Abstract
Genetic alterations, including the overexpression of epidermal growth factor receptor (EGFR) (in approximately 70% of ovarian tumors), play a crucial role in the signal transduction pathways that regulate key cellular functions, such as cell survival and proliferation, and are responsible for compromising traditional chemotherapy. 3,3′-Diindolylmethane (DIM) is an indole compound present in Brassica vegetables. In our previous studies, we demonstrated that BR-DIM, a formulated version of DIM, suppressed the growth of ovarian cancer cells by causing cell cycle arrest and apoptosis. In the present study, we delineated the mechanism by which DIM suppressed the growth of SKOV-3, OVCAR-3, and TOV-21G human ovarian cancer cells. DIM treatment caused significant down-regulation of the constitutive EGFR protein level as well as phosphorylation of EGFR at Tyr1068, Tyr992, Tyr845, and Tyr1173 in various ovarian cancer cells. To determine whether DIM suppressed the activation of EGFR by activating phosphorylation, cells were treated with epidermal growth factor. Epidermal growth factor treatment significantly blocked the DIM-mediated inhibition of EGFR activation and apoptosis in both SKOV-3 and OVCAR-3 cells. In addition, DIM treatment drastically reduced the phosphorylation of mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated kinase (ERK), which are downstream to EGFR, without affecting their protein levels. DIM treatment also inhibited the kinase activity of ERK, as observed by the down-regulation of phospho-E twenty-six like transcription factor 1 (p-ELK1) in all three ovarian cancer cell lines. DIM significantly suppressed the growth of ovarian tumors in vivo. Tumor growth suppressive effects of DIM in SKOV-3 tumor xenografts were associated with reduced phosphorylation of EGFR, MEK, and ERK. These results indicate that DIM induces apoptosis in ovarian cancer cells by inhibiting the EGFR-ERK pathway in vitro and in vivo.
Introduction
Ovarian carcinoma is the second leading gynecological malignancy in the Unites States and European countries. Approximately 22,800 cases are estimated to occur each year (Zeineldin et al., 2010; Jemal et al., 2011). Ovarian cancer is rarely detected in its early stages due to lack of screening tests. However, the majority of patients with this malignancy are detected in stages III to IV, and hardly 20% of them survive >5 years (Bast et al., 2009). Platinum and taxane drugs that are used in the clinic to treat ovarian cancer are associated with severe systemic toxicity and side effects. Moreover, tumors acquire resistance to these drugs at certain stages of the treatment (Ozols et al., 2006). The most common form of ovarian cancer that arises from ovarian surface epithelium expresses epidermal growth factor receptor (EGFR) (Zeineldin et al., 2010).
EGFR is a transmembrane receptor tyrosine kinase that is important in cell growth and proliferation. Various ligands, such as epidermal growth factor (EGF), transforming growth factor-α, and epiregulin, bind to EGFR and activate it. The activated receptor dimerizes and results in the autophosphorylation of several tyrosine sites (Yarden and Sliwkowski, 2001). Different phosphorylation sites in EGFR act as docking sites for a variety of proteins that are upstream of several signaling cascades and involved in proliferation, differentiation, migration, and antiapoptosis (Turner et al., 1996). Some of these signaling pathways include mitogen-activated protein kinase (MAPK), protein kinase C, and phosphatidylinositol 3-kinase (Bier, 1998). EGFR plays a significant role in neural development and the formation of skin (Sibilia et al., 2007). In addition, it is important for the development of lung, liver, and bone (Sibilia et al., 2007). However, mutations in EGFR lead to malignant tumor formation in all of those organs. Accumulated literature strongly suggests that EGFR is activated or overexpressed in several cancers, including ovarian cancer.
Almost 70% of ovarian tumors express high levels of EGFR (Alper et al., 2001). In addition to its role in transducing signals that lead to cell survival, EGFR plays a significant role in the epithelial-to-mesenchymal transition of tumor cells and angiogenesis, thereby causing metastasis to distant organs (Casanova et al., 2002). Because EGFR is involved in various aspects of cancer growth, including tumor initiation, angiogenesis, and metastasis, it represents an attractive target for therapeutic intervention.
