Abstract
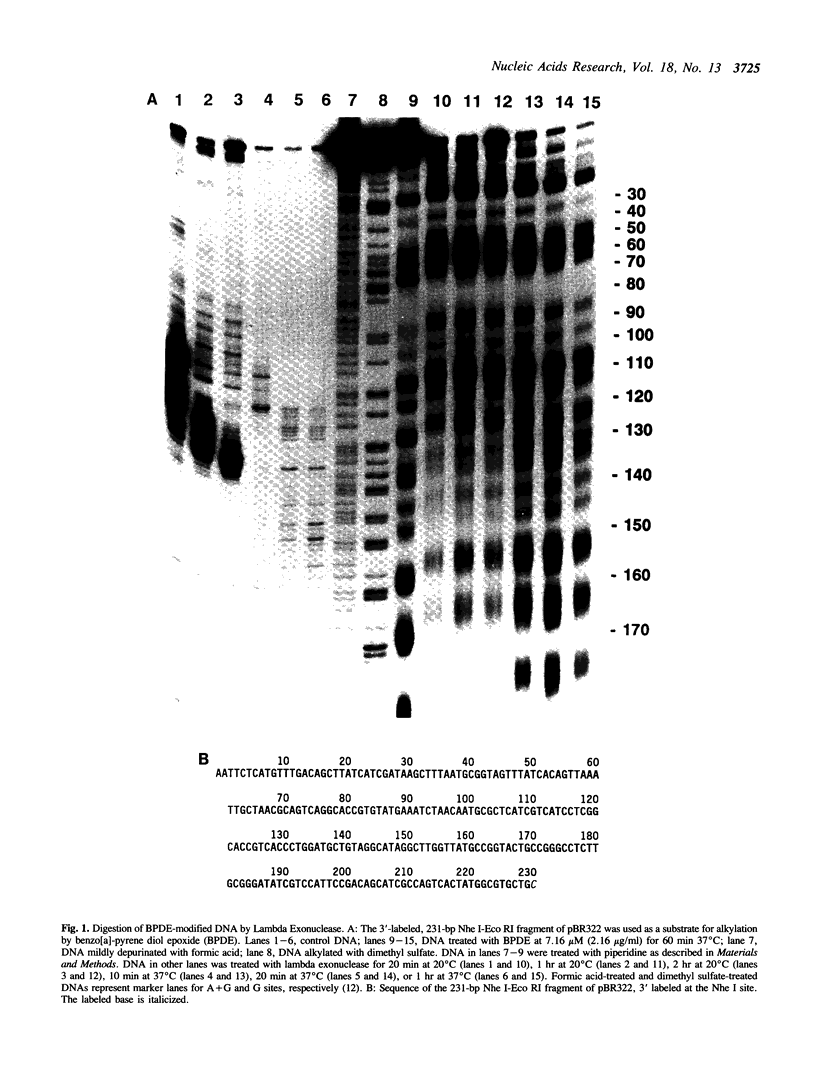
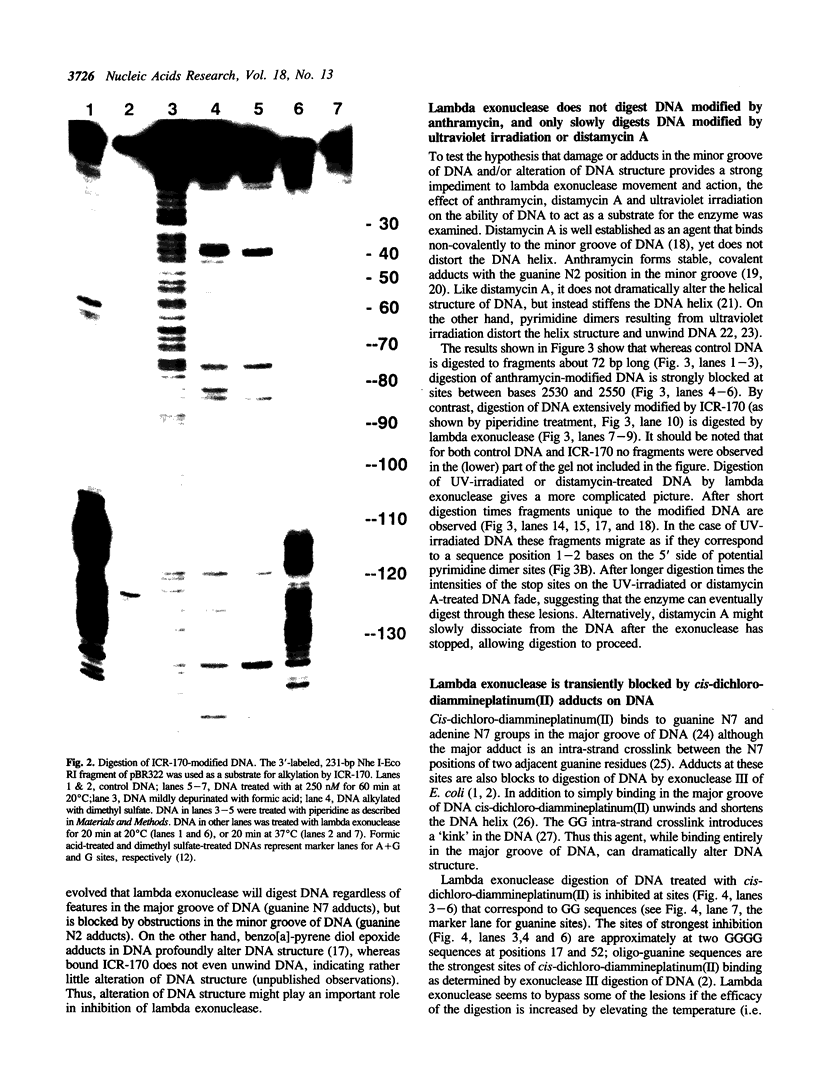
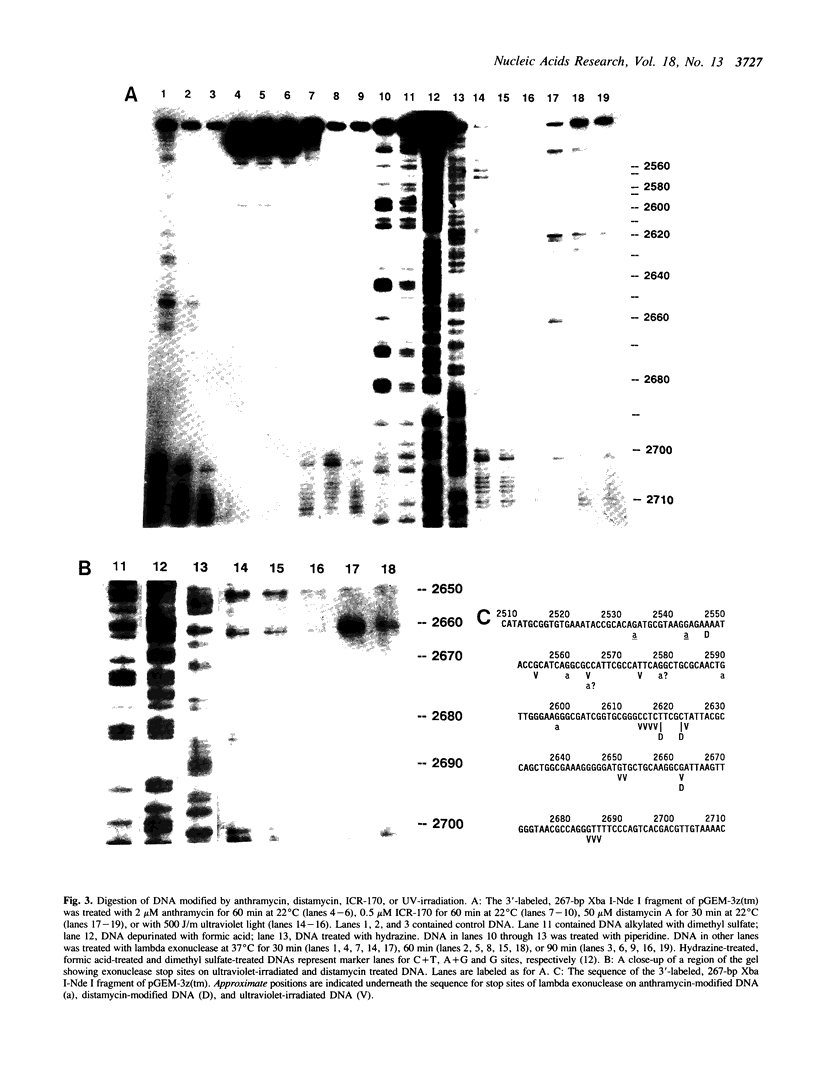
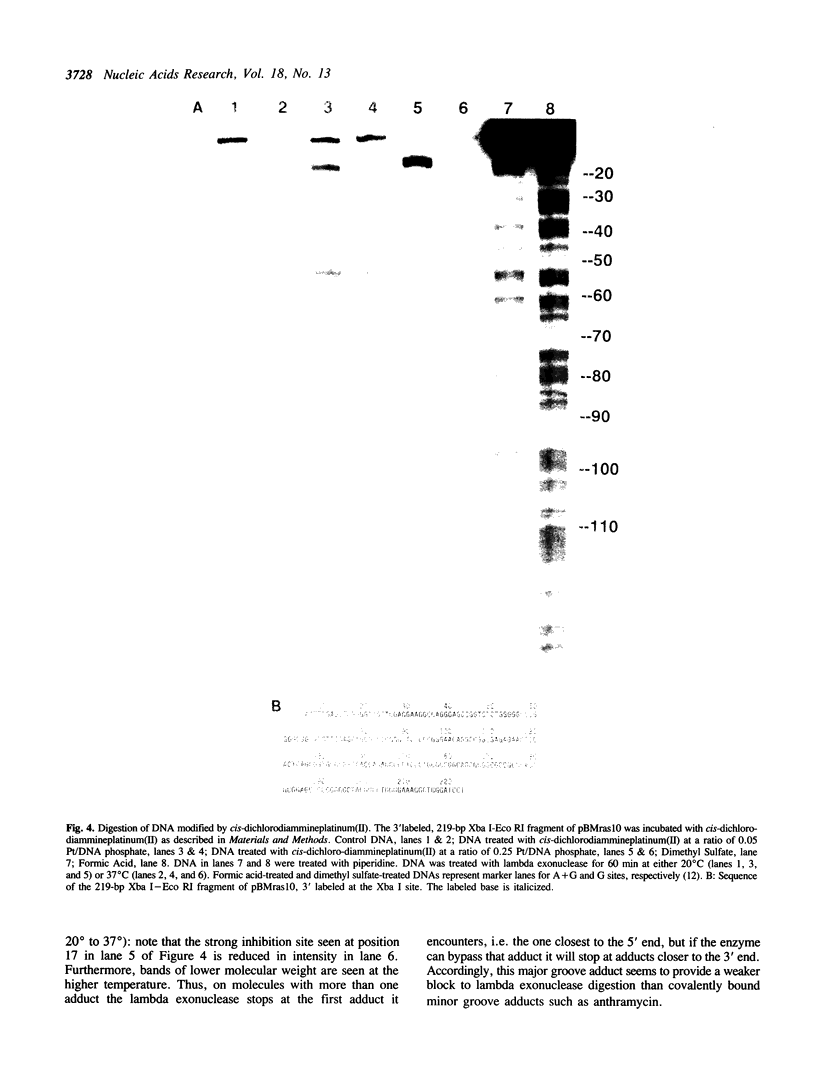
Various kinds of DNA damage block the 3' to 5' exonuclease action of both E. coli exonuclease III and T4 DNA polymerase. This study shows that a variety of DNA damage likewise inhibits DNA digestion by lambda exonuclease, a 5' to 3' exonuclease. The processive degradation of DNA by the enzyme is blocked if the substrate DNA is treated with ultraviolet irradiation, anthramycin, distamycin, or benzo[a]-pyrene diol epoxide. Furthermore, as with the 3' to 5' exonucleases, the enzyme stops at discrete sites which are different for different DNA damaging agents. On the other hand, digestion of treated DNA by lambda exonuclease is only transiently inhibited at guanine residues alkylated with the acridine mustard ICR-170. The enzyme does not bypass benzo[a]-pyrene diol epoxide or anthramycin lesions even after extensive incubation. While both benzo[a]-pyrene diol epoxide and ICR-170 alkylate the guanine N-7 position, only benzo[a]-pyrene diol epoxide also reacts with the guanine N-2 position in the minor groove of DNA. Anthramycin and distamycin bind exclusively to sites in the minor groove of DNA. Thus lambda exonuclease may be particularly sensitive to obstructions in the minor groove of DNA; alternatively, the enzyme may be blocked by some local helix distortion caused by these adducts, but not by alkylation at guanine N-7 sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. E., Camier S., Sentenac A., Hall B. D. Gene size differentially affects the binding of yeast transcription factor tau to two intragenic regions. