Abstract
The mechanism for activation of extracellular transglutaminase 2 (TG2) in the small intestine remains a fundamental mystery in our understanding of celiac sprue pathogenesis. Using the T84 human enterocytic cell line, we show that interferon-γ (IFN-γ), the predominant cytokine secreted by gluten-reactive T cells in the celiac intestine, activates extracellular TG2 in a dose-dependent manner. IFN-γ mediated activation of TG2 requires phosphatidylinositol-3-kinase (PI3K) activity, but is uninfluenced by a number of other kinases reported to be active in T84 cells. Pharmacological inhibition of PI3K in the presence of IFN-γ prevents TG2 activation as well as the previously characterized increase in transepithelial permeability. Our findings therefore establish PI3K as an attractive target for celiac sprue therapy, a possibility that is underscored by the encouraging safety profiles of several PI3K inhibitors undergoing human clinical trials.
Introduction
Celiac sprue is a widespread inflammatory disease of the small intestine (Green and Jabri, 2006; Green and Cellier, 2007; Kagnoff, 2007). The primary genetic factors (HLA-DQ2 or, less frequently, HLA-DQ8) (Sollid et al., 1989; Karell et al., 2003) and environmental factors (dietary gluten) (Dicke et al., 1953) responsible for the onset of this lifelong illness have been well established. It has also been demonstrated that toxic gluten peptides elicit a strong immune response in the celiac intestine after regiospecific deamidation by an endogenous extracellular enzyme, transglutaminase 2 (TG2) (Molberg et al., 1998; van de Wal et al., 1998; Anderson et al., 2000). However, under normal physiological conditions, extracellular TG2 in the small intestinal mucosa is predominantly inactive (Siegel et al., 2008; Stamnaes et al., 2010) and must therefore be activated before gluten peptides can be deamidated. The mechanism by which TG2 is activated in the celiac small intestine remains unknown. A better understanding of this mechanism could facilitate the discovery of drugs that protect celiac patients from gluten-induced immunotoxicity by blocking TG2 activity in the small intestine (Sollid and Khosla, 2011).
We have shown that inflammatory signals, such as activation of toll-like receptor 3, rapidly induced TG2 activity in the mouse small intestine (Siegel et al., 2008). However, in celiac disease, inflammation is triggered by T cells that recognize toxic gluten peptides in an HLA-DQ2 (or, less frequently, HLA-DQ8)-dependent manner. Because the primary proinflammatory cytokine secreted by these T-helper cells is IFN-γ, (Nilsen et al., 1995, 1998) we hypothesized the existence of an alternate signal transduction pathway for extracellular TG2 activation, one that is induced by IFN-γ. The major goal of the current study was therefore to test this hypothesis. To do so, we investigated the human intestinal epithelial cell line T84, because of a large body of evidence suggesting that these cells were responsive to IFN-γ (Madara and Stafford, 1989; Bruewer et al., 2003, 2005; Utech et al., 2005). In particular, when the basolateral side of a cultured monolayer of T84 cells is exposed to IFN-γ, its permeability increases, as measured by the transepithelial flux of gluten peptides (Bethune et al., 2009b). Using this assay, we have not only verified our hypothesis, but have also identified a promising kinase target for celiac sprue therapy.
Materials and Methods
Materials.
Reagents for peptide synthesis were from Chem-Impex (Wood Dale, IL), Peptides International Inc. (Louisville, KY), AnaSpec, Inc. (San Jose, CA), and EMD Biosciences (San Diego, CA). Cy3 and Cy5-N-hydroxysuccinimide ester were from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Recombinant IFN-γ was from Peprotech (Rocky Hill, NJ). Cell culture medium, antibiotics, trypsin-EDTA, sterile PBS, goat anti-rabbit (H-L) and goat anti-mouse (H-L) secondary antibodies, streptavidin Alexa Fluor 647, streptavidin-HRP, and goat anti-mouse HRP were from Invitrogen (Carlsbad, CA). Primary antibodies mouse anti-TG2 (CUB 7402 + TG100) and rabbit anti-E-cadherin were from Thermo Fisher Scientific (Waltham, MA) and Cell Signaling Technology (Danvers, MA), respectively. Fetal bovine serum was from Atlanta Biologicals (Norcross, GA). T84 cells were from American Type Culture Collection (Manassas, VA). Permeable supported culture transwells were from Corning Life Sciences (Lowell, MA). Radioimmunoprecipitation assay buffer was from Thermo Fisher Scientific. Hybond ECL nitrocellulose and ECL detection reagents were from GE Healthcare. The TG2 inhibitor 2-[(3-bromo-4,5-dihydro-isoxazol-5-ylmethyl)-carbamoyl]-pyrrolidine-1-carboxylic acid quinolin-3-ylmethyl ester (ERW1041E) was synthesized as described previously (Watts et al., 2006). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) was from Cell Signaling Technology. 2-amino-1-[(3S)-hexahydro-3-methyl-4-[(4-methyl-5-isoquinolinyl)sulfonyl]-1H-1,4-diazepin-1-yl]ethanone (Glycyl-H-1152), triciribine, and bisindolylmaleimide-II were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Compound 15e, 4-[2,3-dihydro-3-methyl-2-oxo-8-(3-quinolinyl)-1H-imidazo[4,5-c]quinolin-1-yl]-α,α-dimethyl-benzeneacetonitrile (BEZ235), and 5-[5-(4-fluoro-2-hydroxy-phenyl)-furan-2-ylmethylene]-thiazolidine-2,4-dione (AS-252424) were from Cayman Chemical (Ann Arbor, MI). Dorsomorphin was from Sigma-Aldrich (St. Louis, MO). 2-[(6-Amino-9H-purin-9-yl)methyl]-5-methyl-3-(2-methylphenyl)-4(3H)-quinazolinone (IC-87114) was from EMD Biosciences (San Diego, CA), and 7-methyl-2-(morpholin-4-yl)-9-(1-phenylaminoethyl)-pyrido[1,2-a]-pyrimidin-4-one (TGX-221) was from Selleck Chemicals LLC (Houston, TX). Tetramethylbenzidine ready mix substrate was from Sigma-Aldrich. Vectashield Mounting Media was purchased from Vector Laboratories (Burlingame, CA). Zeiss Immersol 518 F fluorescence free oil was from Thermo Fisher Scientific.
Peptide Synthesis, Labeling, and Purification.
