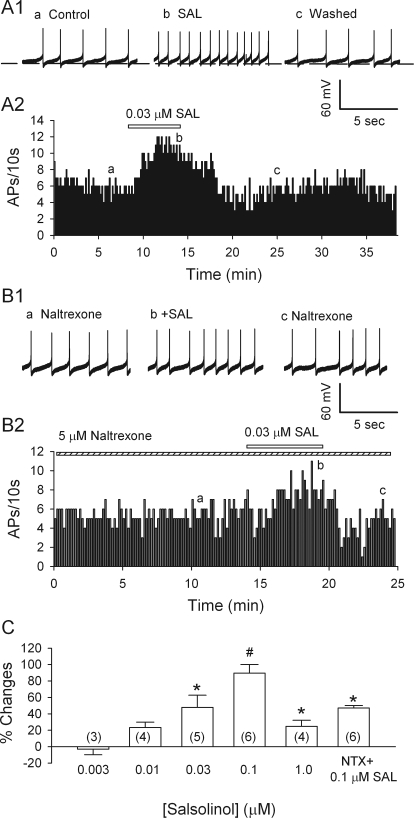
Using whole-cell current clamp, we recorded in the p-VTA (from 5.3 to 6.0 mm posterior to the bregma (Paxinos and Watson, 2007) because a previous in vivo study found that salsolinol has an effect on posterior but not anterior VTA (Rodd et al., 2008). Most neurons fired spontaneously and slowly (0.5–2 Hz) in a regular pacemaker mode. The slow firing of dopamine neurons allowed us to estimate the resting membrane potential that is between the action potential threshold and after hyperpolarization (Grace and Onn, 1989). As the basal firing rate varied, each neuron served as its own control. After recording spontaneous firing for 5 to 10 min in the absence of salsolinol, we switched the perfusion to a solution containing salsolinol for 5 to 10 min, before returning to the control solution. As illustrated in Fig. 1A1, 0.03 μM salsolinol robustly increased the firing of the putative dopamine neurons by 48 ± 14.8% (n = 5; p < 0.05; by paired t test). This effect was reversible upon washout of salsolinol. Figure 1C illustrates the biphasic dose dependence of salsolinol's effect: for salsolinol concentrations of 0.003, 0.01, and 0.1 μM, the firing rate increased by −3.1 ± 6.8% (n = 3; p = 0.7), 23.4 ± 6.6% (n = 4; p = 0.07), and 89.6 ± 10.6% (n = 6; p = 0.003), respectively; but with 1 μM salsolinol, the increase diminished sharply to only 25.3 ± 7.5% (n = 4; p = 0.046). In keeping with the acceleration of firing, 0.1 μM salsolinol depolarized cells by 2.6 ± 0.47 mV, from −50.5 ± 1.8 to −47.5 ± 2.1 mV (n = 6 cells; p = 0.003). The salsolinol-induced increase in firing was also observed while recording in the loose-patch cell-attached configuration (data not shown).
Fig. 1.
Salsolinol (SAL) accelerates spontaneous firing of dopamine neurons in the VTA by an MOR-dependent action. A1, traces illustrate spike discharge at the times indicated in A2. A2, time course of acceleration of pacemaking firing rate of a dopamine neuron by 0.03 μM salsolinol. B1, traces obtained at the times indicated in B2. B2, time course of the effect of salsolinol on the firing rate of a dopamine neuron in the presence of naltrexone, a μ-opioid antagonist (5 μM). C, dose-dependent potentiation of the firing rate of dopamine neurons. NTX, naltrexone. Means and S.E.M. are shown, with numbers of cells in parentheses. *, p < 0.05; #, p < 0.01; by Student's t test, for differences from control.
Naltrexone, an Antagonist of MORs, Attenuated Salsolinol-Induced Excitation.
In agreement with our previous report (Xiao et al., 2007), naltrexone (5 μM) significantly reduced ongoing firing of putative dopamine neurons by 29 ± 10.3% (n = 6; p = 0.04). Evidently an opioidergic influence tonically facilitated dopamine cell firing, presumably by suppressing GABAergic inhibition. After the response to naltrexone had stabilized, adding salsolinol (0.1 μM) increased the firing rate by only 47.2 ± 2.9% (n = 6), which was significantly less than the acceleration produced by 0.1 μM salsolinol alone (89.6 ± 10.6%) (Student's unpaired t test; salsolinol versus naltrexone + salsolinol; p < 0.05). These data, illustrated in Fig. 1, B1 and B2, provide further support for the hypothesis that salsolinol does not excite dopamine neurons directly, but rather by reinforcing opioidergic suppression of GABAergic inhibition.