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8768–8772. doi: 10.1073/pnas.84.24.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Boyer V., Moustacchi E., Sage E. Sequence specificity in photoreaction of various psoralen derivatives with DNA: role in biological activity. Biochemistry. 1988 Apr 19;27(8):3011–3018. doi: 10.1021/bi00408a052. [DOI] [PubMed] [Google Scholar]
- Burnouf D., Duane M., Fuchs R. P. Spectrum of cisplatin-induced mutations in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3758–3762. doi: 10.1073/pnas.84.11.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrocchi G., Pedrini A. M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982 Feb 25;155(2):177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Chan G. L., Haseltine W. A. T4 DNA polymerase (3'-5') exonuclease, an enzyme for the detection and quantitation of stable DNA lesions: the ultraviolet light example. Nucleic Acids Res. 1985 May 10;13(9):3285–3304. doi: 10.1093/nar/13.9.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. DNA binding spectrum of the carcinogen N-acetoxy-N-2-acetylaminofluorene significantly differs from the mutation spectrum. J Mol Biol. 1984 Jul 25;177(1):173–180. doi: 10.1016/0022-2836(84)90063-9. [DOI] [PubMed] [Google Scholar]
- Glaubiger D., Kohn K. W., Charney E. The reaction of anthramycin with DNA. 3. Properties of the complex. Biochim Biophys Acta. 1974 Sep 13;361(3):303–311. doi: 10.1016/0005-2787(74)90373-6. [DOI] [PubMed] [Google Scholar]
- Graves D. E., Stone M. P., Krugh T. R. Structure of the anthramycin-d(ATGCAT)2 adduct from one- and two-dimensional proton NMR experiments in solution. Biochemistry. 1985 Dec 17;24(26):7573–7581. doi: 10.1021/bi00347a011. [DOI] [PubMed] [Google Scholar]
- Hogan M. E., Dattagupta N., Whitlock J. P., Jr Carcinogen-induced alteration of DNA structure. J Biol Chem. 1981 May 10;256(9):4504–4513. [PubMed] [Google Scholar]
- Hurley L. H., Petrusek R. Proposed structure of the anthramycin-DNA adduct. Nature. 1979 Nov 29;282(5738):529–531. doi: 10.1038/282529a0. [DOI] [PubMed] [Google Scholar]
- Husain I., Griffith J., Sancar A. Thymine dimers bend DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2558–2562. doi: 10.1073/pnas.85.8.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. H., Salnikow J., Moore S., Stein W. H. Bovine pancreatic deoxyribonuclease A. Isolation of cyanogen bromide peptides; complete covalent structure of the polypeptide chain. J Biol Chem. 1973 Feb 25;248(4):1489–1495. [PubMed] [Google Scholar]
- Little J. W. An exonuclease induced by bacteriophage lambda. II. Nature of the enzymatic reaction. J Biol Chem. 1967 Feb 25;242(4):679–686. [PubMed] [Google Scholar]