D8mer and 33mer peptides were synthesized by using tert-butoxy-carbonyl/O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate chemistry on solid-phase N-R-t-Boc-l-aminoacyl phenylacetamidomethyl resin as described previously (Xia et al., 2006; Bethune et al., 2009a,b). The membrane-permeable myosin light chain kinase (MLCK) inhibitor, PIK, was synthesized by using a fluorenylmethyloxycarbonyl/O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate chemistry on solid-phase fluorenylmethyloxycarbonyl-Nε-Boc-l-lysine-aminomethyl rink amide aminomethyl resin as described previously (Zolotarevsky et al., 2002). After cleavage of the D8mer and 33mer from the phenylacetamidomethyl resin by using trifluoroacetic acid (TFA)/trifluoromethanesulfonic acid/thioanisole (10:1:1, v/v/v) for 4 h, or the PIK peptide from the rink amide acetoxymethyl ester resin by using TFA/triisopropylsilane/water (95:2.5:2.5, v/v/v) for 1 h, the crude peptides were precipitated in cold ether and dissolved in 1:1 (v/v) acetonitrile/water. The peptides were purified by reverse-phase HPLC on a semipreparative C18 column by using a water-acetonitrile gradient in 0.1% (v/v) TFA. Their identities were confirmed by electrospray mass spectrometry and analytical reverse-phase HPLC. The peptides were lyophilized and stored at −20°C before use. For transepithelial transport assays, purified D8mer and 33mer peptides were labeled by using Cy3 or Cy5-N-hydroxysuccinimide ester in DMSO according to the manufacturer's (GE Healthcare) instructions, repurified by HPLC, lyophilized, and stored at −20°C. The correct mass of all peptides was confirmed by liquid chromatography-assisted mass spectrometry. Before use, peptides were resuspended in 50 mM sodium phosphate, pH 7.0, supplemented with 0.02% (w/v) NaN3. The concentrations of unlabeled, Cy3-labeled, and Cy5-labeled peptides were determined at pH 7.0 by spectrophotometric measurement of A280 (D8mer ε280 = 1280 M−1 · cm−1; 33mer ε280 = 3840 M−1 · cm−1; PIK ε280 = 2560 M−1 · cm−1); A550 (ε550 = 150,000 M−1 · cm−1); and A650 (ε650 = 250,000 M−1 · cm−1), respectively. D8mer = easasysa (784.8 g/mol), 33mer = LQLQPF(PQPQLPY)3PQPQPF (3911.5 g/mol), and PIK = RKKYKYRRK-NH2 (1324.6 g/mol).
Cell Culture.
T84 epithelial cells were grown in Dulbecco's modified Eagle's medium/Ham's F-12 (1:1) supplemented with antibiotics (penicillin/streptomycin) and 5% (v/v) fetal bovine serum. Cells were grown at 37°C and 5% CO2. Medium was changed every alternate day. When the cells reached more than 90% confluence, they were passaged using trypsin-EDTA.
Peptide Flux Translocation Assays.
Transwell-permeable supports (5-μm pore size, 6.5 mm in diameter) were coated in rat tail collagen followed by overnight sterilization under ultraviolet light. Cultured T84 cells were seeded on the sterile, collagen-coated permeable supports at 3 × 104 cells/well, and the medium was exchanged every other day for 2 weeks while the cells grew to confluence and formed tight junctions. After this maturation period, culture media in the apical and basolateral chambers were replaced with fresh medium containing kinase inhibitors, as needed, for 1 h. This was followed by 1- to 72-h incubation with 0 to 1000 U/ml IFN-γ added to the basolateral side. Thereafter, the media in both the apical and basolateral chambers were replaced with fresh medium, and equimolar concentrations of Cy3-D8mer and Cy5-33mer were added to the apical side. Both sides were sampled at the 0-h time point. Additional samples were withdrawn every hour from the basolateral side over a 4-h experiment. Fluorescence in collected samples was measured in a 96-well format on a FlexStation II 384 (Molecular Devices, Sunnyvale, CA), monitoring two channels (excitation 540 nm and emission 575 nm for Cy3; excitation 640 nm and emission 675 nm for Cy5). The slope of basolateral fluorescence units versus time (from 1–4 h) was calibrated to the initial apical fluorescence and divided by the permeable support area (0.33 cm2) to yield the transepithelial flux (pmol/cm2/h). Peptide flux fold change was normalized to control samples lacking IFN-γ. Each set of conditions was tested at least in triplicate (n = 3) wells.
Confocal Fluorescence Microscopy.
T84 cells were grown to maturity as described above. For fluorescence staining experiments, T84 transwells were treated with IFN-γ in the basolateral side for 48 h. Afterward, the medium in both chambers was replaced with fresh medium containing 200 μM 5-biotinamido pentylamine (5BP) for 4 h. As a control, TG2 activity was inhibited with 25 μM ERW1041E for 1 h after IFN-γ exposure but before 5BP incubation. ERW1041E was also maintained in the media during the 5BP incubation. After the 5BP incubation, monolayers were washed with warm PBS three times and fixed with 4% (w/v) paraformaldehyde in PBS for 15 min. Cells were then washed three times with PBS and blocked overnight at 4°C by using 5% (w/v) bovine serum albumin in PBS supplemented with 0.1% (v/v) Tween 20. Primary antibodies, rabbit anti-E-cadherin IgG monoclonal antibody (1:200 dilution in blocking buffer), and mouse anti-TG2 IgG monoclonal antibody (1:200 dilution in blocking buffer), were used to label E-cadherin and TG2, respectively, in an overnight incubation at 4°C. E-cadherin was used as a marker of cell-cell contacts in the enterocyte monolayer. Cells were washed three times with PBS + 0.1% (v/v) Tween 20 and incubated once again overnight at 4°C with secondary antibodies, goat anti-rabbit (H-L) IgG Alexa Fluor 555 conjugate (2 μg/ml in blocking buffer), and goat anti-mouse (H-L) IgG Alexa Fluor 488 conjugate (2 μg/ml in blocking buffer). To visualize TG2 activity, streptavidin Alexa Fluor 647 conjugate (2 μg/ml in blocking buffer) was also added. Subsequently, cells were washed four times with PBS + 0.1% (v/v) Tween 20. Permeable supports were cut from their frames with a sterile razor blade and mounted on slides by using Vectashield mounting media. Coverslips were sealed onto the mounting slides with multiple coats of clear nail polish. Sealed slides were stored in the dark at 4°C and imaged using a Carl Zeiss Inc. (Thornwood, NY) LSM 510 meta confocal microscope, Zeiss Immersol 518F oil, and a 40× Plan-Neo/1.3 NA Oil objective lens with digital image correlation capability.
Western Blot Analysis of T84 Cell Lysates.