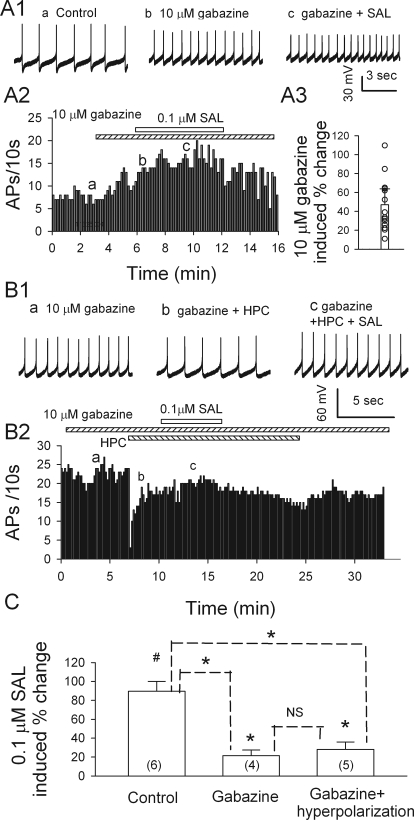
Salsolinol-Induced Excitation of VTA Dopamine Neurons Is Much Attenuated by a GABAA Receptor Antagonist.
Because most MORs in the VTA are expressed on GABAergic inhibitory neurons, the activation of MORs should reduce GABAergic activity. Therefore, the blockade of GABAA receptors should attenuate salsolinol-induced excitation. To test this prediction, we compared salsolinol's effects in the absence and presence of the GABAA antagonist gabazine. When applied alone, gabazine (10 μM) also increased dopamine cell firing (Fig. 2, A1-A3), by 47 ± 16.8% (n = 14; p = 0.03), in agreement with our previous report (Xiao et al., 2007). Thus, dopamine neurons were under strong tonic inhibition, mediated via GABAA receptors (Johnson and North, 1992). When applied in the continued presence of gabazine, 0.1 μM salsolinol further enhanced firing, but only by 21.5 ± 5.9% (Fig. 2, A1-A3; n = 4; p = 0.037), much less than the 89.6 ± 10.6% increase induced by 0.1 μM salsolinol alone (n = 6; p = 0.003). This indicates that salsolinol occludes the increase in firing with gabazine.
Fig. 2.
Gabazine prevents SAL-induced excitation of dopamine neurons. A1a, ongoing firing, recorded from a current-clamped dopamine neuron under control condition. A1b, the firing was increased by gabazine. A1c, in the presence of gabazine, salsolinol had minimal effect. These traces were recorded at the times indicated in A2. A2, time course of changes. A3, a scatter plot summarizes all the responses to 10 μM gabazine. B, Data obtained in other tests, where salsolinol was applied in the presence of gabazine (10 μM) and after injecting a small hyperpolarizing current (HPC, −20 pA) to restore the initial firing rate; note: salsolinol-induced excitation was suppressed. B1, traces illustrate spike discharge at the times indicated in B2. B2, time course of the effect of salsolinol on the firing rate of a putative dopamine neuron in the presence of gabazine and HPC. C, Summary (means ± SEM) of increases in firing rate produced by 0.1 μM salsolinol in the absence, or presence of gabazine or gabazine + HPC. In brackets are numbers of recorded neurons. #, p < 0.05, paired t test for salsolinol versus presalsolinol condition. *, p < 0.05, t test for salsolinol versus pre-salsolinol condition or versus without gabazine or gabazine + HPC, as indicated. NS, not significant, t test for salsolinol-induced increase in firing in gabazine versus in gabazine + HPC.
In the presence of gabazine, the stimulating effect of salsolinol may be underestimated owing to nonlinear summation of excitation. We therefore repeated such tests of salsolinol after restoring the firing frequency to the pregabazine level by a hyperpolarizing current injection. Under these conditions, 0.1 μM salsolinol increased firing by 28 ± 7.8% (n = 5; p = 0.014), which was not significantly different from the increase seen without the hyperpolarizing current, yet is still much less than the increase when gabazine was not present (89.6 ± 10.6%; unpaired t test; p < 0.05). Thus, salsolinol-induced excitation of VTA dopamine neurons is largely driven by a change in GABAergic inhibition.
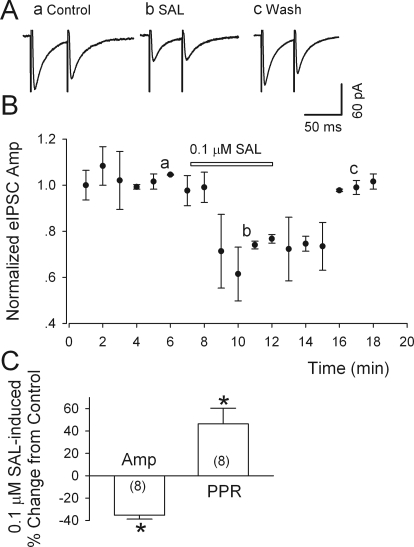
Salsolinol Depresses GABAA Receptor-Mediated IPSCs of Putative Dopamine Neurons in the VTA.
