Abstract
Long-term use of opioids is hindered by respiratory depression and the possibility for fatal overdose in drug abusers. This is attributed to higher levels of tolerance that develops against antinociception than to respiratory depression. Identifying important mechanisms that would increase morphine respiratory depression and overdose tolerance could lead to the safer use of opioids. Because protein kinase C (PKC) activity mediates the development and maintenance of morphine antinociceptive tolerance, we hypothesized that activating PKCα or PKCε at the pre-Bötzinger complex (preBötC) can increase morphine tolerance in respiration and overdose. Laser microdissection and quantitative reverse transcriptase-polymerase chain reaction were used to compare the relative mRNA abundances of PKCα, γ, and ε between ventrolateral periaqueductal gray (vlPAG) and preBötC. To test whether PKCα or ε could enhance morphine tolerance in respiratory depression and overdose, lentivirus carrying the wild type, constitutively activated mutants, and small interference RNA against PKCα or ε was stereotaxically injected into the preBötC. Expression of constitutively active PKC (CAPKC) α or ε, but not wild-type PKC (WTPKC) α or ε, at the preBötC allowed rats to develop tolerance to morphine respiratory depression. In terms of lethality, expression of WTPKCε, CAPKCα, or CAPKCε at preBötC increased morphine tolerance to lethal overdose. CAPKCε-expressing rats developed the highest level of respiratory depression tolerance. Furthermore, when CAPKCε lentivirus was injected into the vlPAG, rats were able to develop significant antinociceptive tolerance at low doses of morphine that normally do not cause tolerance. The approach of increasing morphine respiratory depression and lethality tolerance by increasing PKCα or ε activity at preBötC could be used to make opioids safer for long-term use.
Introduction
Opioids are the most effective analgesics for the treatment of moderate to severe pain, and morphine is the prototypical opiate. The long-term use of morphine is hampered both by the development of analgesic tolerance and the relative lack of tolerance to side effects such as respiratory depression (Marks and Goldring, 1973; Ling et al., 1989; Paronis and Woods, 1997; Athanasos et al., 2006). Moreover, morphine is thought to cause lethality via respiratory depression (White and Irvine, 1999). With long-term use, differential tolerance to morphine analgesia and respiratory depression would decrease the therapeutic index, the ratio between LD50 and ED50 (White and Irvine, 1999). Therefore, morphine would be less lethal if tolerance to respiratory depression could be increased relative to analgesic tolerance. More than 10,000 people died of unintentional nonheroine prescription opioid overdose in the United States in 2007, and the majority of deaths occurred in long-term opioid users (Hall et al., 2008; Okie, 2010). Thus, there is the need to develop a safer opioid that has similar levels of tolerance to analgesia, respiratory depression, and lethal overdose.
The preBötC initiates respiration rhythm and also mediates opioid-induced respiratory depression (Janczewski and Feldman, 2006; Montandon et al., 2011). Somatostatin is both a functional and anatomical marker for preBötC since abrupt silencing of somatostatin-expressing neurons at the preBötC results in apnea and death (Stornetta et al., 2003; Tan et al., 2008). preBötC also stains positive for the μ-opioid receptor (MOR), and microinjection of [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin, a MOR agonist, slows down the respiration rate (Gray et al., 1999). Furthermore, preBötC is involved in mediating an enhanced respiratory drive in response to hypoxic or acidotic conditions (Solomon et al., 2000; Krause et al., 2009). preBötC is sandwiched by Bötzinger complex (BötC) and parafacial respiratory group/retrotrapezoid nucleus (pFRG/RTN) rostrally and the ventral respiratory group (VRG) caudally—all of them involved in respiration. Morphine is a well known respiratory depressant, and it is thought to cause lethality by depressing respiration (Pattinson, 2008). If cellular responses to morphine at preBötC can be altered, it might be possible to increase overdose and respiratory depression tolerance during long-term morphine treatment. One such cellular response is the μ-opioid receptor-mediated PKC activation.
PKC activity is required for both the initiation and maintenance of morphine antinociceptive tolerance. Intracerebroventricular injection of PKC inhibitors before long-term morphine administration prevented the development of antinociceptive tolerance (Gabra et al., 2008). After tolerance has developed, intracerebroventricular injection of either nonspecific PKC inhibitor or subtype-specific inhibitors of α, γ, and ε each reversed morphine antinociceptive tolerance (Javed et al., 2004). Inhibiting PKCα has been shown to block MOR desensitization in locus coeruleus neurons, whereas inhibiting PKCε blocked MOR desensitization in HEK293 cells expressing MOR (Bailey et al., 2009; Chu et al., 2010). Furthermore, development of morphine tolerance was attenuated in PKCγ or ε knockout mice (Zeitz et al., 2001; Newton et al., 2007). Therefore, because of preBötC's role in initiating respiration, mediating opioid respiratory depression, and chemoreception, we reasoned that if PKC activity at the preBötC is increased by expressing constitutively active PKC, tolerance to respiratory depression and lethality will be increased. Although several if not all of the classic or novel PKC subtypes could be involved, we decided to examine one classic subtype, PKCα, and one novel subtype, PKCε, in controlling respiratory depression and lethality tolerance. The reason for choosing these two PKC subtypes over others was also based on their relative abundance within the preBötC and ventrolateral periaqueductal gray (vlPAG) as demonstrated in our current study. By increasing morphine tolerance in respiratory depression and lethal overdose through PKCα or ε activation at the preBötC, the safety of morphine might be improved.
The effect of increasing PKC activity at preBötC on morphine respiratory depression and lethality tolerance was tested in this study. Lentivirus carrying wild type, constitutively active, or small interfering RNA (siRNA) against either PKCα or PKCε was stereotaxically microinjected into the preBötC bilaterally. Activation of PKCα or ε at preBötC increased tolerance to morphine respiratory depression and lethality. We observed that rats expressing constitutively activated PKCε (CAPKCε) developed the highest level of tolerance to morphine respiratory depression in terms of the time to onset of tolerance, level of hemoglobin saturation, and fold increase in ED50. We also observed that wild-type PKCε (WTPKCε) expressing rats developed overdose tolerance but not rats that received WTPKCα lentivirus injections. Therefore, there might be PKC subtype selectivity in morphine respiratory depression and lethality tolerance development.
Materials and Methods
Animals.
Male Sprague-Dawley rats between 250 and 275 g were purchased from Harlan (Indianapolis, IN). All surgeries and behavioral tests were approved by the University of Minnesota Institutional Animal Care and Use Committee (protocol 0902A60283). Rats were housed in a 12-h light/dark cycle with food and water ad libitum. Only well healed rats were used in subsequent experiments because approximately 25% of rodents either died or suffered neurological deficits from stereotaxic brain surgery.
Chemicals.
Morphine sulfate was obtained from the National Institute on Drug Abuse (Bethesda, MD). Ketamine was purchased from Phoenix Pharmaceuticals (Belmont, CA), xylazine was from Lloyd Laboratories (Shenandoah, IA), isoflurane was from Phoenix Pharmaceuticals, and cefazolin was from Apotex Corporation (Weston, FL). Triton X-100, bovine serum albumin, paraformaldehyde, and iodixanol were purchased from Sigma-Aldrich (St. Louis, MO). Toluidine blue was from Ricca Chemical (Arlington, TX). Advanced Dulbecco's modified Eagle's medium-reduced serum medium, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA).
Cell Culture.
NS20Y neuroblastoma cells were grown in advanced Dulbecco's modified Eagle's medium-reduced serum medium (Invitrogen) with 5% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 10% CO2 incubator. For lentiviral transductions, virus was applied at a multiplicity of infection of 500 when the cells were 25% confluent.