3,3′-Diindolylmethane (DIM), an active metabolite of indole-3-carbinol, is present in cruciferous vegetables (Ciska et al., 2009). DIM is formed by the dimerization of indole-3-carbinol. Accumulating epidemiological evidence indicates an inverse relationship between the intake of cruciferous vegetables and the risk of ovarian cancer (Zhang et al., 2002). Several studies, including those from our laboratory, have suggested that DIM possesses chemopreventive and therapeutic properties (Li et al., 2005; Ali et al., 2008; Kandala and Srivastava, 2010; Rajoria et al., 2011). Moreover, DIM was shown to be nontoxic to normal cells (Rahman and Sarkar, 2005). A recently concluded DIM clinical trial demonstrated that 50% of cervical cancer patients showed improvement (Del Priore et al., 2010). It also is currently in clinical trials for prostate cancer (Heath et al., 2010). In our previous study, we showed that DIM exhibits antiproliferative properties in ovarian cancer cells by causing G2/M cell cycle arrest (Kandala and Srivastava, 2010). In the present study, we investigated the mechanism by which DIM inhibits the proliferation of ovarian cancer cells.
Materials and Methods
Chemicals.
BR-DIM was a kind gift from Dr. Michael Zeligs (BioResponse, Boulder, CO). Antibodies against cleaved caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), p-EGFR (Tyr1068), p-EGFR (Tyr992), p-EGFR (Tyr845), EGFR, p-extracellular signal-regulated kinase (ERK) (Thr202/Tyr204), ERK, and p-mitogen-activated protein kinase kinase (MEK) (Ser217), MEK antibodies, and the ERK kinase activity kit were obtained from Cell Signaling Technology (Danvers, MA). The antibody against p-EGFR (Tyr1173) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Actin antibody, EGF, MCDB105 medium, and Medium 199 were procured from Sigma-Aldrich (St. Louis, MO). RPMI 1640 medium and McCoy's 5A medium were purchased from Mediatech (Herndon, VA). Suramin and 2′-amino-3′-methoxyflavone (PD98059) were obtained from Calbiochem (San Diego, CA).
Cell Cultures.
SKOV-3, OVCAR-3, and TOV-21G cells lines were procured from the American Type Culture Collection (Manassas, VA). The three cell lines differ from each other in terms of expressing p53. SKOV-3 has null p53, OVCAR-3 has mutated p53, and TOV-21G expresses wild-type p53. SKOV-3 cells were maintained in McCoy's 5A medium supplemented with 10% fetal bovine serum (FBS). OVCAR-3 cells were maintained in RPMI 1640 medium supplemented with 20% FBS, 10 mM sodium pyruvate, 10 mM HEPES, 10 mg/l bovine insulin, and 4.5 g/l glucose. TOV-21G cells were maintained in a 1:1 mixture of MCDB105 medium and Medium 199 supplemented with 15% FBS. A 1% antibiotic mixture was used in all of the above media. All of the cell lines were maintained at 37°C in a humidified incubator circulated with 5% CO2/95% air.
Western Blot Analysis.
SKOV-3, OVCAR-3, and TOV-21G cells were exposed to varying concentrations of DIM alone or in combination with cisplatin. Cells were collected and lysed, and 20 to 80 μg of protein was subjected to SDS gel electrophoresis followed by immunoblotting as described by us previously (Kandala and Srivastava, 2010).
Annexin V Apoptosis Assay.
SKOV-3 or OVCAR-3 cells were plated at a density of 0.3 × 106 cells per well in a six-well plate and allowed to attach overnight. Cells then were treated with or without DIM. After 24 h, cells were exposed to EGF for 15 min, washed, suspended in binding buffer, and incubated for 15 min with annexin V-fluorescein isothiocyanate (BD Biosciences, San Jose, CA). Fluorescence was measured using a C6 flow cytometer (Accuri, Ann Arbor, MI) with a minimum of 10,000 events per sample as described by us previously (Boreddy et al., 2011).
EGF Treatment.
SKOV-3 or OVCAR-3 cells were treated with 75 μM DIM for 24 h followed by incubation with 50 ng/ml EGF for 15 min. Cells then were processed for apoptosis assay or Western blot analysis as described above.
ERK Activity.