Monosynaptic evoked IPSCs (eIPSCs) were evoked in the presence of APV (50 μM) and DNQX (20 μM) at a holding potential of −65 mV; their total suppression by gabazine (10 μM) confirmed that they were mediated by GABAA receptors. As illustrated in Fig. 3, A and C, salsolinol (0.1 μM) significantly and reversibly diminished the peak amplitude of eIPSCs (by 35.4 ± 3.5%; n = 8; p < 0.001). To determine whether salsolinol inhibits GABAergic synaptic transmission via a presynaptic or a postsynaptic mechanism, we evoked pairs of such eEPSCs, at intervals of 50 ms, and calculated the paired-pulse ratios (PPR = IPSC2/IPSC1) (Fig. 3). It is known that if an agent modifies PPR, it probably acts through a presynaptic mechanism (Debanne et al., 1996). As illustrated in Fig. 3A, 0.1 μM salsolinol reduced the amplitude of IPSC1 to a greater extent than that of IPSC2, so PPR increased by 46.4 ± 14.1% (from 0.81 ± 0.08 to 1.13 ± 0.11) (Fig. 3C). Therefore, salsolinol was probably acting at a presynaptic site to lower GABA release.
Fig. 3.
Salsolinol depresses eIPSCs in putative dopamine neurons. A, salsolinol (0.1 μM) sharply reduced the peak amplitude of both the first and second IPSC evoked by paired stimulation (at 50-ms interval) within the VTA, but the greater relative reduction of the first response increased the ratio of IPSC2/IPSC (PPR). Labels a-c refer to times in B. B, time course of salsolinol-induced changes in eIPSC amplitude (Amp). C, summary of the effects of salsolinol (0.1 μM) on the amplitude and the PPR of eIPSCs; numbers of recorded neurons are in parentheses. *, p < 0.01, paired t test for salsolinol application versus presalsolinol control. All IPSCs were recorded from putative dopamine neurons at a VH of −70 mV in the presence of APV (50 μM) and DNQX (20 μM).
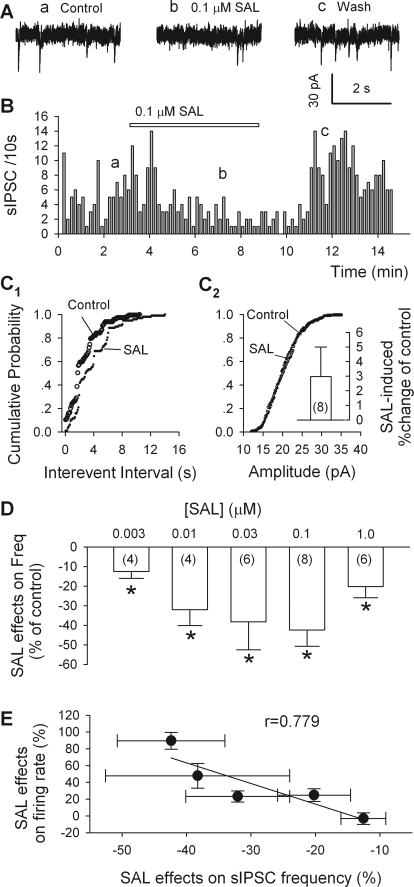
Our observation that salsolinol lowered the frequency but not the amplitude of sIPSCs is further evidence that salsolinol acted presynaptically, by reducing GABA release (Fig. 4, A–D). These sIPSCs, recorded from putative dopamine neurons, were eliminated by gabazine (10 μM) and therefore mediated by GABAA receptors (data not illustrated). By reducing sIPSC frequency, salsolinol (0.1 μM) caused a significant rightward shift of the cumulative probability plot of interevent intervals (Kolmogorov-Smirnov test; p < 0.001; Fig. 4C1). In eight dopamine neurons, 0.1 μM salsolinol decreased sIPSC frequency by 42.2 ± 8.4% (n = 8; p = 0.015; Fig. 4D); however, 0.1 μM salsolinol did not significantly change sIPSC amplitude (3.3 ± 2.0%; n = 8; p > 0.25; from 23.5 ± 0.5 to 24.1 ± 0.7 pA). Like the excitation of dopamine neurons (Fig. 1C), the depression of sIPSC frequency reached a peak with 0.1 μM salsolinol and was not much attenuated by a higher salsolinol concentration (Fig. 4D).
Fig. 4.
Salsolinol reduces sIPSC frequency in putative dopamine neurons. A, traces obtained at the times indicated in B. B, time course of salsolinol's effect on sIPSC frequency. C1 and C2, cumulative probability plots of data from the same cell show effects of salsolinol (0.1 μM) on sIPSC frequency (C1) and amplitude (C2). Insets are pooled data (means ± S.E.M.; n = 8). D, salsolinol-induced percentage changes of sIPSC frequency as a function of salsolinol concentration; all data (n = 4–8) were normalized to values obtained in the absence of salsolinol. *, p < 0.05, t test for salsolinol versus presalsolinol condition. E, plot of salsolinol effects on firing of dopamine neurons against the salsolinol effects on sIPSC frequency. Each point corresponds to the effects of one concentration of salsolinol on cell firing rate and sIPSC frequency. The data were fitted by a linear equation with a correlation coefficient of 0.883 (p < 0.0001).