For measurement of TG2 expression, T84 monolayers were grown to maturity as described above and treated with basolateral IFN-γ (0–1000 U/ml) for 48 h. Cells were washed three times with warm PBS. All chamber contents were removed, and 100 μl of apical radioimmunoprecipitation assay buffer supplemented with 1% (w/v) SDS and complete protease inhibitors (1×) was added to the apical chamber for 15 min at room temperature. Apical contents were removed and placed in Eppendorf tubes. Samples were centrifuged at ∼14,000g for 10 min. Supernatants were collected, and the cell debris was discarded. Forty microliters of Laemmli buffer containing 2-mercaptoethanol was mixed with 10 μl of T84 cell lysate and boiled for 10 min. Forty-microliter protein samples were loaded onto a 4 to 20% SDS-polyacrylamide electrophoresis gel and run for 2 h at 100 V. The gel was removed, washed with deionized water, equilibrated in 4°C transfer buffer, and loaded onto a protein transfer apparatus. Gel protein bands were transferred to a Hybond ECL membrane using transfer buffer [20 mM Tris, pH 8, 150 mM glycine, 20% (v/v) methanol] for 1 h at 80 V and 4°C. The membrane was removed from the apparatus, quickly rinsed with wash buffer [20 mM Tris, pH 7.5, 0.9% (w/v) NaCl, 0.1% (v/v) Tween 20], and blocked using wash buffer supplemented with 5% (w/v) dry milk for 2 h at room temperature. After incubation with primary antibodies [mouse anti-TG2 IgG monoclonal antibody (1:1000 dilution in blocking buffer) and mouse anti-β-actin (1:4000 dilution in blocking buffer)] overnight at 4°C, the membrane was washed with Tris-buffered saline + 0.1% (v/v) Tween 20 (wash buffer) three times each for 5 min at room temperature. The membrane was then incubated with secondary antibody goat anti-mouse IgG HRP (1:2000 in blocking buffer) for 2 h at room temperature and washed with Tris-buffered saline + 0.1% (v/v) Tween 20 (wash buffer) three times each for 5 min at room temperature. The membrane was developed by using ECL detection reagents for 5 min, rinsed, and quantified using a Typhoon fluorescence imager (GE Healthcare).
TG2 Activity Assays in Cell Culture.
For measurement of TG2 activity, T84 monolayers were grown to maturity as described above. Then IFN-γ was added to the basolateral side. After a defined period, the medium in both chambers was replaced with fresh medium containing kinase inhibitors, as needed, for 1 h. Next, 5BP was spiked in both chambers at 200 μM for 4 h. ERW1041E was used as described in the fluorescence microscopy section above. After 5BP exposure, T84 monolayers were washed with warm PBS three times and fixed with 4% (w/v) paraformaldehyde in PBS for 15 min at room temperature. After three washes with PBS, monolayers were blocked overnight at 4°C using 5% (w/v) bovine serum albumin in PBS supplemented with 0.1% (v/v) Tween 20. Then, monolayers were treated with horseradish peroxidase-conjugated streptavidin, diluted according to the manufacturer's recommendations, in blocking buffer overnight at 4°C. Cells were washed four times with PBS + 0.1% Tween 20. A ready mix solution of tetramethylbenzidine substrate was added to the transwell chambers for 5 to 10 min, after which 100 μl was removed and quenched in equal volume of 1 M hydrochloric acid in a 96-well plate format. Absorbance at 450 nm was measured using a 96-well plate reader. The fold change in 5BP incorporation was normalized to the 0 U/ml IFN-γ condition. Each set of conditions was assayed in at least triplicate wells. To convert these measurements into TG2-specific activity, purified recombinant TG2 was spiked into the T84 wells, and the 5BP assay was repeated (data not shown).
Results
IFN-γ Mediated Peptide Flux across T84 Monolayers Is Dominated by Paracellular Transport.
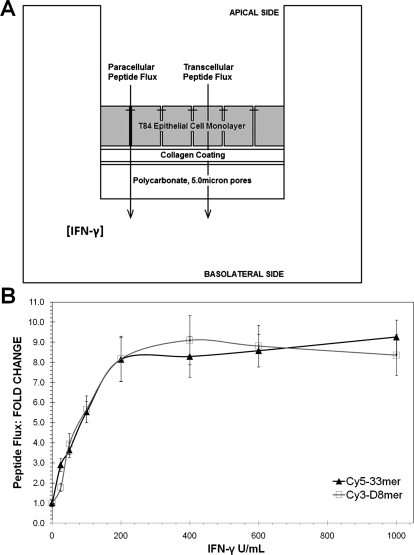
The T84 model used in this study was adapted from the cell culture system described in our previous report, in which the ability of IFN-γ to enhance transepithelial peptide flux was verified (Fig. 1) (Bethune et al., 2009b). Although it is well established that IFN-γ induces actin rearrangement and the internalization of tight junction proteins in mature T84 monolayers (Bruewer et al., 2005; Utech et al., 2005; Beaurepaire et al., 2009), the relative contribution of paracellular versus transcellular transport of gluten peptides in this model system is unclear (Fig. 1A). To address this question, we measured the flux of a Cy5-labeled gluten peptide (LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF, a.k.a. 33mer) (Shan et al., 2002; Bethune et al., 2009b) as well as a Cy3-labeled octapeptide comprised of d-amino acids that is exclusively transported across the intestinal epithelium via the paracellular route (easasysa, a.k.a. D8mer) (Pappenheimer et al., 1994, 1997). Both peptides are highly resistant to proteolysis and therefore remained stable in cell culture for the duration of the experiment. T84 cells, grown to maturity on collagen-coated supports, were treated with IFN-γ on the basolateral side for 48 h. The apical-to-basolateral flux of Cy5-33mer and Cy3-D8mer was quantified by sampling the basolateral chamber every hour for 4 h. IFN-γ increased the flux of Cy5-33mer and Cy3-D8mer peptides across the T84 monolayer by as much as 10-fold (Fig. 1B). Within experimental error, the observed increase in flux was identical for both peptides (Fig. 1C), implying that paracellular transport is the dominant pathway for gluten peptide translocation across the T84 epithelial cell monolayer. In the context of celiac sprue, paracellular peptide transport would allow gluten peptides to gain access to TG2 in the extracellular matrix of the small intestine.
Fig. 1.
T84 translocation assay used to measure flux of fluorescently labeled peptides. A, transwell schematic illustrates paracellular and transcellular transport of peptides. If DP′ = paracellular mass flux of D8mer upon exposure to IFN-γ, DP = basal paracellular mass flux of D8mer, PP′ = paracellular mass flux of 33mer in response to IFN-γ, PP = basal paracellular mass flux of 33mer, PT′ = transcellular mass flux of 33mer upon exposure to IFN-γ, and PT = basal transcellular mass flux of 33mer, then PP′/PP ≈ DP′/DP would suggest that the paracellular route is the dominant transport pathway of 33mer. B, average peptide flux normalized to 0 U/ml IFN-γ (basal) condition. The data are represented as mean ± S.D. In the absence of IFN-γ, the molar fluxes of the Cy3-D8mer (□) and Cy5-33mer (▴) peptides were 1.80 ± 0.41 and 0.97 ± 0.17 pmol/cm2/h, respectively.
IFN-γ Activates TG2 in the Extracellular Matrix of T84 Monolayers.