Control and DIM-treated cells were lysed. Approximately 500 μg of protein was incubated overnight with 15 μl of immobilized antibody bead slurry at 4°C and centrifuged at 14,000g for 30 s. The pellet was washed with phosphate-buffered saline (PBS) and suspended in 50 μl of kinase buffer supplemented with 200 μM ATP substrate and incubated for 30 min at 30°C. The protein was resolved by gel electrophoresis. ERK activity was determined by immunoblotting with phospho-E twenty-six like transcription factor [p-ELK (Ser383)].
Cell Cycle Analysis.
The effect of DIM on cell cycle distribution in the presence and absence of EGF was assessed by flow cytometry after staining the cells with propidium iodide. Stained cells were analyzed using a flow cytometer (Accuri) as described by us previously (Kandala and Srivastava, 2010).
In Vivo Tumor Xenograft.
Four- to 6-week-old female athymic nude mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA). The use of mice and their treatment was approved by the Institutional Animal Care and Use Committee of the Texas Tech University Health Sciences Center, and all of the experiments were carried out in strict compliance with regulations. Mice were fed with antioxidant-free AIN-76A special diet for a week before starting the experiment. Approximately 5 × 106 SKOV-3 cells were injected subcutaneously into each flank (both right and left). Eight mice were assigned randomly to each group. Because each mouse was implanted with two xenografts, each group had 16 tumors. Mice in the control group received PBS, whereas mice in the treatment group received 3 mg of DIM suspended in PBS by oral gavage every day. Tumor growth was monitored until day 48 as described by us previously (Boreddy et al., 2011). At day 48, mice were euthanized, and tumors were removed, weighed, and processed for Western blot analysis.
Quantification and Statistical Analysis.
All of the statistical analyses were performed using Prism 5.0 (GraphPad Software Inc., San Diego, CA). The data represent mean ± S.D. Student's t test was used to compare the control and treated groups. In experiments involving more than three groups, nonparametric analysis of variance followed by a Bonferroni post hoc multiple comparison test was used. All of the statistical tests were two sided. Differences were considered statistically significant when the p value was less than 0.05.
Results
DIM Inhibits the Activation of EGFR in Ovarian Cancer Cells.
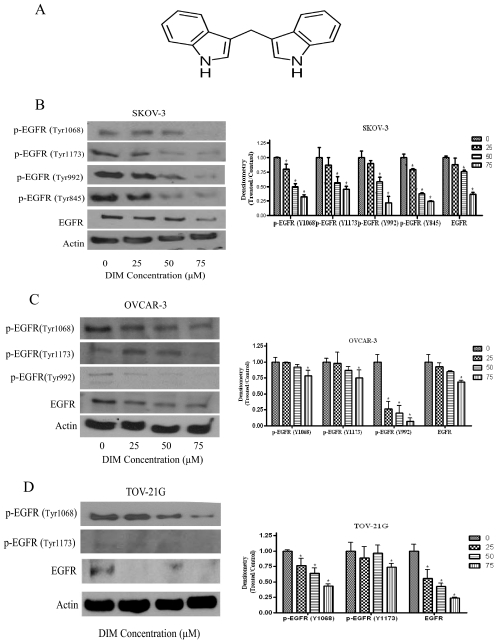
DIM is a dimer of indole-3-carbinol (Fig. 1A). We have demonstrated previously that DIM inhibits the proliferation of ovarian cancer cells by inducing apoptosis (Kandala and Srivastava, 2010). We hypothesized that the growth suppressive effect of DIM in ovarian cancer cells was mediated by inhibiting EGFR activation. To test this hypothesis, we exposed SKOV-3, OVCAR-3, and TOV-21G cells to varying concentrations of DIM for 24 h. We observed that Tyr1068 and Tyr1173 phosphorylation sites on EGFR were expressed in all three cell lines tested. The phosphorylation of EGFR at Tyr992 was prominent in both SKOV-3 and OVCAR-3 cells, whereas Tyr845 was active only in SKOV-3 cells (Fig. 1, B–D). Our results further reveal that DIM substantially inhibits the activation of EGFR by blocking various phosphorylation sites expressed in all three different ovarian cancer cell lines tested (Fig. 1, B–D). For example, a 60% to 70% decrease in the phosphorylation of EGFR at Tyr1068, Tyr1173, Tyr992, and Tyr 845 was observed by DIM treatment in SKOV-3 cells. Almost 30% to 80% inhibition at Tyr1068, Tyr1173, and Tyr992 was observed in OVCAR-3 cells, and a 30% to 60% blockade in EGFR phosphorylation at Tyr1068 and Tyr1173 was observed in TOV-21G cells. DIM also down-regulated the protein expression of EGFR in all three ovarian cancer cells. Taken together, these results demonstrate that DIM blocks the activation of EGFR in ovarian cancer cells without being specific to a particular cell line.