Lentivirus Production.
Plasmids containing CAPKCα (A25E point mutation), CAPKCε (pseudosubstrate inhibitory site amino acid 154–163 deletion), WTPKCα, and WTPKCε were generously provided by Drs. Allen Samarel and Jody Martin from Loyola University (Maywood, IL) (Strait et al., 2001). Deleting or mutating the pseudosubstrate inhibitory site causes PKC to become constitutively activated (Pears et al., 1990). WTPKCα and CAPKCα were cloned into the EcoRI sites of the pCDHGFP vector to generate pCDHGFP-WTPKCα and pCDHGFP-CAPKCα (System Biosciences, Mountain View, CA). Correct gene orientation was confirmed by nucleotide sequencing. WTPKCε and CAPKCε plasmids were amplified with primers 5′-TCAAGCTAGCACCATGGTAGTGTTCAATGGCCT-3′ and 5′-TCATGCGGCCGCTCAGGGCATCAGGTCTTCACC-3′ and cloned into the NheI and NotI sites of pCDHGFP to make pCDHGFP-WTPKCε and pCDHGFP-CAPKCε. Plasmids containing siRNA against PKCα were constructed using Invitrogen's Block-it Pol II RNAi expression kit (5′-AGGTGAAAGACCACAAATTCA-3′). Plasmids containing siRNA against PKCε were either purchased from Open Biosystems (Huntsville, AL; TRCN22759 5′-CCCTTATCTAACCCAACTCTA-3′; TRCN22762 5′-CGTCACTTCGAGGACTGGATT-3′) or constructed using Invitrogen's Block-it Pol II RNAi expression kit (5′-AAGATCGAGCTGGCTGTCTTT-3′; 5′-AGAGCCAATACTTACACTTGT-3′; 5′-GCAGATCAACCAGGAAGAATT-3′; K4935-00). Vector for scramble siRNA was obtained from Open Biosystems (pGIPZ nonsilencing negative control). HEK293T cells were purchased from Open Biosystems. Fifteen-centimeter plates were coated with polylysine for 15 min at 37°C and washed thoroughly with distilled water. Cells (20 million) were plated on each 15-cm dish 24 h before transfection. All plasmids were harvested using QIAGEN′s Endotoxin-free Plasmid Maxi kits and kept in 0.3 M sodium acetate/ethanol until ready to use (QIAGEN, Valencia, CA). On the day of transfection, plasmids were ethanol precipitated and dissolved in Tris-EDTA, pH 8.0. Invitrogen's ViraPower Lentivirus Expression System was used to produce lentivirus (Invitrogen). Three microliters of Lipofectamine 2000 was used to transfect each microgram of the four-plasmid mixture: pLP1, pLP2, pLP/VSVG, and pCDHGFP (ratio 3:1:1.5:5). Supernatants were collected every 24 h for 4 days and stored at 4°C. Cell debris were cleared by centrifuging at 3000 rpm for 5 min and then filtered through 0.45-μm filters (SCHVU01RE; Millipore, Billerica, MA). To concentrate lentivirus, 30 ml of supernatant was put into sterilized ultracentrifuge tubes (Beckman Coulter, Brea, CA), and 200 μl of 60% iodixanol (Sigma-Aldrich) was layered on the bottom using a metal cannula. After ultracentrifugation for 2.5 h at 4°C, 55,000g, the virus appeared as a visible band between the clear iodixanol and red medium. Afterward, medium was removed from the top until the last 3 ml. For larger volumes, ultracentrifugation was repeated until the suspension decreased to less than 30 ml, which underwent a last round of ultracentrifugation for 180 min 4°C at 75,000g using only 30 μl of an iodixanol underlayer. All of the supernatant was removed very carefully until the last 200 μl (Coleman et al., 2003). The concentrated lentivirus solution was measured in aliquots and frozen at −80°C. Lentivirus was titered using flow cytometry with HT-1080 cells following the manufacturer's protocols (K4975-00; Invitrogen). All of the lentivirus used had a titer between 2 and 3 × 109 transducing units/ml.
Morphine Respiratory Depression and Lethal Overdose.
Dixon's up and down method was used to determine the morphine ED50 dose for respiratory depression and lethal overdose (Dixon, 1965). The up and down method was used because it required fewer animals for determining ED50 without compromising accuracy and because its use has been supported by other investigators (Lichtman, 1998; Kao et al., 2010). This method requires consecutive testing of rats using doses spaced at constant log intervals where a negative response elevates the dose for the next animal, but a positive result decreases the dose for the next rat. The log interval was 0.15 for respiration and 0.11 for lethality experiments. The initial dose of morphine used on the first rat in the sequence was an estimation of the ED50, and the second dose would either be a log interval higher or lower than the first dose depending on the response of the first rodent. Each sequence required between six and nine animals to assess ED50. All rats were used only once in each up and down sequence. After completing the sequence with six to nine animals, the equation ED50 = Xf + K × d was used to calculate the ED50, where Xf was the value of the last dose injected, K was a tabular number obtained from a table reported by Dixon, and d was the interval in log scale (Dixon, 1965).
To determine the ED50 value of morphine to cause respiratory depression, the baseline hemoglobin oxygen saturation (Hb Sat) without any opioid treatment was measured for 5 min for all animals using the MouseOx (Starr Life Sciences, Oakmont, PA). The baseline Hb Sat was consistently between 94% and 98%. Since Dixon's up and down method required a cutoff threshold to differentiate positive responses to morphine from negatives, a decrease in Hb Sat to <85% was deemed to be a positive response for morphine respiratory depression. The threshold of 85% was chosen because it represented a significant and robust decrease in Hb Sat from baseline (P < 0.001). After measuring the baseline Hb Sat, morphine was injected intraperitoneally, and the rats were returned to home cages for 25 min. Hb Sat then was recorded for 3 min with a data collection rate of 60 Hz. With the exclusion of motion artifacts, the average Hb Sat during the 3-min interval was recorded. If the average Hb Sat was <85%, that rat exhibited a positive response to morphine respiratory depression, and the subsequent rodent would be tested with a smaller dose. The ED50 of morphine to cause respiratory depression was determined for both morphine-naive rats and ones that received four daily injections of 20 mg/kg morphine. Morphine tolerance to respiratory depression is defined by one of two methods in this report: a significant increase in ED50 or significant attenuation in the severity of respiratory depression in response to 20 mg/kg morphine as explained in the following paragraph.
In addition to measuring morphine respiratory depression ED50 on the 1st and 5th day, the Hb Sat of rats after injection with 20 mg/kg morphine was measured every day for 5 days. Attenuation in the severity of Hb Sat decrease compared with day 1 would also be considered as respiratory depression tolerance in addition to ED50 changes. Hb Sat was measured at 25 and 50 min after morphine injections, and the lower of the two time points was recorded. Two time points were taken (25 and 50 min) instead of only one (25 min) because rats were measured in parallel instead of sequentially, such as during ED50 determination, allowing more time for data acquisition. Hb Sat was used to monitor respiration as it integrates both the breathing frequency and amplitude.
To determine morphine LD50, Dixon's up and down method was also used. Lethality was defined as death within 24 h of morphine injection. A time of 24 h was chosen as the cutoff for comprehensiveness even though most deaths occurred between 1.5 and 5 h after morphine injection. The LD50 of morphine was determined for both morphine-naive rats and ones that had been chronically treated with 4 days of morphine (5 mg/kg b.i.d.) for a total of eight injections.
Morphine Antinociception.