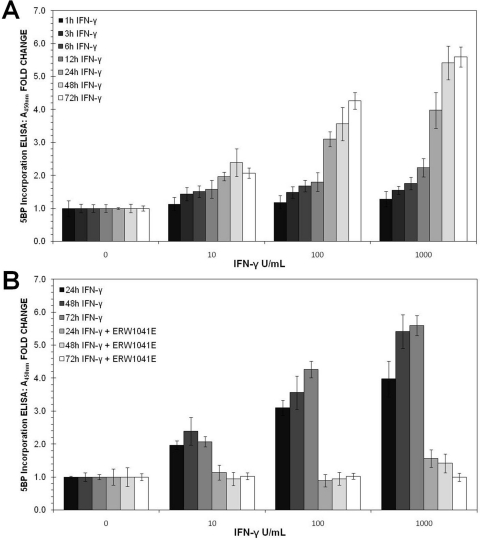
In addition to increasing the paracellular permeability of antigenic gluten peptides, we sought to determine whether physiological concentrations of IFN-γ could activate TG2 in the extracellular matrix of mature T84 monolayers. Again, the basolateral side of these cells was exposed to varying doses of IFN-γ for 1 to 72 h. Thereafter, a small-molecule substrate of TG2, 5BP, was added to the cell culture medium for 4 h. During this period, catalytically active TG2 attached 5BP to proteins such as fibronectin in the extracellular matrix. To quantify the extent to which IFN-γ activated TG2, an ELISA using streptavidin conjugated to horseradish peroxidase was developed. Because T84 cells were fixed but not permeabilized, only extracellular TG2 activity was assayed in this manner. As seen in Fig. 2, TG2 activity increased steadily in response to IFN-γ exposure. Pretreatment with ERW1041E (1; Fig. 3), a potent TG2 inhibitor (Watts et al., 2006), completely blocked 5BP incorporation. Because the observed increase in TG2 activity occurred mainly after 24 h of IFN-γ exposure, and a concomitant increase in TG2 protein was also visualized, de novo gene expression was likely to have played a role in this process. Western blot analysis of whole-cell lysates from T84 monolayers further supports this hypothesis (Supplemental Fig. 1).
Fig. 2.
Dose and time dependence of TG2 activation in response to IFN-γ treatment of a mature monolayer of T84 cells. A, T84 monolayers were treated for 1 to 72 h with 0 to 1000 U/ml IFN-γ. TG2 activity in these cultures was quantified by the amount of cross-linked 5BP, as measured by turnover of tetramethylbenzidine, an HRP substrate. The streptavidin used to detect cross-linked 5BP was conjugated to HRP. B, effect of 25 μM ERW1041E (1), a small-molecule TG2 inhibitor, on IFN-γ induced TG2 activity. Data are reported as mean ± S.D.
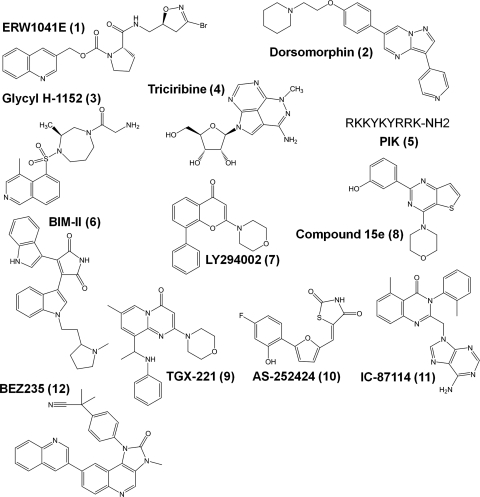
Fig. 3.
Structures of small-molecule and peptide inhibitors used in this study.
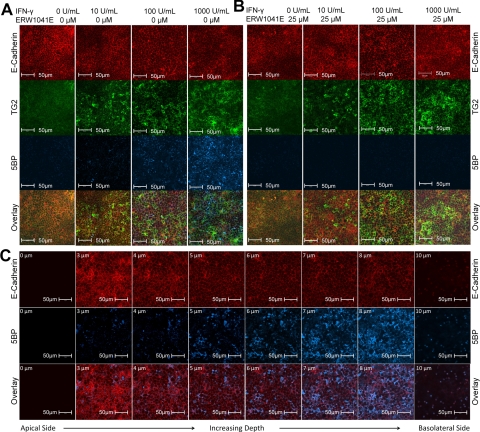
To visualize the spatial distribution of catalytically active TG2 in a T84 monolayer, fixed cells were exposed to streptavidin conjugated to Alexa Fluor 647 as well as control antibodies, and confocal fluorescence microscopy was used to visualize the labeling pattern. As observed in Fig. 4A, TG2 activity in the monolayer increased as the concentration of IFN-γ was raised. Maximum activity was observed at the highest IFN-γ concentration tested (1000 U/ml). Once again, incorporation of 5BP was completely inhibited when T84 monolayers were pretreated with ERW1041E (Fig. 4B).
Fig. 4.
Fluorescence microscopic analysis of T84 monolayers treated with IFN-γ. Cultures were stained with antibodies against E-cadherin (red) and TG2 (green) proteins, as well as with a streptavidin conjugate (blue) that recognizes 5BP incorporated at any site where extracellular TG2 is activated. Images were generated by using a Carl Zeiss 510 Meta confocal microscope with an oil immersion compatible 40× objective capable of digital imaging correlation. A, treatment of T84 monolayers with 0 to 1000 U/ml IFN-γ for 48 h. B, T84 monolayers exposed to 0 to 1000 U/ml IFN-γ for 48 h were pretreated with 25 μM ERW1041E before addition of 5BP. C, single plane images were generated at different depths of a T84 monolayer treated with 1000 U/ml IFN-γ for 48 h. The depth of each focal plane is illustrated in the upper left corner (0–10 μm) of each single plane image. Images are arranged from apical to basolateral side of the T84 monolayer as read left to right. Each transwell was performed in triplicate (n = 3). All images were processed in the same manner.
To establish the precise location of catalytically active extracellular TG2, single plane images were generated at varying depths into the monolayer and compiled. As seen in Fig. 4C, most of the extracellular TG2 activity preferentially localized toward the permeable support and below the E-cadherin marker of tight junctions, which by definition marks the basolateral side of the monolayer. Furthermore, unlike E-cadherin, catalytically active TG2 does not seem to be tightly associated with the cell surface of the columnar cells. Instead, it is distributed as clumps, analogous to observations of extracellular TG2 in the lamina propria of the small intestine (Esposito et al., 2003; Biagi et al., 2006).
Role of Selected Kinases in IFN-γ Mediated Changes in TG2 Activity and Transepithelial Flux.
A number of kinases have been proposed to influence the barrier function of the T84 intestinal epithelial cell line. We therefore sought to identify which kinases have the greatest influence on the observed increases in transepithelial peptide flux and TG2 activity in response to IFN-γ. Candidate kinases included AMP-activated protein kinase (Scharl et al., 2009), Rho-associated protein kinase (ROCK) (Samarin et al., 2007), the serine-threonine protein kinase AKT (Nava et al., 2010), MLCK (Zolotarevsky et al., 2002; Utech et al., 2005), protein kinase C (PKC) (Song et al., 2001; McKay et al., 2007), and phosphatidylinositide-3-kinase (PI3K) (Choudhury, 2004; McKay et al., 2007).