After pooling all of the results obtained with the various concentrations of salsolinol, we plotted mean changes in dopamine cell firing against corresponding mean changes in sIPSC frequency (Fig. 4E). The slope of the best-fitting line was very close to −1.0 (r = 0.883; p < 0001), indicating that the firing rate was inversely proportional to sIPSC frequency, in keeping with the idea that salsolinol-induced changes in the firing rate of dopamine neurons are determined mainly by changes in GABAergic inputs.
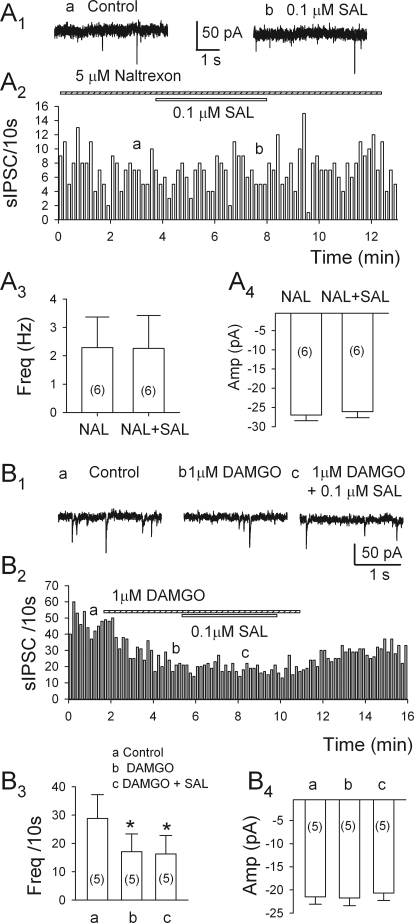
Naltrexone or DAMGO Prevents Salsolinol's Action on sIPSCs.
In the VTA, MORs are expressed mostly on the somata and dendrites of nondopamine neurons (Mansour et al., 1994, 1995; Garzón and Pickel, 2001), with only 8% situated on inhibitory axonal terminals (Dilts and Kalivas, 1989; Garzón and Pickel, 2001). This suggests that the observed μ-opioid actions are mediated principally by GABAergic neurons situated in the VTA (Johnson and North, 1992). As illustrated in Fig. 5, A1 to A4, in six of eight cells tested 5 μM naltrexone alone significantly affect sIPSC frequency (10.5 ± 2.4%; n = 6; p = 0.007); in its presence, 0.1 μM salsolinol failed to alter the frequency or the amplitude of sIPSCs [the frequency of sIPSCs changed by −7.3 ± 4.5% (n = 6; p = 0.18); and the amplitude changed from 27.3 ± 2.4 to 27.2 ± 1.4 pA in naltrexone and 26.3 ± 1.6 pA after the addition of salsolinol (n = 6; p = 0.14)]. Thus, salsolinol seems to lower sIPSC frequency largely by activating MORs directly or indirectly. Moreover, as illustrated in Fig. 5, B1 to B4, 1 μM DAMGO, an MOR agonist, sharply reduced the frequency of sIPSCs (by 42.1 ± 10.6%; n = 5; p = 0.016), and adding 0.1 μM salsolinol did not change the frequency or amplitude of sIPSCs (Fig. 5, B1-B4). These results show that salsolinol's action can be blocked not only by a selective MOR antagonist, but also by a selective agonist, in the second case presumably by occlusion.
Fig. 5.
Salsolinol-induced depression of sIPSC frequency is prevented by naltrexone (NAL) or DAMGO. A1, current traces obtained at the times indicated in A2. A2, incidence of sIPSCs during application of salsolinol (0.1 μM) in the presence of naltrexone. A3 and A4, pooled data (means ± S.E.M.; n = 6) show the lack of effect of salsolinol in the presence of naltrexone (5 μM) (frequency, A3; amplitude, A4). Numbers of cells are indicated in parentheses. B1, current traces obtained at the times indicated in A2. B2, time course of depression of sIPSC frequency by DAMGO (1 μM), which cancels the effect of salsolinol. B3 and B4, pooled data (means ± S.E.M.; n = 5) summarize these results (frequency, B3; amplitude, B4). *, p < 0.05, paired t test for DAMGO (b) or DAMGO + SAL versus pretreatment condition.
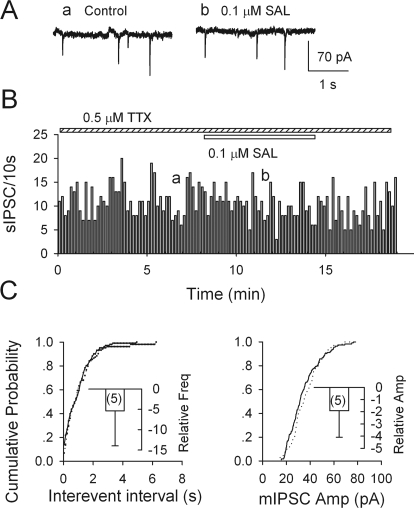
Salsolinol Does Not Alter mIPSCs in VTA Dopamine Neurons.