Dixon's up and down method was used again to determine the ED50 of morphine to elicit antinociception because this approach required fewer animals. Antinociception was measured with the hot-plate test. The hot plate was set to 55°C, and the cut off time was 30 s. Thirty minutes after morphine injection, rats were tested on the hot plate. Licking of the hind paw was deemed a positive nociceptive response. Percentage maximal possible effect (%MPE) was calculated according to the formula: (test latency-baseline latency)/(cut off-baseline latency) × 100, where >50% MPE was noted as a positive morphine antinociceptive response. This threshold was instituted because Dixon's up and down method to assess ED50 required a binary outcome of positives and negatives. The threshold of 50% MPE was chosen because it represented robust morphine antinociception. The ED50 of morphine to cause antinociception was determined for both morphine-naive rodents, and rats that were chronically treated with 4 days of morphine (5 or 1.5 mg/kg b.i.d.). Morphine antinociception and respiratory depression were measured in different groups of animals.
Stereotaxic Injections.
For microinjections at the preBötC, rats between 250 and 275 g were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine. Depth of anesthesia was monitored every 15 min, and additional doses were given as needed. After mounting on a Stoelting stereotaxic frame (Stoelting, Wood Dale, IL), a midsagittal incision was made to expose the bregma and lambda. Bregma was positioned 5 mm below lambda, and the muscles on the back of the head were removed to uncover the skull. The connective tissue was cut away with a scissor, and a dental drill cleared away the skull to expose the obex. With obex as zero, coordinates for preBötC were anteroposterior (AP) 0.9 mm, mediolateral (ML) ±2 mm, and dorsoventral (DV) 2.7 mm (Tan et al., 2008). One microliter of the lentiviral solution was injected on each side over 5 min for a total of two injections, and the cannula was kept in place for another 5 min before withdrawal to prevent backflow (Quintessential Stereotaxic Injector; Stoelting). Viral solution levels in the cannula tubing were marked before and after the injection to confirm virus delivery. For lentivirus microinjections at the vlPAG, four injections of 1 μl each (two injections/side) were made to each rat. Coordinates were AP −8.16 mm and AP −7.44 mm, ML ±0.8 mm, DV −6 mm with respect to bregma. Four injections were made to cover the entire rostrocaudal extent of vlPAG as the structure is longer in the anterior-posterior axis. Rats received 5 mg/kg i.p. b.i.d. ketoprofen for antinociception and 20 mg/kg i.p. t.i.d. cefazolin for antimicrobial prophylaxis after surgeries.
Immunohistochemistry.
Rats were anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine, perfused with phosphate-buffered saline, and then with 4% paraformaldehyde (Sigma-Aldrich). Brains were postfixed in 4% paraformaldehyde overnight at 4°C, immersed in 30% sucrose until they sank to the bottom, and frozen in OCT (Sakura Finetek, Tokyo, Japan), and 25-μm thick sections were cut on a cryostat (Leica Microsystems, Wetzlar, Germany). Brains of rats that died from morphine overdose were removed from the skull, directly frozen in OCT, cut into 25-μm thick sections with a cryostat, and fixed with 4% paraformaldehyde for 3 min before staining. Sections were blocked with 10% normal goat serum (Jackson ImmunoResearch, West Grove, PA), 0.3% Triton X-100 (Sigma-Aldrich), and 0.5% BSA (Sigma-Aldrich) for 1 h at room temperature. All primary antibodies were dissolved in TBS (25 mM Tris, 3 mM KCl, 140 mM NaCl, 0.05% Tween 20, pH 7.4) containing 0.3% Triton X-100 and 0.5% BSA. GFP was stained with mouse anti-GFP 1:1000 (Invitrogen), somatostatin was stained with rabbit anti-somatostatin 1:600 (ImmunoStar, Hudson, WI), and MOR was stained with rabbit anti-MOR 1:10,000 (custom antibody; GeneTex, Irvine, CA). To characterize the rabbit anti-MOR, rat and wild-type mice with cervical spinal cords served as the positive control, whereas MOR knockout mice served as negative control (Supplemental Fig. 1). Sections were incubated with primary antibodies with gentle agitation overnight at 4°C and washed three times, 15 min each. Secondary antibodies were goat anti-mouse Alexa Fluor 488 1:200 (Invitrogen) and goat anti-rabbit Alexa Fluor 594 1:200 (Invitrogen) and were diluted in TBS with 0.3% Triton X-100 and 0.5% BSA. Tissue was incubated in secondary antibody for 1 h at room temperature and then washed three times for 15 min each before mounting on slides using Fluoromount-G (SouthernBiotech, Birmingham, AL). Fluorescence images were captured with a Leica DMIRE2 microscope connected to a BD CARVII confocal imager and a Hamamatsu EM CCD camera with laser light sources from Spectral Applied Research (green: 473 nm; red: 561 nm; Richmond Hill, ON, Canada). Successful lentivirus stereotaxic microinjection to the preBötC or vlPAG was confirmed through immunohistochemical analyses after behavioral studies.
Laser Microdissection and Quantitative Reverse Transcription-Polymerase Chain Reaction.
Rats were anesthetized with 5% isoflurane/95% O2 and decapitated. The brains were immediately removed and frozen in OCT (Sakura Finetek). On the day of microdissection, 8-μm-thick sections were cut with a cryostat, mounted on 2.0-μm-thick polyethylene naphthalate membrane coated slides (Leica Microsystems), stained with 1% toluidine blue (Ricca Chemical, Arlington, TX) for 5 s, fixed in acetone at 4°C for 3 min, and stored on dry ice until microdissection. Laser microdissection was performed with a Leica AS LMD (Leica Microsystems), and mRNA was isolated using TRI Reagent according to manufacturer's protocol (Molecular Research Center, Cincinnati, OH). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using QIAGEN′s Quantitect SYBR Green RT-PCR kit and the Bio-Rad iCycler (Bio-Rad, Hercules, CA). qRT-PCR primers to amplify PKCα were 5′-GTTTACCCGGCCAACGACT-3′ and 5′-GGGCGATGAATTTGTGGTCTT-3′; qRT-PCR primers to amplify PKCγ were 5′-GACCCTCGCAACAAGCACAAG-3′ and 5′-GATTTCCAGTTGCAGACGTCC-3′; qRT-PCR primers to amplify PKCε were 5′-AGCCGGCTTCTGGAAACTCCC-3′ and 5′-AGCTGCCTTTGCCTAACACCTTGAT-3′; and qRT-PCR primers to amplify β-actin were 5′-GACGATATGGAGAAGATTTGGCAC-3′ and 5′-GAGGCATACAGGGACAACACAGC-3′. To translate Ct values into absolute copy numbers, standard curves were obtained for PKCα, γ, and ε and β-actin. Constructing the standard curve required a 10-fold serial dilution of templates. The templates were longer and contained the segments that were amplified in qRT-PCR analyses mentioned above. Because the templates were longer, a different set of primers was required first to make the template for each gene. To obtain the templates to make the standard curve, the following primers were used to amplify rat whole-brain cDNA: PKCα 5′-GGGACCATGGCTGACGTTTAC-3′ and 5′-CCCTCTTCTCTGTGTGATCCATTC-3′; PKCγ 5′-CCACAAGTTCACCGCTCGTTTC-3′ and 5′-CATCCACAGGAGCCTTGAGTAGC-3′; PKCε 5′-TACGAAGTGCGCTGGGCTAAAG-3′ and 5′-GTCAGCCAGCTTGCAGTGACC-3′; and β-actin 5′-GATGGTGGGTATGGGTCAGAAG-3′ and 5′-ACGATTTCCCTCTCAGCTGTGG-3′. A 10-fold serial dilution of the templates was then amplified using the same primers for qRT-PCR PKCα, γ, and ε and β-actin mRNA abundance analysis. Using the standard curve, Ct values for each gene was translated into absolute copy numbers, and the copy number was then normalized to the absolute quantity of β-actin. To a minimal degree, cryostat sections from five rats were laser dissected and used in quantification of the transcripts.