- Malinge J. M., Schwartz A., Leng M. Characterization of the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II), ethidium bromide and nucleic acids. Nucleic Acids Res. 1987 Feb 25;15(4):1779–1797. doi: 10.1093/nar/15.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W., Matheson D. W. GC-rich regions in genomes as targets for DNA alkylation. Carcinogenesis. 1988 Nov;9(11):2065–2072. doi: 10.1093/carcin/9.11.2065. [DOI] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. Mechanism of DNA strand breakage by piperidine at sites of N7-alkylguanines. Biochim Biophys Acta. 1986 Oct 16;868(1):71–76. doi: 10.1016/0167-4781(86)90088-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mokul'skii M. A., Kapitonova K. A., Mokul'skaya T. D. Secondary structure of phage T2 DNA. Mol Biol. 1972 Nov-Dec;6(6):716–731. [PubMed] [Google Scholar]
- Osborne M. R., Jacobs S., Harvey R. G., Brookes P. Minor products from the reaction of (+) and (-) benzo[a]-pyrene-anti-diolepoxide with DNA. Carcinogenesis. 1981;2(6):553–558. doi: 10.1093/carcin/2.6.553. [DOI] [PubMed] [Google Scholar]
- Ostrander E. A., Karty R. A., Hallick L. M. High resolution psoralen mapping reveals an altered DNA helical structure in the SV40 regulatory region. Nucleic Acids Res. 1988 Jan 11;16(1):213–227. doi: 10.1093/nar/16.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi G. B., Walker I. G. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3'-5' exonuclease action of T4 DNA polymerase. Biochemistry. 1990 Feb 27;29(8):2122–2126. doi: 10.1021/bi00460a023. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Detection of neocarzinostatin chromophore-deoxyribose adducts as exonuclease-resistant sites in defined-sequence DNA. Biochemistry. 1985 Jul 16;24(15):4035–4040. doi: 10.1021/bi00336a035. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Regulation of lambda exonuclease. I. Properties of lambda exonuclease purified from lysogens of lambda T11 and wild type. J Mol Biol. 1966 Jul;18(2):235–250. doi: 10.1016/s0022-2836(66)80243-7. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Rice J. A., Crothers D. M., Pinto A. L., Lippard S. J. The major adduct of the antitumor drug cis-diamminedichloroplatinum(II) with DNA bends the duplex by approximately equal to 40 degrees toward the major groove. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4158–4161. doi: 10.1073/pnas.85.12.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E., Moustacchi E. Sequence context effects on 8-methoxypsoralen photobinding to defined DNA fragments. Biochemistry. 1987 Jun 16;26(12):3307–3314. doi: 10.1021/bi00386a010. [DOI] [PubMed] [Google Scholar]
- Suck D., Oefner C. Structure of DNase I at 2.0 A resolution suggests a mechanism for binding to and cutting DNA. Nature. 1986 Jun 5;321(6070):620–625. doi: 10.1038/321620a0. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Cross-linking and relaxation of supercoiled DNA by psoralen and light. Biochim Biophys Acta. 1978 Dec 21;521(2):529–546. doi: 10.1016/0005-2787(78)90295-2. [DOI] [PubMed] [Google Scholar]