After blocking action potential-generated sIPSCs with 0.5 μM TTX, the frequency of spontaneous events dropped by 71.1 ± 6.7% (from 1.0 ± 0.5 to 0.3 ± 0.1 Hz; n = 5; p < 0.001), but their mean amplitude did not differ significantly (21.9 ± 0.6 versus 20.9 ± 0.9 pA; n = 5; p = 0.31) (data not illustrated). Thus, mIPSCs make up nearly 30% of sIPSCs. In contrast to its effect on sIPSCs, 0.1 μM salsolinol did not significantly change the frequency (−5.4 ± 8.6%; n = 5; p = 0.56) and the amplitude of mIPSCs (−1.9 ± 2.2%; n = 5; p = 0.43; Fig. 6). Further tests of salsolinol on mIPSCs in the presence of naltrexone or DAMGO were equally negative. Thus, at least in isolated slices, the action of salsolinol is mainly to reduce the firing of GABAergic neurons (presumably in the VTA) rather than to depress GABA release from inhibitory terminals.
Fig. 6.
Salsolinol does not affect spontaneous miniature IPSCs. mIPSCs were recorded in VTA dopamine neurons in the presence of 0.5 μM TTX. A and B, individual traces (A) and time course (B) are illustrated. C, salsolinol did not change the cumulative probability of interevent intervals (left) and mIPSC amplitudes (right). Insets show mean relative changes (± S.E.M.; n = 5 neurons) induced by 0.1 μM salsolinol.
Does Salsolinol Mediate Ethanol's Excitatory Action in Slices?
In an attempt to answer this question, we used two procedures that should prevent the formation of salsolinol by the combination of acetyldehyde and dopamine. First, we tested ethanol on DA neurons in p-VTA slices pretreated with 10 μM reserpine for >2 h to deplete dopamine. In six cells, 86 mM ethanol significantly increased firing by 56 ± 21% (n = 6; p < 0.01), which was not significantly different (p = 0.65) from the effect of ethanol seen without pretreatment in reserpine (44 ± 6%; n = 5). We also applied ethanol to midbrain slices pretreated with d-penicillamine, a highly selective agent for sequestering acetaldehyde in vivo (Font et al., 2005). Brain slices were pretreated for >90 min with d-penicillamine (10 mM in ACSF). Ethanol (43 mM) increased firing by 29 ± 9% (n = 5) in d-penicillamine-treated cells and 25 ± 7% in control slices (n = 5), and ethanol (86 mM) increased firing by 44 ± 11% in d-penicillamine-treated cells (n = 4) and 44 ± 6% in control cells (n = 5). There was no significant difference between test and control data (by t test; p = 0.7 and 0.97, respectively). These results suggest that, in slices, salsolinol does not mediate the excitation of DA neurons by ethanol.
Discussion
We present here the first electrophysiological evidence of the effect of salsolinol on putative dopamine neurons of the VTA in acute midbrain slices. Salsolinol at moderate doses stimulated these neurons and inhibited GABA release onto these cells. Furthermore, the salsolinol-induced excitation of dopamine neurons was attenuated by gabazine and naltrexone, indicating the involvement of GABAA receptors and MORs.
Salsolinol Excites VTA Dopamine Neurons by a MOR-Dependent Mechanism.
The present data indicate that salsolinol excites the VTA dopamine neurons, in agreement with our previous behavioral and neurochemical findings, notably that salsolinol, microinjected directly into the posterior region of the VTA, induced dose-dependent locomotor activity of Wistar rats (Hipólito et al., 2012). The dose-response curve for salsolinol had an inverted U-shaped profile, with a peak at 30 pM. Even more recently, we reported that this dose of salsolinol can induce: 1) a strong place preference in a classic conditioning paradigm and 2) a MOR-dependent increase in dopamine levels in the ipsilateral NAcc shell. The fact that both were completely prevented by the local pretreatment with the irreversible antagonist of MORs, β-Funaltrexamine hydrochloride, clearly suggested the involvement of these receptors in the mechanism of the action of salsolinol (Hipólito et al., 2011).