Statistical Analysis.
Results were analyzed with GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). Data is expressed as mean ± S.E.M. Two-way ANOVA with Bonferroni's post-test was used when there were two factors (i.e., gene and nuclei). One-way ANOVA with Bonferroni's post-test was used to compare one factor among three or more groups. Student's t test was used to compare one factor between two groups. P < 0.05 was considered to be statistically significant.
Results
The Relative Level of PKC Subtypes at the vlPAG versus preBötC.
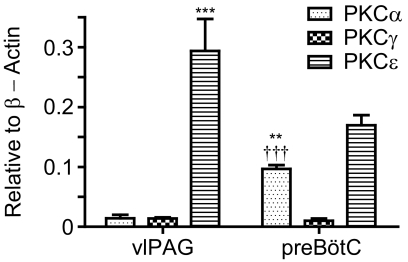
Because PKCα, γ, and ε are the three subtypes involved in mediating morphine antinociceptive tolerance (Smith et al., 2007), we decided to compare their relative abundances in the vlPAG and preBötC. The vlPAG is an important center for morphine antinociception, whereas the preBötC is involved in opioid respiratory depression (Siuciak and Advokat, 1987; Lane et al., 2005; Montandon et al., 2011). The relative abundance of the three subtypes at vlPAG versus preBötC might provide some insight into why morphine develops more tolerance in antinociception than respiratory depression or lethal overdose. vlPAG or preBötC was laser microdissected, and the relative quantities of PKCα, γ, and ε and β-actin mRNAs were determined via qRT-PCR (Supplemental Fig. 2). Standard curves were constructed for each gene to translate Ct values into the copy number. The copy numbers for the three PKC subtypes were normalized to that of β-actin. Two-way ANOVA analyses indicated that PKC abundances were significantly different [F(6,41) = 84.21; P < 0.0001] (Fig. 1). Bonferroni's post-tests were used after two-way ANOVA analysis. In vlPAG, PKCα and γ transcripts exist at comparable levels, which are significantly lower than the PKCε abundance (P < 0.001). At the preBötC, PKCε was again the highest followed by PKCα, and PKCγ was the least abundant subtype (P < 0.001). Furthermore, preBötC had more PKCα transcripts than vlPAG (P < 0.01), whereas preBötC had less PKCε than vlPAG (P < 0.001). PKCγ transcripts were present at similar levels in the two nuclei (Fig. 1). Although all three PKC subtypes have been implicated in morphine antinociceptive tolerance development (Smith et al., 2007), we focused on the probable roles of PKCα and PKCε in current studies because these two PKC subtypes represent the classic and novel PKC subtypes, respectively. Furthermore, the higher PKCε levels in vlPAG compared with preBötC (P < 0.001) might reflect PKCε's role on the observed greater morphine tolerance in antinociception than respiratory depression or lethal overdose (Fig. 1).
Fig. 1.
Relative abundance of PKC subtypes at the vlPAG and preBötC. vlPAG and preBötC were laser microdissected to compare the relative quantities of PKCα, γ, and ε and β-actin transcripts in the two nuclei. Copy numbers were normalized to that of β-actin, which was set as 1. ***, P < 0.001, compared with PKCα vlPAG, PKCγ vlPAG, and PKCε preBötC. †††, P < 0.001, compared with PKCγ preBötC and PKCε preBötC. **, P < 0.01 compared with PKCα vlPAG. Data were analyzed with two-way ANOVA with Bonferroni's post-test and presented as mean ± S.E.M (n ≥ 5).
Lentiviral Expression of PKC at the preBötC.
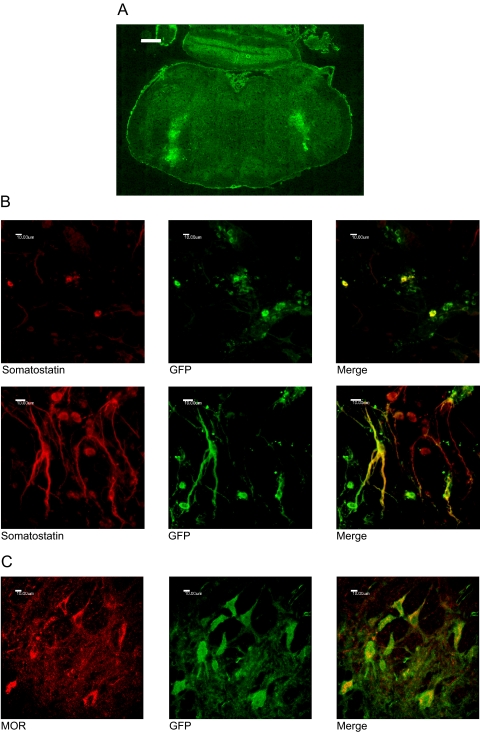
Immunohistochemistry was carried out to confirm transduction of PKC in the preBötC. preBötC is located in the ventrolateral medulla, and somatostatin is both a functional and anatomical marker for preBötC neurons (Stornetta et al., 2003; Tan et al., 2008). GFP delivered by the lentivirus confirms transgene expression in the ventrolateral medulla cells (Fig. 2A). Higher magnification colocalization studies indicated that a subset of GFP-positive cells also expressed the μ-opioid receptor and somatostatin, markers for preBötC neurons (Fig. 2, B and C). The intracellular staining of MOR is attributed to permeabilization of the plasma membrane with 0.3% Triton X-100, which was required in costaining with GFP and is in accord with earlier reports (Arvidsson et al., 1995). By the 2nd week after lentivirus injection, robust GFP expression was readily observed. Such GFP expression continued for at least 6 months. All experiments were completed within the time frame between 2 weeks to 6 months after lentivirus injection.
Fig. 2.
Lentiviral transduction of somatostatin and MOR-positive preBötC neurons. Lentivirus was stereotaxically microinjected into the preBötC, and the animals were perfused for immunohistochemistry 9 weeks after surgery. A, GFP-positive cells due to lentiviral transduction at the ventrolateral medulla corresponding to the location of the preBötC. Magnification is 100×, and scale bar is 500 μm. B, significant colocalization between lentiviral expressed GFP and endogenous somatostatin. Magnifications are 400× and 630×. Scale bars are 10 μm. C, colocalization between exogenous GFP and endogenous MOR-expressing neurons. Magnification is 400×, and the scale bar is 10 μm.
The Role of PKCα in Tolerance to Morphine-Induced Respiratory Depression.