Fig. 1.
DIM inhibits the activation of EGFR in ovarian cancer cells. A, structure of DIM. B–D, representative blots and their densitometric analyses, showing the concentration-dependent effect of DIM on p-EGFR (Tyr1068), p-EGFR (Ser1173), p-EGFR (Tyr992), or p-EGFR (Tyr845) and EGFR in SKOV-3 (B), OVCAR-3 (C), and TOV-21G (D) ovarian cancer cells. Actin was used as a loading control. Each experiment was repeated three times independently. *, p < 0.05 compared with control.
DIM Treatment Blocks EGFR Downstream Signaling.
Activation of EGFR leads to the phosphorylation of MEK at Ser217, which in turn activates ERK by phosphorylating it at Thr202/Tyr204. Because we observed a significant blockade in EGFR activation by DIM treatment, we sought to determine the effects of DIM on molecules that were downstream to EGFR. Exposure of SKOV-3, OVCAR-3, or TOV-21G cells to different concentrations of DIM for 24 h resulted in significant inhibition of the activation of MEK and ERK (Fig. 2). An approximately 50% reduction was observed in the phosphorylation of MEK at Ser217, whereas 70% inhibition was observed in the phosphorylation of ERK at Thr202/Tyr204 in SKOV-3 cells. ERK phosphorylation was reduced 70 and 90% in OVCAR-3 and TOV-21G cells, respectively. Likewise, up to 60 and 70% reduction in the phosphorylation of MEK was observed in OVCAR-3 and TOV-21G cells, respectively. The constitutive protein levels of MEK and ERK were not altered by DIM treatment. These results indicate that DIM modulates downstream molecules of the EGFR pathway.
Fig. 2.
DIM inhibits the MEK-ERK axis in ovarian cancer cells. A–C, representative blots and their densitometric analyses, showing the concentration-dependent effects of DIM on p-MEK (Ser217), MEK, p-ERK (Thr202/Tyr204), and ERK in SKOV-3 (A), OVCAR-3 (B), and TOV-21G (C) ovarian cancer cells. Actin was used as a loading control. Each experiment was repeated three times independently. *, p < 0.05 compared with control.
Effects of DIM on EGFR Signaling Were Inhibited by EGF.
EGFR can be activated by growth factors and its ligand, EGF. When EGF binds, EGFR is phosphorylated and in turn phosphorylates its downstream molecules, such as MEK and ERK. Treatment of SKOV-3 or OVCAR-3 cells with 50 ng/ml EGF resulted in 3-fold activation of EGFR (Fig. 3, A and B). EGF treatment significantly blocked DIM-mediated suppression of EGFR phosphorylation at Tyr1068 (Fig. 3, A and B). Phosphorylation of ERK that was reduced by DIM treatment was prevented by EGF treatment. Nonetheless, EGF did not show any effect on the protein levels of EGFR or ERK in SKOV-3 and OVCAR-3 cells. Because AKT also is regulated by EGFR, we tested the effects of DIM on p-AKT (Ser473). DIM treatment reduced the activation of AKT by blocking its phosphorylation at Ser473. However, EGF treatment blocked the effects of DIM on AKT. Because EGF treatment blocked the effects of DIM, we hypothesized that EGF treatment would abrogate DIM-induced apoptosis. The effects of EGF and DIM on apoptosis by flow cytometry and cleavage of caspase-3 and PARP thus were evaluated. From Fig. 3, A and B, it is clear that EGF treatment substantially decreased the cleavage of caspase-3 and PARP induced by DIM in SKOV-3 and OVCAR-3 cells compared with that induced by DIM treatment alone. In support of these data, our flow cytometry results also showed that EGF treatment significantly blocked DIM-induced apoptosis in both SKOV-3 and OVCAR-3 cells (Fig. 3, C and D). These results confirm that DIM treatment induces apoptosis through the EGFR pathway.