MORs are enriched in the VTA and are located primarily on nondopamine neurons (Mansour et al., 1995). Morphine increases the discharge of dopamine neurons but inhibits nondopamine neurons in slices (Johnson and North, 1992). These findings led to the hypothesis that the activation of MORs on GABA neurons results in the disinhibition of the dopamine neurons. Previous evidence suggested that salsolinol may activate MORs. Our observation of pronounced effects of naltrexone on GABAergic IPSCs is strong evidence of ongoing opioid release in the VTA. Moreover, naltrexone largely eliminated the effects of salsolinol on dopamine neurons. The simplest explanation of our results is that salsolinol activates the MORs of GABAergic interneurons. Overall, our findings in vitro fully support the hypothesis that the effects of salsolinol are mediated, at least partly, by MORs (Hipólito et al., 2011), with salsolinol probably activating MORs directly. Given that the excitation of dopamine neurons was not completely eliminated by naltrexone, salsonilol's action may involve some other mechanisms. This possibility is supported by our preliminary observation that the activating effect of salsolinol on dopamine neurons could also be attenuated by glutamate antagonists (G. Xie and J.-H. Ye, unpublished data).
The GABAergic System in the VTA Plays a Critical Role in the Reinforcement of Salsolinol Consumption.
VTA dopamine neurons are often considered essential components in the reward pathways. Their principal inhibitory inputs are GABAergic, including axons from medium spiny neurons of the NAcc (Kalivas, 1993), the ventral pallidum, and the rostromedial tegmental nucleus (Jhou et al., 2009). Because blocking GABAAR both in vivo and in vitro strongly increases dopamine cell firing (Johnson and North, 1992; Xiao et al., 2007), GABAergic IPSCs must normally dampen dopamine neuron excitability. In the acute coronal slices, however, the critical inhibitory regulation is probably done by collaterals of GABAergic neurons situated in the VTA.
It is noteworthy that, in our current study, there was dissociation between the changes in spontaneous IPSC frequency (but not amplitude), on one hand, and the absence of any changes in mini-IPSC frequency (or amplitude). This suggests that salsolinol acts by inhibiting GABA neuron firing rather than by directly impairing GABA release, presumably because the GABAergic terminals are sufficiently far from the site of action potential initiation (at or near the cell body) to be unaffected by changes in somatic excitability. The reduction in paired-pulse depression may be explained by diminished autoregulation of GABA release (Waldmeier et al., 1988) when the first IPSC is made much smaller by salsolinol's indirect action.
Several lines of evidence have linked ethanol-induced reinforcement to the GABAergic system in the VTA. Both systemic and intra-VTA administration of GABAR agonists facilitate, whereas antagonists decrease, voluntary ethanol drinking in rats (Smith et al., 1992). Rodents readily self-administer GABAR antagonists infused focally into the VTA (David et al., 1997; Ikemoto et al., 1997b). GABAR blockade in the VTA increases dopamine levels in the NAcc (Ikemoto et al., 1997a) and is strongly rewarding (Laviolette and van der Kooy, 2001). VTA GABA neurons become hyperexcitable during ethanol withdrawal (Gallegos et al., 1999). Although we have learned much about the role of GABA in ethanol abuse, we know much less about the effect of salsolinol on GABAergic transmission. Our study shows that local GABAergic inhibition plays a critical role in salsolinol's excitatory effects in the VTA.
Relevance of These Findings for Ethanol's Rewarding Properties.
We reported previously that ethanol not only excites putative VTA dopamine neurons but even more potently inhibits putative VTA GABA neurons (Xiao et al., 2007; Xiao and Ye, 2008). Because acute ethanol administration increases the release in the rat brain of β-endorphin, an endogenous ligand for MORs (Jarjour et al., 2009), we postulated that the inhibition of GABAergic IPSCs by ethanol might be caused by an increase in β-endorphin levels in the VTA (Xiao et al., 2007; Xiao and Ye, 2008). The present results suggest that salsolinol, generated by the metabolism of ethanol, also activates VTA dopamine neurons indirectly via MORs on inhibitory neurons.
We tested salsolinol in the concentration range of 0.01 to 1 μM, because 0.03 to 0.3 μM salsolinol is readily self-administered when injected directly into the p-VTA of Wistar rats (Rodd et al., 2008), and such concentrations are within a pharmacologically relevant range (Matsubara et al., 1987; Haber et al., 1999). Our data showed that, at such concentrations, salsolinol significantly stimulates dopamine neurons. The inverted U-shaped concentration response curve in our in vitro study is remarkably similar to findings in vivo (Rodd et al., 2008). In contrast to the strong activation by 0.1 μM salsolinol (Fig. 1), only at concentrations of 100 to 200 mM does ethanol produces a similar potentiation of dopamine (Brodie et al., 1990; Xiao et al., 2007). Thus, salsolinol is 1 million- to 2 million-fold more effective than ethanol as a stimulator of dopamine neurons in the posterior VTA. Our findings in vitro further support the hypothesis that the production of salsolinol contributes to the development of alcohol addiction (Rodd et al., 2008).