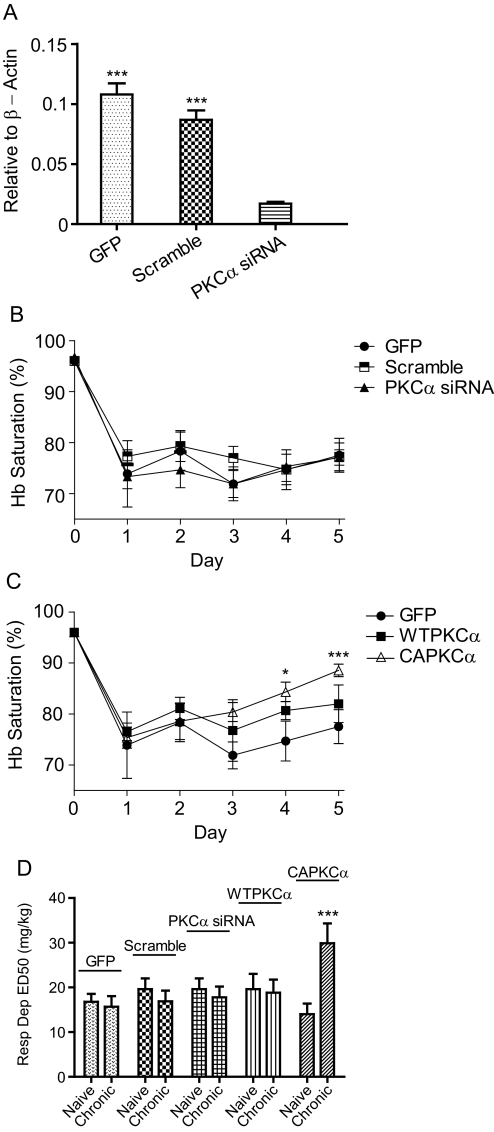
PKCα is known to be important in maintaining morphine antinociceptive tolerance (Smith et al., 2007). Whether PKCα is involved in respiratory depression tolerance was investigated by expressing the wild-type, constitutively active, or siRNA against PKCα at preBötC through lentivirus injection. siRNA knockdown of PKCα was confirmed by laser-microdissecting rat preBötC, and its mRNA abundance was quantitated via qRT-PCR. Lentiviral expressed PKCα siRNA knocked down PKCα by 85% relative to GFP (P < 0.001) and 80.4% relative to scramble siRNA (P < 0.001) [Fig. 3A and Supplemental Fig. 2; F(3,19) = 5314; P < 0.0001]. Rats receiving GFP lentivirus, scramble siRNA, or siRNA against PKCα consistently had their Hb Sat depressed to less than 80% after daily injections of 20 mg/kg morphine, indicating a lack of respiratory depression tolerance (Fig. 3B). Relative to day 1, rodents that received CAPKCα began to develop respiratory depression tolerance to 20 mg/kg morphine on the 4th day with Hb Sat of 84.3 (P < 0.05) and 88.9% (P < 0.001) on the 5th day [Fig. 3C; F(3,19) = 4.32; P < 0.05]. Because CAPKCα is a constitutively active mutant that bypasses the normal regulation for PKC activation, WTPKCα lentivirus was injected into preBötC to determine whether overexpression of the PKCα level was enough to increase respiration tolerance. In contrast to CAPKCα, WTPKCα-expressing rats did not develop tolerance to morphine respiratory depression throughout the 5 days of treatment (Fig. 3C). In addition to the onset of tolerance, the magnitude of tolerance was quantified by increases in ED50 after long-term treatment. Rats that received CAPKCα increased their ED50 for respiratory depression significantly from 14 ± 2.37 to 29.9 ± 4.42 mg/kg (P < 0.001) after four daily injections of 20 mg/kg morphine [Fig. 3D; F(10,61) = 9.04; P < 0.0001]. In contrast, GFP, scramble siRNA, PKCα siRNA, and WTPKCα rats did not have significantly increased respiratory depression ED50 after four injections of 20 mg/kg morphine (Fig. 3D). These results indicate that increased activity of PKCα and not the mere overexpression of WTPKCα are needed to enhance tolerance to morphine respiratory depression.
Fig. 3.
PKCα in morphine respiratory depression tolerance. Rats were injected with lentivirus carrying GFP, scramble siRNA, PKCα siRNA, WTPKCα, or CAPKCα at the preBötC bilaterally. A, siRNA knockdown efficiency. After injection of PKCα siRNA lentivirus at the preBötC, PKCα knockdown was assessed through laser microdissection and qRT-PCR. β-Actin was set as 1. ***, P < 0.001 compared with PKCα siRNA (one-way ANOVA). B and C, respiratory depression due to 20 mg/kg morphine. Data were analyzed using two-way ANOVA. *, P < 0.05 compared with day 1 CAPKCα. ***, P < 0.001 compared with day 1 CAPKCα. D, morphine respiratory depression ED50. Respiratory depression (Resp Dep) ED50 values was determined before and after chronic morphine treatment. ***, P < 0.001 compared with MS naive CAPKCα (two-way ANOVA). Data are presented as mean ± S.E.M. (n ≥ 6).
PKCε Activation Increases Morphine Respiratory Depression Tolerance.
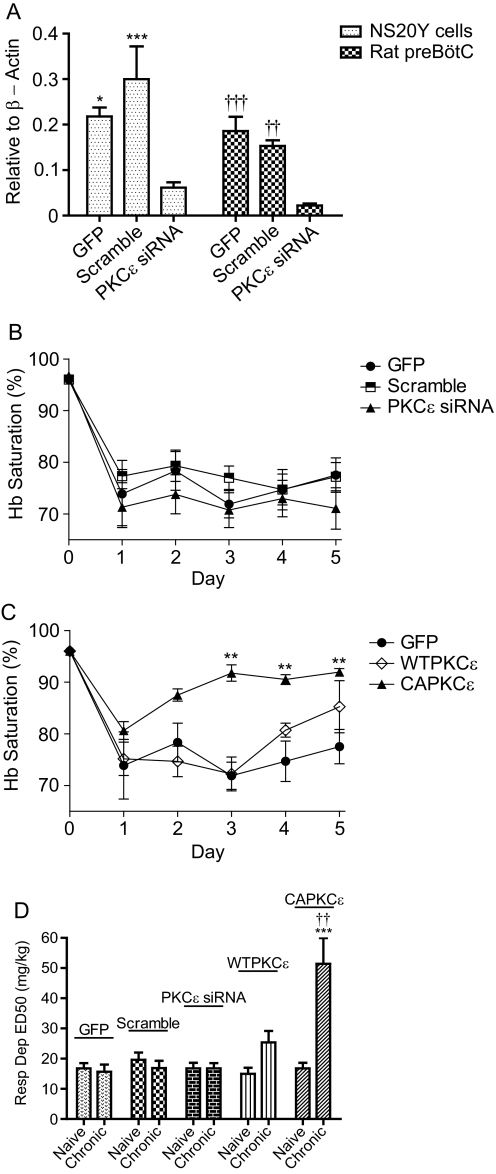
To test whether activating PKCε at the preBötC would increase respiratory depression tolerance, we expressed the constitutively active, wild type, or siRNA against PKCε at preBötC by stereotaxically injecting lentivirus containing the respective genes. Because the siRNA sequences used matched both mouse and rat PKCε mRNA, the ability of lentivirus-delivered PKCε siRNA for knockdown was confirmed both in mouse NS20Y neuroblastoma cells and rat preBötC. Knockdown in rat preBötC was 88.2% compared with GFP (P < 0.001) and 85.6% compared with scramble siRNA (P < 0.01) [Fig. 4A; F(3,20) = 216.1; P < 0.0001]. To study respiratory depression tolerance, 20 mg/kg morphine was injected intraperitoneally once a day for 5 days, and the Hb Sat was measured. In rats that received GFP, scramble siRNA, or PKCε siRNA lentivirus at the preBötC, 20 mg/kg morphine consistently depressed Hb saturation below 79% throughout the five daily administrations, indicating a lack of respiration tolerance (Fig. 4B). In contrast, CAPKCε-injected rats started to develop significant tolerance to morphine respiratory depression on the 3rd day with Hb Sat of 91.7% (P < 0.01) [Fig. 4C; F(3,18) = 26.15; P < 0.0001]. CAPKCε also developed tolerance faster than CAPKCα as CAPKCα rats exhibited tolerance from the 4th day instead of the 3rd, similar to CAPKCε (Figs. 3C and 4C). Alternatively, tolerance can be quantified as increases in ED50; morphine-naive ED50 for respiratory depression was compared with the ED50 after four injections of 20 mg/kg morphine sulfate. Rats that received CAPKCε at preBötC demonstrated a 3-fold increase in respiratory depression ED50 from 16.8 ± 1.81 to 51.4 ± 8.55 mg/kg (P < 0.001) (Fig. 4D). When both were treated with morphine long term, the ED50 of CAPKCε was significantly higher than that of WTPKCε (P < 0.01) [Fig. 4D; F(10,60) = 8.74; P < 0.01]. Injection of GFP, scramble siRNA, PKCε siRNA, and WTPKCε into preBötC did not result in significant increases in the respiratory depression ED50 after long-term morphine treatment (Fig. 4D). Thus, similar to PKCα, activation of PKCε rather than simple overexpression is needed to cause morphine tolerance in respiratory depression.