Fig. 3.
EGF overrides the effects of DIM. Effects of DIM on EGFR activation and apoptosis. A and B, SKOV-3 (A) or OVCAR-3 (B) cells were stimulated with 50 ng/ml EGF for 15 min after treatment with 75 μM DIM for 24 h. Whole-cell lysates were resolved by 10% SDS polyacrylamide gel electrophoresis for the analysis of the phosphorylation of EGFR at Tyr705, phosphorylation of ERK at Ser202/Tyr204, phosphorylation of AKT at Ser473, EGFR, ERK, and cleavage of caspase-3 and PARP. Actin was used as a loading control. C and D, effects of EGF on DIM-induced apoptosis also were determined in SKOV-3 (C) and OVCAR-3 (D) cells by flow cytometry. E and F, effects of suramin (E) and PD98059 (F) on EGFR signaling and apoptosis. The experiments were repeated three times, and similar results were obtained. The differences among all of the groups were compared by analysis of variance with Bonferroni post hoc comparisons or Student's t test. Statistical tests were two sided. *, p < 0.05 compared with control; #, p < 0.05 compared with control.
We previously reported that DIM induces G2/M cell cycle arrest in ovarian cancer cells (Kandala and Srivastava, 2010). To test the role of EGFR in DIM-induced cell cycle arrest, we treated SKOV-3 cells with 50 ng/ml EGF, followed by treatment with DIM for 24 h, and analyzed the cells for cell cycle distribution by flow cytometry. Our results showed that DIM-treated SKOV-3 cells were arrested in the G2/M phase (Supplemental Fig. 1). However, EGF treatment failed to block G2/M cell cycle arrest induced by DIM (Supplemental Fig. 1).
We also confirmed our observations using inhibitors of EGFR and ERK. Suramin, an EGFR inhibitor, not only blocked the activation of EGFR, MEK, ERK, and AKT but also induced apoptosis in SKOV-3 cells, as indicated by the cleavage of caspase-3 (Fig. 3Ei). Likewise, treatment of SKOV-3 cells with PD98059, an inhibitor of MEK, inhibited the activation of MEK and ERK and increased the cleavage of caspase-3 (Fig. 3Fi). Apoptosis-inducing effects of suramin and PD98059 were further confirmed by the annexin V apoptosis assay, showing that inhibitors alone induce apoptosis (Fig. 3, Eii and Fii). Taken together, these results suggest that the EGFR-ERK pathway is critical for the growth of ovarian cancer cells and a target for therapeutic intervention.
DIM Treatment Inhibits ERK Activity in Ovarian Cancer Cells.
ERK is a major MAPK molecule that is downstream of the EGFR pathway. Phosphorylation of ERK leads to the activation of its downstream transcription factor ELK-1, which is responsible for cell proliferation. ERK is reported to be activated in a majority of ovarian tumors (Ahmed et al., 2002; Steinmetz et al., 2004). Because we observed substantially reduced phosphorylation of ERK by DIM treatment, we wanted to confirm these observations by ERK kinase activity. ERK activity was determined by evaluating the phosphorylation of its downstream substrate ELK-1 at Ser383. Our results demonstrate that DIM treatment strongly suppressed ERK activity in a concentration-dependent manner in all three ovarian cancer cell lines (Fig. 4A). DIM treatment reduced ERK activity by approximately 70% in SKOV-3 or OVCAR-3 cells (Fig. 4B), whereas in TOV-21G cells inhibition was 80% (Fig. 4B). These results establish that ERK is a major target of DIM in our model.
Fig. 4.
ERK activity was inhibited significantly by DIM. A, concentration-dependent effects of DIM for 24 h on ERK activity represented by the phosphorylation of ELK at Ser383 in SKOV-3, OVCAR-3, or TOV-21G cells. B, densitometric analyses of various blots representing the effects of DIM on ERK activity represented by p-ELK (Ser383) in SKOV-3, OVCAR-3, and TOV21G cells. The experiments were repeated twice, and similar results were obtained. The differences between the groups were compared by Student's t test. Statistical tests were two sided. *, p < 0.05 compared with control.