The AMP-activated protein kinase inhibitor dorsomorphin (2; Fig. 3) (Zhou et al., 2001) failed to alter the effect of IFN-γ on transepithelial peptide flux or TG2 activity (Supplemental Fig. 2). Likewise, although pharmacological inhibition of ROCK prevented the internalization of apical junctional and tight junctional proteins into the cytosol (Samarin et al., 2007), the ROCK inhibitor Glycyl-H-1152 (3; Fig. 3) (Tamura et al., 2005) had no influence on IFN-γ-induced T84 peptide permeability or TG2 activity (Supplemental Fig. 3). AKT is also activated in response to IFN-γ in T84 cells (Nava et al., 2010); however, inhibition of AKT with triciribine (4; Fig. 3) (Yang et al., 2005) failed to block IFN-γ-induced peptide permeability or TG2 activity (Supplemental Fig. 4). The membrane-permeable peptide inhibitor of MLCK, PIK (5; Fig. 3) (Zolotarevsky et al., 2002), also failed to modulate IFN-γ-mediated peptide flux or TG2 activity increases in T84 cells (Supplemental Fig. 5). Last, but not least, PKC has been implicated in the control of T84 paracellular permeability (Song et al., 2001; McKay et al., 2007). However, inhibition of PKC using the highly potent and selective compound BIM-II (6; Fig. 3) (Komander et al., 2004) did not influence peptide permeability or TG2 activity in our assays (Supplemental Fig. 6).
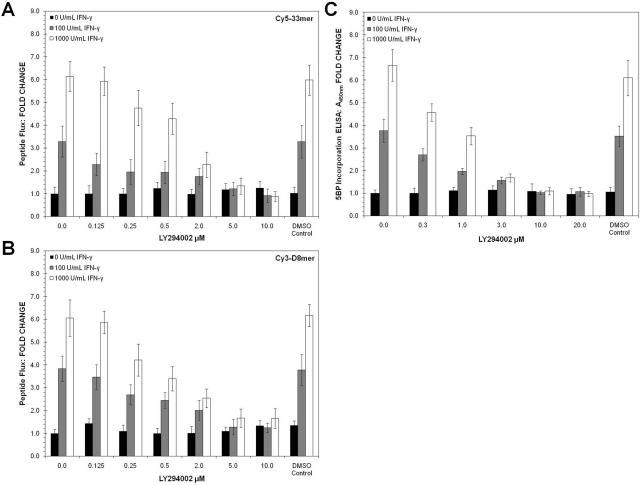
In contrast to all of the above kinases, pharmacological inhibition of PI3K completely reversed both of the above phenotypic consequences of treating T84 monolayers with IFN-γ. PI3K activation in response to IFN-γ is well established in multiple cell lines (Choudhury, 2004; Boivin et al., 2009). For example, earlier studies have demonstrated that the PI3K inhibitor LY294002 (7; Fig. 3; Vlahos et al., 1994) reverses the effects of IFN-γ on transepithelial resistance and 51Cr-EDTA flux (McKay et al., 2007). We therefore first verified that LY294002 negates IFN-γ-induced increase in the flux of Cy5-33mer (Fig. 5A) and Cy3-D8mer (Fig. 5B). More significantly, activation of TG2 by IFN-γ was also completely suppressed by LY294002 (Fig. 5C). As further support for the role of PI3K activity in the IFN-γ-induced phenotypes, a structurally unrelated pan-PI3K inhibitor, BEZ235 (12; Fig. 3) (Maira et al., 2008), was also able to block the IFN-γ-induced increase in permeability and TG2 activation (Supplemental Fig. 7). Thus, of all the kinases known to be active in T84 cells, PI3K is uniquely capable of fully suppressing both responses to IFN-γ that are relevant to celiac disease: increase in paracellular peptide flux and up-regulation of TG2 activity.
Fig. 5.
Effects of the PI3 kinase inhibitor LY294002 on paracellular peptide transport and extracellular TG2 activity in a T84 monolayer treated with IFN-γ. A and B, permeability of Cy5–33mer (A) and Cy3-D8mer (B) across T84 monolayers treated with IFN-γ for 48 h. C, T84 monolayers were treated for 24 to 72 h at 0 to 1000 U/ml IFN-γ with or without 10 μM LY294002. DMSO levels were kept below 0.1% (v/v) in media. DMSO controls show no influence on peptide flux or TG2 activity. Data shown are normalized to 0 U/ml IFN-γ condition represented by mean ± S.D.
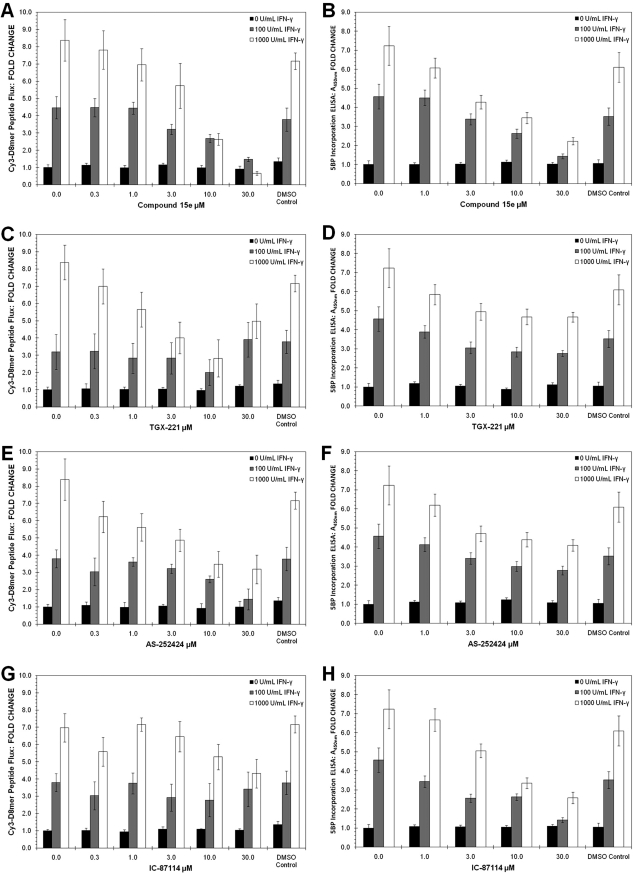
Given the relatively broad specificity of LY294002 for different PI3K isozymes (Gharbi et al., 2007), we also tested a set of more specific class I PI3K inhibitors. Isozymes PI3K-α, -β, -γ, and -δ were targeted by using compound 15e (8; Fig. 3) (Hayakawa et al., 2006), TGX-221 (9; Fig. 3) (Jackson et al., 2005), AS-252424 (10; Fig. 3) (Pomel et al., 2006), and IC-87114 (11; Fig. 3) (Sadhu et al., 2003), respectively. Of these small-molecule inhibitors, compound 8 was the only agent able to mimic the effect of LY294002 on IFN-γ-induced peptide flux (Fig. 6A) and TG2 activation (Fig. 6B). However, because full inhibition was observed only at high inhibitor concentrations (≥10 μM), it is likely that multiple PI3K isozymes were inhibited under these conditions. Compounds 9 and 10 affected the IFN-γ-induced peptide flux (Fig. 6C) but not TG2 activity (Fig. 6D), whereas compound 11 was able to reduce TG2 activity (Fig. 6H) without substantially blocking peptide flux (Fig. 6G). Thus, it seems that PI3K inhibitors with broader isozyme specificity, such as LY294002, BEZ235, and compound 15e, are required to modulate both the paracellular permeability and extracellular TG2 activity of T84 monolayers. The identification of inhibitors that block permeability increases without altering TG2 activity or vice versa also implies that the PI3K isozymes involved in these two outcomes could be different.