An important question is whether salsolinol brain levels are significantly enhanced after chronic ethanol exposure (see Hipólito et al., 2012 for review). Many laboratories have tested the role of the ethanol metabolite acetaldehyde, by impairing its formation (see Correa et al., 2011 for review) or using acetaldehyde sequestering agents such as d-penicilamine (Enrico et al., 2009; Martí-Prats et al., 2010; Pautassi et al., 2010). All of these in vivo studies have shown that the behavioral, neurochemical, and electrophysiological properties of ethanol are impaired when acetaldehyde, and consequently salsolinol, cannot be formed (Enrico et al., 2009; Martí-Prats et al., 2010). In our experiments, however, neither d-penicilamine nor dopamine depletion reduced the effect of ethanol, suggesting that in slices the excitation of dopamine cells by ethanol may not be mediated by salsolinol. This would be in agreement with previous evidence that salsolinol levels in the brain are raised after chronic but not acute ingestion of ethanol (Hipólito et al., 2012).
In summary, our study shows that very low concentrations of salsolinol excite dopamine neurons of the VTA, evidently by activating MORs on local GABA interneurons, in agreement with previous behavioral and neurochemical findings on salsolinol's effects. The increased dopamine neuron activity induced by salsolinol may be a cellular mechanism underlying salsolinol's reinforcing property.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA016964, AA016618] (to J.-H.Y.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- NAcc
- nucleus accumbens
- APV
- 2-amino-5-phosphono-valeric acid
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- MOR
- μ-opioid receptor
- VTA
- ventral tegmental area
- p-VTA
- posterior VTA
- TTX
- tetrodotoxin
- ACSF
- artificial cerebrospinal fluid
- IPSC
- inhibitory postsynaptic current
- sIPSC
- spontaneous IPSC
- mIPSC
- miniature IPSC
- eIPSC
- evoked IPSC
- HPC
- hyperpolarizing current
- PPR
- paired-pulse ratio
- SAL
- salsolinol
- APs
- action potentials
- GABAR
- GABA receptor
- SR-95531
- 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)pyridazinium bromide.
Authorship Contributions
Participated in research design: Xie, Hipólito, and Ye.
Conducted experiments: Xie and Zuo.
Performed data analysis: Xie and Zuo.
Wrote or contributed to the writing of the manuscript: Xie, Hipólito, Polache, Granero, Krnjević, and Ye.
References
- Brodie MS, Shefner SA, Dunwiddie TV. (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508:65–69 [DOI] [PubMed] [Google Scholar]
- Correa M, Salamone JD, Segovia KN, Pardo M, Longoni R, Spina L, Peana AT, Vinci S, Acquas E. (2011) Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neurosci Biobehav Rev 36:404–430 [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. (1997) Self-administration of the GABAA antagonist bicuculline into the ventral tegmental area in mice: dependence on D2 dopaminergic mechanisms. Psychopharmacology (Berl) 130:85–90 [DOI] [PubMed] [Google Scholar]
- Davis VE, Walsh MJ. (1970) Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science 167:1005–1007 [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. (1996) Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol 491:163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XS, Deitrich RA. (2008) Putative role of brain acetaldehyde in ethanol addiction. Curr Drug Abuse Rev 1:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. (1989) Autoradiographic localization of μ-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res 488:311–327 [DOI] [PubMed] [Google Scholar]
- Duncan C, Deitrich RA. (1980) A critical evaluation of tetrahydroisoquinoline induced ethanol preference in rats. Pharmacol Biochem Behav 13:265–281 [DOI] [PubMed] [Google Scholar]
- Enrico P, Sirca D, Mereu M, Peana AT, Lintas A, Golosio A, Diana M. (2009) Acetaldehyde sequestering prevents ethanol-induced stimulation of mesolimbic dopamine transmission. Drug Alcohol Depend 100:265–271 [DOI] [PubMed] [Google Scholar]
- Faraj BA, Camp VM, Davis DC, Lenton JD, Kutner M. (1989) Elevation of plasma salsolinol sulfate in chronic alcoholics as compared to nonalcoholics. Alcohol Clin Exp Res 13:155–163 [DOI] [PubMed] [Google Scholar]
- Fertel RH, Greenwald JE, Schwarz R, Wong L, Bianchine J. (1980) Opiate receptor binding and analgesic effects of the tetrahydroisoquinolines salsolinol and tetrahydropapaveroline. Res Commun Chem Pathol Pharmacol 27:3–16 [PubMed] [Google Scholar]
- Font L, Miquel M, Aragon CM. (2005) Prevention of ethanol-induced behavioral stimulation by d-penicillamine: a sequestration agent for acetaldehyde. Alcohol Clin Exp Res 29:1156–1164 [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. (1999) Adaptive responses of γ-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther 291:1045–1053 [PubMed] [Google Scholar]
- Garzón M, Pickel VM. (2001) Plasmalemmal μ-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse 41:311–328 [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. (1989) Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9:3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber H, Dumaual N, Bare DJ, Melzig MF, McBride WF, Lumeng L, Li TK. (1999) The quantitative determination of R- and S-salsolinol in the striatum and adrenal gland of rats selectively bred for disparate alcohol drinking. Addict Biol 4:181–189 [DOI] [PubMed] [Google Scholar]