Fig. 4.
PKCε in tolerance to morphine respiratory depression. Rats were microinjected bilaterally at the preBötC with either lentivirus carrying GFP, scramble siRNA, PKCε siRNA, WTPKCε, or CAPKCε. A, PKCε lentiviral siRNA knockdown. In vitro, NS20Y cells were transduced with PKCε siRNA lentivirus. In vivo, rat preBötC injected with siRNA lentivirus was laser microdissected for qRT-PCR analysis. β-Actin was set as 1.*, P < 0.05 and ***, P < 0.001 compared with NS20Y PKCε siRNA (one-way ANOVA). ††, P < 0.01 and †††, P < 0.001 compared with rat preBötC PKCε siRNA (one-way ANOVA). B and C, respiratory depression due to 20 mg/kg morphine. **, P < 0.01 compared with day 1 CAPKCε (two-way ANOVA). D, morphine respiratory depression (Resp Dep) ED50. The ED50 values to cause respiratory depression were determined before and after long-term morphine treatment. ***, P < 0.001 compared with naive CAPKCε and ††, P < 0.01 compared with chronic WTPKCε using two-way ANOVA. Data are presented as mean ± S.E.M. (n ≥ 6).
PKCα and PKCε Increase Morphine Tolerance to Lethal Overdose.
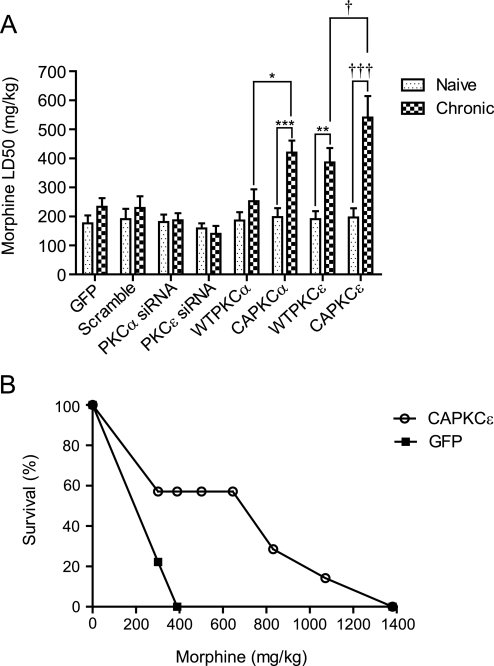
Lethal overdose is the most feared adverse effect during opioid treatment, and respiratory depression is thought to be the cause of morphine lethality (White and Irvine, 1999). Increasing tolerance to overdose would make morphine a safer drug for long-term use. Given that activating PKCα and PKCε increased tolerance to respiratory depression, tolerance to lethal overdose might also be increased. In rats that received GFP, scramble siRNA, PKCα siRNA, PKCε siRNA, or WTPKCα lentivirus injections at preBötC bilaterally, LD50 did not increase significantly after 4 days of 5 mg/kg b.i.d. morphine. When rats received siRNA against PKCε at the preBötC, there was a nonstatistically significant decrease in LD50 after long-term use of morphine. With the same 4 days of 5 mg/kg b.i.d. morphine, rats injected with WTPKCε had their LD50 value increased by 2.02-fold (P < 0.01), whereas those injected with CAPKCα and CAPKCε had their LD50 value increased by 2.12- (P < 0.001) and 2.76-fold (P < 0.001), respectively, compared with the morphine-naive LD50 values within the same gene [Fig. 5A; F(16,107) = 6.49; P < 0.001]. In addition, after long-term morphine treatment, the LD50 of CAPKCα was significantly higher than WTPKCα, and the LD50 of CAPKCε was higher than WTPKCε (P < 0.05) (Fig. 5A). To complement LD50 quantifications, a Kaplan-Meier curve illustrates how GFP and CAPKCε-expressing rats survived when injected with increasing doses of morphine. GFP and CAPKCε rodents were first treated with 5 mg/kg b.i.d. morphine for 4 days, and then both groups were challenged to increasing doses of morphine each day to measure the percentage survival. CAPKCε expressing rats survived higher doses of morphine than GFP-expressing rats (Fig. 5B). Seventy-eight percent of the rats injected with GFP virus died at 301 mg/kg, and the remaining 22% died when injected with 390 mg/kg the next day. In contrast, only 43% of CAPKCε-expressing rats died when injected with 301 mg/kg morphine, and the remaining 57% all survived dosage of 645 mg/kg with one rat able to survive dosage of 1071 mg/kg. When morphine caused lethality, most rats died between 1.5 and 5 h after morphine injection.
Fig. 5.
PKCα and ε increase morphine tolerance in lethal overdose. Rats were microinjected with lentivirus carrying GFP only, scramble siRNA, PKCα siRNA, PKCε siRNA, WTPKCα, WTPKCε, CAPKCα, or CAPKCε at the preBötC bilaterally. A, morphine LD50 for naive and chronically treated rats.*, P < 0.05 compared with chronic WTPKCα. ***, P < 0.001 compared with naive CAPKCα. **, P < 0.01 compared with naive WTPKCε. †, P < 0.05 compared with chronic WTPKCε. †††, P < 0.001 compared with naive CAPKCε. Data were analyzed with two-way ANOVA and presented as mean ± S.E.M. (n ≥ 6). B, survival to increasing doses of morphine. GFP and CAPKCε expressing rats were treated with 5 mg/kg b.i.d morphine for 4 days before they were challenged to increasing doses of morphine each day. CAPKCε (n = 7) and GFP (n = 9).
Expressing CAPKCε at the vlPAG Decreased the Dose Required to Develop Morphine Antinociceptive Tolerance.