DIM Suppresses the Growth of Ovarian Tumor Xenografts by Inhibiting the EGFR Pathway.
To determine whether oral administration of DIM can suppress the growth of ovarian tumors in vivo in mice, we implanted 5 × 106 SKOV-3 tumor cells in both flanks of female athymic nude mice and randomly divided the mice into two groups. Mice in the control group received PBS, whereas mice in the treatment group received 3 mg/day DIM by oral gavage. Our results indicate that the oral administration of 3 mg of DIM everyday substantially suppressed the growth of ovarian tumors in vivo. For example, at day 48, the average weight of the tumors in control mice was approximately 325 mg, whereas the average weight of the tumors in mice from the treatment group was approximately 205 mg, indicating a suppression of approximately 33% (Fig. 5A). To further establish that the inhibition of the EGFR pathway was mediating the tumor growth suppressive effects of DIM, tumors were lysed and subjected to Western blot analysis as described by us in our previous study (Pramanik et al., 2011). Our results reveal that the expression of p-EGFR (Tyr1068), EGFR, p-MEK (Ser217), and p-ERK (Tyr202/Thr204) were reduced significantly with DIM treatment compared with the control (Fig. 5, B and C). These results establish that DIM reduces the growth of ovarian tumor cells in vitro and in vivo by targeting EGFR.
Fig. 5.
DIM suppressed ovarian tumor growth by blocking the EGFR pathway. SKOV-3 tumor cells were implanted into athymic nude mice and randomized into two groups. Mice received PBS or 3 mg/day DIM by oral gavage everyday. A, effect of DIM on tumor weight on day of sacrifice. *, p < 0.05 compared with control. B, inhibition of EGFR signaling in the tumors of mice administered with DIM. Tumors from control and treated mice were excised at day 48 after implantation, lysed, and analyzed by Western blot for p-EGFR (Tyr1068), EGFR, p-MEK (Ser217), p-ERK (Thr202/Tyr204), ERK, cleaved caspase-3, and cleaved PARP. Blots were stripped and reprobed with an actin antibody to verify equal protein loading. Each lane represents a different tumor sample. C, densitometric quantification of Western blots represented above. The differences between the groups were compared by Student's t test. Statistical tests were two sided. *, p < 0.05 compared with control.
Discussion
The Erbb or EGFR family of proteins is important for cell growth. In response to various growth factors, EGFR is activated by autocrine or paracrine signaling by phosphorylation at various tyrosine residues (Yarden and Sliwkowski, 2001). Activated EGFR mediates many diverse biological processes, including cell survival, differentiation, invasion, angiogenesis, and apoptosis (Wieduwilt and Moasser, 2008). Aberrant expression of EGFR was reported in various cancers, including ovarian cancers. Almost 70% of ovarian tumors express high levels of EGFR. Inhibition of EGFR activation has been shown to suppress the growth of human malignant cells, and hence the targeted disruption of EGFR could be one potential approach to treat ovarian cancer.
In this study, we clearly demonstrated that DIM-induced apoptotic death of human ovarian cancer cells was associated with substantial reductions in activated EGFR (Tyr1068, Tyr1173, Tyr998, and Tyr845) and protein levels of EGFR. The loss of EGFR expression in response to DIM treatment was observed in three different ovarian cancer cell lines differing in their p53 status, indicating that the actions of DIM were not specific to any cancer cell line and independent of p53. In addition, DIM-induced suppression of EGFR activation was abrogated significantly by EGF treatment. EGF treatment also significantly blocked DIM-induced apoptosis in ovarian cancer cells, thus confirming the involvement of EGFR. A recent finding suggests that the EGF-ligand-EGFR axis is an important mechanism that supports the autocrine growth of approximately 70% of ovarian tumors (Kohler et al., 1989). Moreover, 17 ovarian carcinoma cell lines examined expressed high levels of EGFR, and their growth was stimulated by EGF (Alper et al., 2001). Our results indicate that EGFR is a molecular target of DIM in ovarian cancer cells. Our results are in agreement with recent studies that showed that resveratrol, capsaicin, DIM, and silibinin suppress the growth of prostate, breast, and lung cancer cells by targeting EGFR (Stewart and O'Brian, 2004; Li et al., 2005; Ali et al., 2008; Rho et al., 2010; Thoennissen et al., 2010; Rajoria et al., 2011).