- Haber H, Winkler A, Putscher I, Henklein P, Baeger I, Georgi M, Melzig MF. (1996) Plasma and urine salsolinol in humans: effect of acute ethanol intake on the enantiomeric composition of salsolinol. Alcohol Clin Exp Res 20:87–92 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Martí-Prats L, Sánchez-Catalán MJ, Polache A, Granero L. (2011) Induction of conditioned place preference and dopamine release by salsolinol in posterior VTA of rats: Involvement of μ-opioid receptors. Neurochem Int 59:559–562 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Sánchez-Catalán MJ, Martí-Prats L, Granero L, Polache A. (2012) Revisiting the controversial role of salsolinol in the neurobiological effects of ethanol: old and new vistas. Neurosci Biobehav Rev 36:362–378 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Sánchez-Catalán MJ, Zornoza T, Polache A, Granero L. (2010) Locomotor stimulant effects of acute and repeated intrategmental injections of salsolinol in rats: role of μ-opioid receptors. Psychopharmacology (Berl) 209:1–11 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. (1997a) GABAA receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem 69:137–143 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. (1997b) Self-infusion of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci 111:369–380 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. (2009) Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin Exp Res 33:1033–1043 [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev 18:75–113 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. (2001) GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci 13:1009–1015 [DOI] [PubMed] [Google Scholar]
- Lee J, Ramchandani VA, Hamazaki K, Engleman EA, McBride WJ, Li TK, Kim HY. (2010) A critical evaluation of influence of ethanol and diet on salsolinol enantiomers in humans and rats. Alcohol Clin Exp Res 34:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi L, Bosio A, Spano PF, Trabucchi M. (1982) Action of ethanol and salsolinol on opiate receptor function. Brain Res 232:506–510 [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29 [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. (1994) mu-Opioid receptor mRNA expression in the rat CNS: comparison to μ-receptor binding. Brain Res 643:245–265 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577:907–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Prats L, Sánchez-Catalán MJ, Hipólito L, Orrico A, Zornoza T, Polache A, Granero L. (2010) Systemic administration of d-penicillamine prevents the locomotor activation after intra-VTA ethanol administration in rats. Neurosci Lett 483:143–147 [DOI] [PubMed] [Google Scholar]
- Matsubara K, Fukushima S, Fukui Y. (1987) A systematic regional study of brain salsolinol levels during and immediately following chronic ethanol ingestion in rats. Brain Res 413:336–343 [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. (2000) Involvement of μ-opioid receptor in the salsolinol-associated place preference in rats exposed to conditioned fear stress. Alcohol Clin Exp Res 24:366–372 [PubMed] [Google Scholar]
- Myers RD, McCaleb ML, Ruwe WD. (1982) Alcohol drinking induced in the monkey by tetrahydropapaveroline (THP) infused into the cerebral ventricle. Pharmacol Biochem Behav 16:995–1000 [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Fabio MC, Spear NE. (2010) An acetaldehyde-sequestering agent inhibits appetitive reinforcement and behavioral stimulation induced by ethanol in preweanling rats. Pharmacol Biochem Behav 97:462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2007) The Rat Brain in Stereotaxic Coordinates, 6th ed, Academic Press, New York [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Goldstein A, Zaffaroni A, McBride WJ, Li TK. (2003) Salsolinol produces reinforcing effects in the nucleus accumbens shell of alcohol-preferring (P) rats. Alcohol Clin Exp Res 27:440–449 [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan G, Bell RL, Li TK, McBride WJ. (2008) The reinforcing properties of salsolinol in the ventral tegmental area: evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcohol Clin Exp Res 32:230–239 [DOI] [PubMed] [Google Scholar]
- Rojkovicova T, Mechref Y, Starkey JA, Wu G, Bell RL, McBride WJ, Novotny MV. (2008) Quantitative chiral analysis of salsolinol in different brain regions of rats genetically predisposed to alcoholism. J Chromatogr B Analyt Technol Biomed Life Sci 863:206–214 [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Sllström Baum S, Dufeu P, Schmidt LG. (1995) Determination of (R)- and (S)-salsolinol sulfate and dopamine sulfate levels in plasma of nonalcoholics and alcoholics. Alcohol 12:309–315 [DOI] [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. (1992) GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol 27:227–231 [PubMed] [Google Scholar]
- Starkey JA, Mechref Y, Muzikar J, McBride WJ, Novotny MV. (2006) Determination of salsolinol and related catecholamines through on-line preconcentration and liquid chromatography/atmospheric pressure photoionization mass spectrometry. Anal Chem 78:3342–3347 [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Wicki P, Feldtrauer JJ, Baumann PA. (1988) Potential involvement of a baclofen-sensitive autoreceptor in the modulation of the release of endogenous GABA from rat brain slices in vitro. Naunyn Schmiedebergs Arch Pharmacol 337:289–295 [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. (2008) Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of μ-opioid receptors. Neuroscience 153:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjević K, Ye JH. (2007) Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res 31:1106–1113 [DOI] [PubMed] [Google Scholar]