Fig. 6.
Effects of PI3 kinase inhibitors 8 to 11 on IFN-γ-treated T84 monolayers. Cells were treated with IFN-γ for 48 h in the presence of isozyme-specific PI3K inhibitors. The flux of Cy3-D8mer peptide and the incorporation of 5BP were measured as described under Materials and Methods. A and B, the effect of compound 15e (8) on IFN-γ-induced peptide permeability (A) and TG2 activation (B). C and D, the effect of TGX-221 (9) on IFN-γ-induced peptide permeability (C) and TG2 activation (D). Elevated concentrations of TGX-221 (≥10 μM) increased peptide permeability, likely due to toxicity. E and F, the effect of AS-252424 (10) on IFN-γ-induced peptide permeability (E) and TG2 activation (F). G and H, the effect of IC-87114 (11) on IFN-γ-induced peptide permeability (G) and TG2 activation (H). DMSO controls show no influence on peptide flux or TG2 activity. Data shown are normalized to the 0 U/ml IFN-γ condition represented by mean ± S.D.
Discussion
Knowledge of the earliest molecular events in the celiac small intestine in response to dietary gluten could lead to the identification of new therapeutic targets. Whereas our understanding of the T-helper cell response to gluten is fairly advanced (Abadie et al., 2011), other facets of this complex immune disorder are less clear. In this article, we have sought to explain a fundamental mystery associated with celiac sprue pathogenesis, the mechanism by which TG2 is activated in a patient's small intestinal mucosa. Not only is this extracellular enzyme responsible for converting protoantigens into high-affinity T cell epitopes, but it is also the primary target of secreted autoantibodies in patients with active celiac disease (Alaedini and Green, 2008).
Our studies have exploited the widely used T84 enterocytic cell line as a model, because of its well known ability to respond to IFN-γ. IFN-γ is the most abundant cytokine released by activated gluten-responsive T-helper cells in the celiac intestine. In the presence of this cytokine, the tight junctions responsible for the barrier properties of a cultured T84 monolayer are disrupted, leading to increased permeability of peptides and other immunogens. Analogously, untreated celiac patients exhibit increased permeability of the small intestine (Bjarnason and Peters, 1984; Smecuol et al., 1997). Using a d-amino acid-derived peptide as a reference for paracellular transport, we have verified that the paracellular pathway is the predominant mechanism for the uptake of antigenic gluten peptides across T84 monolayers (Fig. 1). Such transport of gluten peptides would allow access to active TG2 within the extracellular matrix of epithelial cells.
The principal finding of our study is that, in addition to the observed increase in peptide permeability, IFN-γ triggers extracellular TG2 activity in a T84 monolayer (Figs. 2 and 4). Both TG2 protein and activity were up-regulated in response to IFN-γ in a time- and dose-dependent manner. Given the comparable change in IFN-γ-induced TG2 expression and TG2 activity in T84 cells (Supplemental Figs. 1 and 2, respectively), the main cause of increased TG2 activation in response to IFN-γ is likely due to up-regulated TG2 expression. TG2 protein levels are also known to be up-regulated in the small intestinal mucosa of patients with active celiac disease (Esposito et al., 2003; Biagi et al., 2006). Our confocal fluorescence microscopic analysis suggests that IFN-γ predominantly induces TG2 activity on the basolateral side of T84 monolayers (Fig. 4).
Using the T84 model system, we have also shown that IFN-γ-mediated TG2 activation occurs via a PI3 kinase-dependent pathway (Figs. 5 and 6). It is noteworthy that some PI3K inhibitors such as LY294002 (7), compound 15e (8), and BEZ-235 (12) can suppress both TG2 activation and the IFN-γ-induced permeability increase in T84 monolayers, whereas other inhibitors cannot. Multiple PI3K isoforms are found in mammals (Kong and Yamori, 2009). Therefore, our findings are either indicative of a mismatch between the isoform specificity of the latter subset of inhibitors and the major PI3K that responds to IFN-γ in T84 cells, or they suggest that multiple PI3K isoforms are operational in this cell line. Regardless, PI3K could be an attractive target for nondietary therapy of celiac disease. Other than strict, lifelong gluten exclusion, no therapeutic option is available to a celiac patient. Thus far, interest in TG2 as a drug target has focused on enzyme inhibitors (Siegel and Khosla, 2007). PI3K inhibition, in contrast, would not only be expected to suppress TG2 activity in the small intestinal mucosa of celiac patients, but also prevent an increase in antigenic peptide permeability. The attractiveness of treating celiac disease in this manner is underscored by the fact that several systemic inhibitors of PI3K, including (3S)-4-[[(1S)-1-carboxy-2-hydroxyethyl]amino]-3-[[2-[[(2S)-5-(diaminomethylideneamino)-2-[[4-oxo-4-[[4-(4-oxo-8-phenylchromen-2-yl)morpholin-4-ium-4-yl]methoxy]butanoyl] amino]pentanoyl]amino]acetyl]amino]-4-oxobutanoic acid acetate (SF1126, a prodrug of LY294002) (Chiorean et al., 2009), 4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1-yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine (GDC0941) (Folkes et al., 2008), N-[4-[[3-(3,5-dimethoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-3-methoxy-4-methylbenzamide (XL765) (Garcia-Echeverria and Sellers, 2008), and BEZ235 (Maira et al., 2008), have shown promising safety characteristics in human clinical trials.
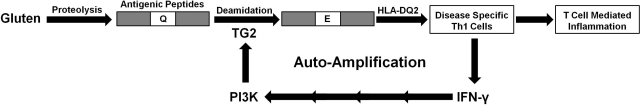
In conclusion, this study has led to a fundamentally new insight into a mechanism by which the T-helper cell response to gluten antigens may be sustained in the celiac small intestine. In this model (Fig. 7), the activation of these disease-specific T cells is autoamplificatory, as long as dietary gluten is present in the lumen of the celiac intestine. Once a small number of T cells are activated, the IFN-γ secreted by them increases the permeability of the epithelial barrier, while simultaneously activating TG2 in the subepithelial space. Together, these changes lead to the supply of larger quantities of antigen to HLA-DQ2-bearing antigen-presenting cells, which in turn activate more T cells.