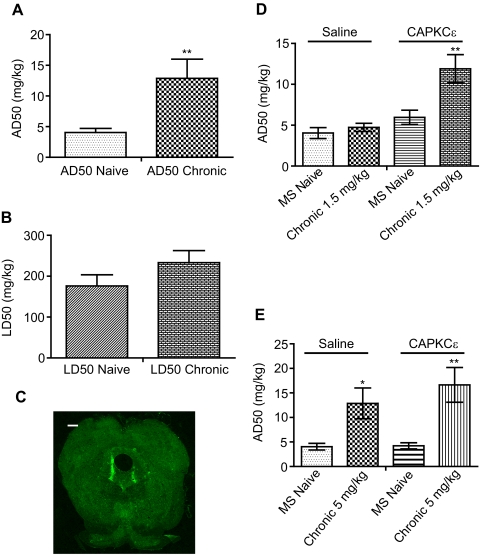
One possible explanation of why expressing PKC at the preBötC increased tolerance to respiratory depression and lethal overdose is that PKC lowered the threshold to develop tolerance. The threshold required to develop morphine tolerance is different for antinociception versus lethality. In wild-type rats treated with 5 mg/kg b.i.d. morphine for 4 days, the ED50 of morphine to cause antinociception increased 3-fold from 4.04 to 12.87 mg/kg (t = 3.17; P < 0.01), but their LD50 did not change significantly (Fig. 6, A and B). Thus, lethality has a higher threshold to develop tolerance than antinociception. Expressing CAPKCε at preBötC might increase tolerance to respiratory depression and lethality by decreasing the threshold to develop tolerance. If this is the case, injection of CAPKCε could also alter tolerance development to morphine's other effects. Hence, to determine whether CAPKCε is able to decrease the threshold for morphine antinociceptive tolerance development, we injected either saline or CAPKCε lentivirus into the vlPAG (Fig. 6C). When both groups were treated with 1.5 mg/kg b.i.d. for 4 days, only the CAPKCε-expressing rats developed tolerance—ED50 increased 2-fold to 11.9 ± 1.73 mg/kg (P < 0.01) [Fig. 6D; F(4,26) = 20.04; P < 0.001]. Since CAPKCε decreased the threshold to develop morphine tolerance, we sought to investigate whether CAPKCε could further increase tolerance after morphine antinociceptive tolerance developed. Rats given saline injections or CAPKCε at the vlPAG were treated with 5 mg/kg b.i.d. morphine for 4 days, a regimen that results in significant antinociceptive tolerance. Both saline- and CAPKCε-injected rats developed similar levels of antinociceptive tolerance (P < 0.05 for saline and P < 0.01 for CAPKCε) [Fig. 6E; F(4,26) = 22.48; P < 0.0001]. Thus, CAPKCε expression at the vlPAG allowed rats to develop significant antinociceptive tolerance when injected with morphine doses that normally do not cause tolerance, but CAPKCε would not increase the degree of tolerance once antinociceptive tolerance occurred.
Fig. 6.
PKCε activation decreased the dose required to develop morphine antinociceptive tolerance. A and B, morphine differential tolerance. The ED50 of morphine to cause antinociception (AD50) or lethality (LD50) was determined before and after chronic morphine. **, P < 0.01, compared with AD50 MS naive (t test). C, lentiviral transduction. CAPKCε lentivirus was injected into the vlPAG. Magnification is 100×, and scale bar is 500 μm. D, CAPKCε rats became tolerant to long-term treatment of 1.5 mg/kg morphine. ED50 was determined in saline or CAPKCε vlPAG microinjected rats. **, P < 0.01 compared with CAPKCε MS naive (two-way ANOVA). E, CAPKCε does not increase the magnitude of morphine tolerance. ED50 values were determined before and after long-term treatment of 5 mg/kg morphine in rats injected with either saline or CAPKCε at the vlPAG.*, P < 0.05 compared with saline MS naive. **, P < 0.01 compared with CAPKCε MS naive (two-way ANOVA). Data are presented as mean ± S.E.M. (n ≥ 6).
Discussion
The hypothesis that activating PKCα or ε at the preBötC could lead to increased morphine tolerance in respiratory depression and lethal overdose was tested. Expression of CAPKCα and ε at preBötC increased morphine tolerance to respiratory depression and lethal overdose. The level of morphine tolerance in respiratory depression was higher in CAPKCε rats than CAPKCα lentivirus-injected rodents. Past studies on PKC's role in morphine tolerance have focused on inhibiting PKC to decrease antinociceptive tolerance. Our current study is the first report that has investigated PKC's ability to increase morphine tolerance in respiratory depression and lethal overdose. Because respiration and overdose tolerance is increased, augmenting PKC activity at the preBötC could be one approach to improve the safety of morphine in long-term use.
The goal of this study was to test whether enhancing PKC activity at an important respiration control center could increase tolerance to opioid lethal overdose and respiratory depression. Although the stereotaxic coordinates targeted the preBötC, our lentivirus carried the cytomegalovirus promoter, which meant that exogenous PKC expression was not limited to the preBötC. Although immunohistochemistry showed that some viral transduced GFP-positive cells also stained for somatostatin and MOR, colocalization was not 100%. Caudal neighbors to preBötC include the rostral and caudal VRG. The rostral VRG is involved in inspiration by sending projections to the phrenic nucleus, whereas caudal VRG is implicated in expiration by projecting to expiratory motor neurons (Kirkwood and Sears, 1973; Dobbins and Feldman, 1994). Rostral to preBötC are BötC and pFRG/RTN. BötC neurons are glycinergic and project to the ventral respiratory group and phrenic nucleus. The inhibitory input from BötC suppresses inspiratory neurons during expiration (Dobbins and Feldman, 1994; Ezure et al., 2003). pFRG/RTN is involved in both expiration and CO2 chemosensation (Janczewski and Feldman, 2006). Dorsal to preBötC is the ambiguous nucleus, which controls muscles of the larynx and pharynx and contains premotor parasympathetic neurons that regulate cardiac function (Dergacheva et al., 2010). Respiratory neurons in the ventrolateral medulla are known to project extensively to each other, and preBötC is known to have reciprocating projections with VRG, BötC, and pFRG/RTN (Ellenberger and Feldman, 1990; Tan et al., 2010). Because many adjacent neurons to preBötC are involved in respiration, unintended viral transduction of preBötC neighbors would still allow us to test the ability of PKC in enhancing morphine tolerance to respiratory depression and lethal overdose.
WTPKCα or ε rodents did not develop significant morphine tolerance in respiratory depression; however, but CAPKCα or ε expressing rats did. These results can be explained by the higher PKC activity of the constitutively active mutants. It is well known that deleting or mutating the PKC pseudosubstrate inhibitory region increased PKC activity both in vitro and in vivo as judged by increased translocation to the plasma membrane (Pears et al., 1990; Takeishi et al., 2000). Although μ-opioid receptor-mediated activation of PKC has been reported in cell models (Chu et al., 2010), our current result implies that lower PKC activation by morphine at preBötC could be one of the reasons for less morphine respiration and overdose tolerance than antinociception. At the preBötC, the lack of effect from PKCα and ε siRNA lentivirus indicated that PKC activation was minimal, and PKC might not be an intrinsic mechanism to regulate MOR (Figs. 3, B and D, and 4, B and D). Both CAPKCα and CAPKCε were able to increase respiration and overdose tolerance, and this is in agreement with both subtypes' role in mediating receptor desensitization after morphine treatment (Bailey et al., 2009; Chu et al., 2010). Whether other members of the classic and novel PKC subtypes would elicit similar responses remains to be demonstrated. In addition, CAPKCε was able to increase respiratory depression tolerance to a higher extent than CAPKCα, which can be caused by differences in kinase activity levels or downstream phosphorylation targets. An alternative explanation for this difference could stem from the nonspecific promoter, cytomegalovirus, used to drive the expression of the transgenes causing the expression of the constitutively active PKC in nonopioid receptor and nonsomatostatin expressing neurons (Fig. 2). Whether CAPKCα and CAPKCε were expressed at identical neurons after injection was assumed and not determined.
Several mechanisms have been hypothesized as causing morphine tolerance, and this article investigated PKC because of the kinase's role in opioid receptor signaling when morphine was the agonist. Morphine is known to cause PKC activation, which in turn participates in opioid receptor desensitization and tolerance (Smith et al., 2007; Bailey et al., 2009; Chu et al., 2010). In addition to PKC, β-arrestin-mediated opioid receptor desensitization and opioid receptor recycling have been proposed as mechanisms to explain opioid antinociceptive tolerance (He and Whistler, 2005). β-Arrestin 2 knockouts are known to experience less respiratory depression and constipation than wild-type animals, with single morphine injections; however, it is not known whether the same applies to long-term treatment or respiratory depression tolerance (Raehal et al., 2005). More work needs to be done to determine the effects of β-arrestin and opioid receptor recycling on morphine tolerance in respiratory depression or lethal overdose.
There is correlation between the development of tolerance to morphine respiratory depression and lethality for CAPKCα and CAPKCε. In contrast, when tolerance did not develop to respiratory depression, such as in WTPKCα, there was a corresponding lack of tolerance to morphine lethality. This correlation between respiratory depression and lethality agrees with the widely believed idea that respiratory depression is the main cause of morphine lethality (White and Irvine, 1999). The majority of morphine lethality occurred between 1.5 and 5 h after injection, which is later than the morphine peak effect at 30 min for intraperitoneal injections. This observation is consistent with the fact that morphine does not cause complete cessation of breathing even at lethal doses but a precipitous reduction instead. Although total respiratory arrest would probably cause death close to the peak effect of morphine at 30 min after injection, a longer time course is required to cause death when rats are breathing at less than adequate minute ventilation. The cumulated effect of depressed respiration such as respiratory acidosis did eventually cause lethality but with slower kinetics than complete respiratory arrest.
Although therapeutic applicability of the findings in this article is limited by current technology, our results indicate that PKCε activation could greatly narrow or equalize tolerance between morphine lethality and antinociception. Morphine lethality normally has a higher threshold to develop tolerance than antinociception (Fig. 6, A and B); however, expressing CAPKCε would lower the threshold and allow morphine lethality tolerance to develop. On the other hand, morphine antinociceptive tolerance would be unchanged as antinociceptive tolerance easily develops, and CAPKCε could not further increase the magnitude of tolerance after it has occurred (Fig. 6E). Equalizing antinociceptive and lethality tolerance would improve the safety of current opioids.
This article tested the hypothesis that selective PKC activation in preBötC could increase tolerance to morphine respiratory depression and lethal overdose. Future opioid therapeutics that take advantage of differences in PKC subtype distribution and morphine-induced PKC activity between analgesic versus respiratory centers could maintain or improve the therapeutic index of morphine during long-term pain treatment. As annual lethal overdoses from prescription opioid analgesics have increased to more than 10,000 in the United States, developing approaches to improve the safety of opioids for long-term use is an important health care issue (Okie, 2010).
Supplementary Material
Acknowledgments
We thank Dr. Martin Wessendorf for the many discussions and recommendations on this project. We also thank Drs. Allen Samarel and Jody Martin for kindly providing the PKC subtype plasmids, Dr. George Wilcox for loaning us the Mouseox, Jinghua Xi for assistance in producing the rabbit anti-MOR, and Dr. Hui Zheng for technical assistance in siRNA lentivirus preparation.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA016674, DA000564, DA011806].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- preBötC
- pre-Bötzinger complex
- MOR
- μ-opioid receptor
- CAPKCα
- constitutively active PKCα
- CAPKCε
- constitutively active PKCε
- WTPKCε
- wild-type PKCε
- PKC
- protein kinase C
- vlPAG
- ventrolateral periaqueductal gray
- siRNA
- small interfering RNA
- Hb Sat
- hemoglobin oxygen saturation
- HEK
- human embryonic kidney
- VRG
- ventral respiratory group
- pFRG/RTN
- parafacial respiratory group/retrotrapezoid nucleus
- BötC
- Bötzinger complex
- MOR
- μ-opioid receptor
- WT
- wild type
- CA
- constitutively active
- OCT
- optimal cutting temperature
- BSA
- bovine serum albumin
- %MPE
- percentage maximal possible effect
- AP
- anteroposterior
- ML
- mediolateral
- DV
- dorsoventral
- TBS
- Tris-buffered saline
- GFP
- green fluorescent protein
- ANOVA
- analysis of variance
- qRT-PCR
- quantitative reverse transcription-polymerase chain reaction.
Authorship Contributions
Participated in research design: Lin, Law, and Loh.
Conducted experiments: Lin.
Performed data analysis: Lin and Law.
Wrote or contributed to the writing of the manuscript: Lin, Law, and Loh.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. (1995) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15:3328–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasos P, Smith CS, White JM, Somogyi AA, Bochner F, Ling W. (2006) Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain 120:267–275 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. (2010) Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization. Cell Signal 22:684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Huentelman MJ, Kasparov S, Metcalfe BL, Paton JF, Katovich MJ, Semple-Rowland SL, Raizada MK. (2003) Efficient large-scale production and concentration of HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics 12:221–228 [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. (2010) Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol 174:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. (1965) The up-and-down method for small samples. J Am Stat Assoc 60:967–978 [Google Scholar]
- Dobbins EG, Feldman JL. (1994) Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347:64–86 [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. (1990) Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res 513:35–42 [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Kondo M. (2003) Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci 23:8941–8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabra BH, Bailey CP, Kelly E, Smith FL, Henderson G, Dewey WL. (2008) Pre-treatment with a PKC or PKA inhibitor prevents the development of morphine tolerance but not physical dependence in mice. Brain Res 1217:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286:1566–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. (2008) Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA 300:2613–2620 [DOI] [PubMed] [Google Scholar]
- He L, Whistler JL. (2005) An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol 15:1028–1033 [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. (2006) Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570:407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed RR, Dewey WL, Smith PA, Smith FL. (2004) PKC and PKA inhibitors reverse tolerance to morphine-induced hypothermia and supraspinal analgesia in mice. Eur J Pharmacol 492:149–157 [DOI] [PubMed] [Google Scholar]
- Kao JH, Chen SL, Ma HI, Law PY, Tao PL, Loh HH. (2010) Intrathecal delivery of a mutant micro-opioid receptor activated by naloxone as a possible antinociceptive paradigm. J Pharmacol Exp Ther 334:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. (1973) Proceedings: monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol (Lond) 234:87P–89P [PubMed] [Google Scholar]
- Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B. (2009) Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol 106:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. (2005) Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience 135:227–234 [DOI] [PubMed] [Google Scholar]
- Lichtman AH. (1998) The up-and-down method substantially reduces the number of animals required to determine antinociceptive ED50 values. J Pharmacol Toxicol Methods 40:81–85 [DOI] [PubMed] [Google Scholar]
- Ling GS, Paul D, Simantov R, Pasternak GW. (1989) Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci 45:1627–1636 [DOI] [PubMed] [Google Scholar]
- Marks CE, Jr, Goldring RM. (1973) Chronic hypercapnia during methadone maintenance. Am Rev Respir Dis 108:1088–1093 [DOI] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. (2011) PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci 31:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. (2007) Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav 6:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S. (2010) A flood of opioids, a rising tide of deaths. N Engl J Med 363:1981–1985 [DOI] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. (1997) Ventilation in morphine-maintained rhesus monkeys. II: Tolerance to the antinociceptive but not the ventilatory effects of morphine. J Pharmacol Exp Ther 282:355–362 [PubMed] [Google Scholar]
- Pattinson KT. (2008) Opioids and the control of respiration. Br J Anaesth 100:747–758 [DOI] [PubMed] [Google Scholar]
- Pears CJ, Kour G, House C, Kemp BE, Parker PJ. (1990) Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem 194:89–94 [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM. (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201 [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. (2003) A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol 455:499–512 [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. (1987) Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res 424:311–319 [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127:129–139 [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. (2000) Pre-Bötzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol 83:2854–2868 [DOI] [PubMed] [Google Scholar]
- Strait JB, 3rd, Martin JL, Bayer A, Mestril R, Eble DM, Samarel AM. (2001) Role of protein kinase C-epsilon in hypertrophy of cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 280:H756–H766 [DOI] [PubMed] [Google Scholar]
- Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA. (2000) Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy. Circ Res 86:1218–1223 [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. (2008) Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat.Nat Neurosci 11:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. (2010) Projections of preBötzinger complex neurons in adult rats. J Comp Neurol 518:1862–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ. (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972 [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94:245–253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.