Fig. 7.
A self-amplificatory loop for inflammation caused by disease-specific T cells that recognize dietary gluten antigens in celiac sprue. Gluten peptides are incompletely proteolyzed in the small intestine, due to their Pro- and Gln-rich character. Some of the resulting metastable peptides undergo regiospecific deamidation by TG2 and are then presented to disease-specific T cells by HLA-DQ2. Activated T cells secrete the cytokine IFN-γ, which increases intestinal permeability, thereby allowing an increased flux of immunotoxic peptides across the enterocyte barrier. IFN-γ also activates TG2. Both processes are mediated by PI3K activity.
Notwithstanding the potential significance of IFN-γ-induced activation of extracellular TG2, we note that intracellular TG2 activation by toxic gluten peptides has also been implicated in the development of the celiac lesion via degradation of the anti-inflammatory peroxisome proliferator-activated receptor γ (De Re et al., 2010; Luciani et al., 2010). It is possible that IFN-γ also increases intracellular TG2 activity in enterocytes, causing increased peroxisome proliferator-activated receptor γ degradation. We also note that, despite the plethora of evidence supporting the dominant role of the T cell-mediated adaptive immune response in celiac disease pathogenesis (Abadie et al., 2011), the innate immune system has also been implicated (Jabri and Sollid, 2005; Jabri et al., 2009). Major innate immune responses to gluten include the up-regulation of interleukin 15 and major histocompatibility complex class I polypeptide-related molecules and the destruction of epithelial tissue by natural killer cells. It remains to be determined whether these phenomena can be reconstituted in a simple system such as the T84 cell line.
Supplementary Material
Acknowledgments
We thank Kathy Siemers (Stanford University) for supplying rat tail collagen; Evelyn Resurreccion (Digestive Disease Center at Stanford University) and Laila Dafik for assisting with confocal microscopy; Laila Dafik for providing ERW1041E; and Michael Bethune for assisting with the T84 peptide translocation assay.
This research was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK063158] (to C. Kh.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- TG2
- transglutaminase 2
- 5BP
- 5-biotinamido pentylamine
- IFN-γ
- interferon-γ
- PI3K
- phosphatidylinositol-3-kinase
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- ECL
- enhanced chemiluminescence
- MLCK
- myosin light chain kinase
- PIK
- permeant inhibitor of MLCK
- TFA
- trifluoroacetic acid
- HPLC
- high-performance liquid chromatography
- DMSO
- dimethyl sulfoxide
- ROCK
- Rho-associated protein kinase
- PKC
- protein kinase C
- ELISA
- enzyme-linked immunosorbent assay
- ERW1041E
- 2-[(3-bromo-4,5-dihydro-isoxazol-5-ylmethyl)-carbamoyl]-pyrrolidine-1-carboxylic acid quinolin-3-ylmethyl ester
- LY294002
- 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- Glycyl-H-1152
- 2-amino-1-[(3S)-hexahydro-3-methyl-4-[(4-methyl-5-isoquinolinyl)sulfonyl]-1H-1,4-diazepin-1-yl]ethanone
- BEZ235
- 4-[2,3-dihydro-3-methyl-2-oxo-8-(3-quinolinyl)-1H-imidazo[4,5-c]quinolin-1-yl]-α,α-dimethyl-benzeneacetonitrile
- AS-252424
- 5-[5-(4-fluoro-2-hydroxy-phenyl)-furan-2-ylmethylene]-thiazolidine-2,4-dione
- IC-87114
- [(6-amino-9H-purin-9-yl)methyl]-5-methyl-3-(2-methylphenyl)-4(3H)-quinazolinone
- TGX-221
- 7-methyl-2-(morpholin-4-yl)-9-(1-phenylaminoethyl)-pyrido[1,2-a]-pyrimidin-4-one
- GDC0941
- 4-[2-(1H-indazol-4-yl)-6-[(4-methylsulfonylpiperazin-1-yl)methyl]thieno[3,2-d]pyrimidin-4-yl]morpholine.
Authorship Contributions
Participated in research design: DiRaimondo and Klöck.
Conducted experiments: DiRaimondo and Klöck.
Contributed new reagents or analytic tools: DiRaimondo and Klöck.
Performed data analysis: DiRaimondo and Klöck.
Wrote or contributed to the writing of the manuscript: DiRaimondo, Klöck, and Khosla.
References
- Abadie V, Sollid LM, Barreiro LB, Jabri B. (2011) Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 29:493–525 [DOI] [PubMed] [Google Scholar]
- Alaedini A, Green PH. (2008) Autoantibodies in celiac disease. Autoimmunity 41:19–26 [DOI] [PubMed] [Google Scholar]
- Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. (2000) In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med 6:337–342 [DOI] [PubMed] [Google Scholar]
- Beaurepaire C, Smyth D, McKay DM. (2009) Interferon-γ regulation of intestinal epithelial permeability. J Interferon Cytokine Res 29:133–144 [DOI] [PubMed] [Google Scholar]
- Bethune MT, Crespo-Bosque M, Bergseng E, Mazumdar K, Doyle L, Sestak K, Sollid LM, Khosla C. (2009a) Noninflammatory gluten peptide analogs as biomarkers for celiac sprue. Chem Biol 16:868–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethune MT, Siegel M, Howles-Banerji S, Khosla C. (2009b) Interferon-γ released by gluten-stimulated celiac disease-specific intestinal T cells enhances the transepithelial flux of gluten peptides. J Pharmacol Exp Ther 329:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi F, Campanella J, Laforenza U, Gastaldi G, Tritto S, Grazioli M, Villanacci V, Corazza GR. (2006) Transglutaminase 2 in the enterocytes is coeliac specific and gluten dependent. Dig Liver Dis 38:652–658 [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Peters TJ. (1984) In vitro determination of small intestinal permeability: demonstration of a persistent defect in patients with coeliac disease. Gut 25:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MA, Roy PK, Bradley A, Kennedy JC, Rihani T, Ma TY. (2009) Mechanism of interferon-γ-induced increase in T84 intestinal epithelial tight junction. J Interferon Cytokine Res 29:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171:6164–6172 [DOI] [PubMed] [Google Scholar]
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. (2005) Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19:923–933 [DOI] [PubMed] [Google Scholar]
- Chiorean EG, Mahadevan D., Harris WB, Von Hoff DD, Younger AE, Rensvold DM, Shelton CF, Hennessy BT, Garlich JR, Ramanathan RK. (2009) Phase I evaluation of SF1126, a vascular targeted PI3K inhibitor, administered twice weekly IV in patients with refractory solid tumors. J Clin Oncol 27 (Suppl):122s [Google Scholar]
- Choudhury GG. (2004) A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cε, and MAPK in mesangial cells regulates interferon-γ-induced STAT1 transcriptional activation. J Biol Chem 279:27399–27409 [DOI] [PubMed] [Google Scholar]
- De Re V, Simula M.P., Notarpietro A, Canzonieri V, Cannizzaro R, Toffoli G. (2010) Do gliadin and tissue transglutaminase mediate PPAR downregulation in intestinal cells of patients with coeliac disease? Gut 59:1730–1731 [DOI] [PubMed] [Google Scholar]
- Dicke WK, Weijers HA, Van De Kamer JH. (1953) Coeliac disease. II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr 42:34–42 [DOI] [PubMed] [Google Scholar]
- Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, Auricchio S, Troncone R. (2003) Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol 98:1813–1820 [DOI] [PubMed] [Google Scholar]
- Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, et al. (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51:5522–5532 [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C, Sellers WR. (2008) Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 27:5511–5526 [DOI] [PubMed] [Google Scholar]
- Green PH, Cellier C. (2007) Celiac disease. N Engl J Med 357:1731–1743 [DOI] [PubMed] [Google Scholar]
- Green PH, Jabri B. (2006) Celiac disease. Annu Rev Med 57:207–221 [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Okada M, Ohta M, Tsukamoto S, Parker P, Workman P, et al. (2006) Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem 14:6847–6858 [DOI] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, et al. (2005) PI 3-kinase p110β: a new target for antithrombotic therapy. Nat Med 11: 507–514 [DOI] [PubMed] [Google Scholar]
- Jabri B, Kasarda DD, Green PH. (2005) Innate and adaptive immunity: the yin and yang of celiac disease. Immunol Rev 206:219–231 [DOI] [PubMed] [Google Scholar]
- Jabri B, Sollid LM. (2009) Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol 9:858–870 [DOI] [PubMed] [Google Scholar]
- Kagnoff MF. (2007) Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest 117:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J, and European Genetics Cluster on Celiac Disease (2003) HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol 64:469–477 [DOI] [PubMed] [Google Scholar]
- Komander D, Kular GS, Schüttelkopf AW, Deak M, Prakash KR, Bain J, Elliott M, Garrido-Franco M, Kozikowski AP, Alessi DR, et al. (2004) Interactions of LY333531 and other bisindolyl maleimide inhibitors with PDK1. Structure 12:215–226 [DOI] [PubMed] [Google Scholar]
- Kong D, Yamori T. (2009) Advances in development of phosphatidylinositol 3-kinase inhibitors. Curr Med Chem 16:2839–2854 [DOI] [PubMed] [Google Scholar]
- Luciani A, Villella VR, Vasaturo A, Giardino I, Pettoello-Mantovani M, Guido S, Cexus ON, Peake N, Londei M, Quaratino S, et al. (2010) Lysosomal accumulation of gliadin p31–43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARγ downregulation in intestinal epithelial cells and coeliac mucosa. Gut 59:311–319 [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J. (1989) Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 83:724–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, et al. (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 7:1851–1863 [DOI] [PubMed] [Google Scholar]
- McKay DM, Watson JL, Wang A, Caldwell J, Prescott D, Ceponis PM, Di Leo V, Lu J. (2007) Phosphatidylinositol 3′-kinase is a critical mediator of interferon-γ-induced increases in enteric epithelial permeability. J Pharmacol Exp Ther 320:1013–1022 [DOI] [PubMed] [Google Scholar]
- Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Norén O, Roepstorff P, et al. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4:713–717 [DOI] [PubMed] [Google Scholar]
- Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, et al. (2010) Interferon-γ regulates intestinal epithelial homeostasis through converging β-catenin signaling pathways. Immunity 32:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen EM, Jahnsen FL, Lundin KE, Johansen FE, Fausa O, Sollid LM, Jahnsen J, Scott H, Brandtzaeg P. (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon γ in patients with celiac disease. Gastroenterology 115:551–563 [DOI] [PubMed] [Google Scholar]
- Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon γ. Gut 37:766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Dahl CE, Karnovsky ML, Maggio JE. (1994) Intestinal absorption and excretion of octapeptides composed of D amino acids. Proc Natl Acad Sci U S A 91:1942–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Karnovsky ML, Maggio JE. (1997) Absorption and excretion of undegradable peptides: role of lipid solubility and net charge. J Pharmacol Exp Ther 280:292–300 [PubMed] [Google Scholar]
- Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, Burgat-Charvillon F, Valognes D, Camps M, Chabert C, et al. (2006) Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase γ. J Med Chem 49:3857–3871 [DOI] [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. (2003) Essential role of phosphoinositide 3-kinase Δ in neutrophil directional movement. J Immunol 170:2647–2654 [DOI] [PubMed] [Google Scholar]
- Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. (2007) Rho/Rho-associated kinase-II signaling mediates disassembly of epithelila apical junctions. Mol Biol Cell 18:3429–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharl M, Paul G, Barrett KE, McCole DF. (2009) AMP-activated protein kinase mediates the interferon-γ-induced decrease in intestinal epithelial barrier function. J Biol Chem 284:27952–27963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. (2002) Structural basis for gluten intolerance in celiac sprue. Science 297:2275–2279 [DOI] [PubMed] [Google Scholar]
- Siegel M, Khosla C. (2007) Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther 115:232–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. (2008) Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE 3:e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Mauriño E, Meddings JB. (1997) Gastrointestinal permeability in celiac disease. Gastroenterology 112:1129–1136 [DOI] [PubMed] [Google Scholar]
- Sollid LM, Khosla C. (2011) Novel therapies for coeliac disease. J Intern Med 269:604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E.M. (1989) Evidence for a primary association of celiac disease to a particular HLA-DQ α/β heterodimer. J Exp Med 169:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JC, Hanson CM, Tsai V, Farokhzad OC, Lotz M, Matthews JB. (2001) Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am J Physiol Cell Physiol 281:C649–C661 [DOI] [PubMed] [Google Scholar]
- Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM. (2010) Redox regulation of transglutaminase 2 activity. J Biol Chem 285:25402–25409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J, Hidaka H. (2005) Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta 1754:245–252 [DOI] [PubMed] [Google Scholar]
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. (2005) Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16:5040–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wal Y, Kooy Y, van Veelen P, Peña S, Mearin L, Papadopoulos G, Koning F. (1998) Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol 161:1585–1588 [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248 [PubMed] [Google Scholar]
- Watts RE, Siegel M, Khosla C. (2006) Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J Med Chem 49:7493–7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Siegel M, Bergseng E, Sollid LM, Khosla C. (2006) Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from α2-gliadin. J Am Chem Soc 128:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, et al. (2005) Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cell overexpressing Akt. Cancer Res 64:4394–4399 [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. (2002) A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123:163–172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.