Several pathways are affected by EGFR activation; MAPK is the main one. Activated EGFR activates MEK by phosphorylating it at Ser217 (Cowley et al., 1994). MEK in turn phosphorylates ERK at Thr202 and Tyr204, thereby leading to its activation (Crews et al., 1992). Activation of ERK leads to the transcription of several genes, thereby promoting cell survival and cell cycle progression (Roux and Blenis, 2004). Several studies have shown the involvement of MEK and ERK signaling in apoptosis (Cowley et al., 1994; Roux and Blenis, 2004). Moreover, clinical data suggest that MEK and ERK are activated in 70% of ovarian tumors (Wang et al., 2005; Leung and Choi, 2007). The blockade of MEK or ERK has been shown to cause apoptosis in breast cancer and leukemia (Nishioka et al., 2008; Jang et al., 2010). Our study reveals that DIM blocks the activation of both MEK and ERK in all three ovarian cancer cell lines. The DIM inhibition of ERK was further verified by the kinase activity of ERK by determining the phosphorylation of its downstream substrate ELK-1. ERK activity was reduced by DIM in a concentration-dependent manner in all three ovarian cancer cell lines. Several studies demonstrated the association of ERK activity with EGFR activation (Hall and Davis, 2002; Shi et al., 2008). Our results confirm that ERK is a downstream target of DIM in ovarian cancer cells. Furthermore, the exposure of cells to EGF, a ligand of EGFR, substantially increased the activation of MEK and ERK. These observations indicate that EGFR is upstream and pivotal in the activation of MEK and ERK in our model. Our results also show that the reduction in the activation of MEK and ERK by DIM treatment was blocked by EGF. Pharmacological inhibitors of EGFR and MEK also induced apoptosis in ovarian cancer cells, indicating the significance of EGFR in the survival of ovarian cancer cells. These results also support the fact that the inactivation of MEK and ERK by DIM was regulated by blocking EGFR phosphorylation, thus confirming that EGFR is a target of DIM in ovarian cancer cells.
Oral administration of 3 mg of DIM per day substantially reduced the growth of ovarian tumors. The tumors from DIM-treated mice demonstrated inhibition of EGFR signaling and increased apoptosis. DIM is a major indole compound present in cruciferous vegetables and consumed on a daily basis (Grose and Bjeldanes, 1992). DIM is well tolerated not only by healthy volunteers but also by patients with cervical cancer (Reed et al., 2008; Del Priore et al., 2010). In addition, DIM alone demonstrated significant clinical improvement in patients with cervical intraepithelial neoplasia (Del Priore et al., 2010). Several pharmacokinetic studies on DIM stated that up to 300 mg of a single dose of DIM can be tolerated by humans (Reed et al., 2008). Our dose of DIM falls within the accepted and tolerated dose in humans. Nevertheless, further clinical studies are needed to show that DIM has no side effects. Considering the fact that several EGFR-targeted therapies such as monoclonal antibodies, small molecule inhibitors, or receptor tyrosine kinase inhibitors failed to pass phase II clinical trials to treat ovarian cancer (Barrena Medel et al., 2010), it is tempting to speculate that DIM should be tried in clinical trials as a therapeutic option against ovarian cancer based on the success of DIM in other cancer models.
In conclusion, our results establish that DIM induces apoptosis in ovarian cancer cells by inhibiting EGFR signaling. Our results also provide evidence that DIM suppress the phosphorylation of MEK and ERK, which are regulated by EGFR. More importantly, DIM suppressed the growth of ovarian tumors in vivo by inhibiting the EGFR pathway. Taken together, our study provides support for the use of DIM in preclinical and clinical settings in the management of ovarian cancer patients.
Supplementary Material
This work was supported in part by the National Institutes of Health National Cancer Institute [Grants R01-CA106953, R01-CA129038] (to S.K.S.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EGFR
- epidermal growth factor receptor
- DIM
- 3,3′-diindolylmethane
- EGF
- epidermal growth factor
- ERK
- extracellular signal-regulated kinase
- FBS
- fetal bovine serum
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase kinase
- PARP
- poly(ADP-ribose) polymerase
- PBS
- phosphate-buffered saline
- PD98059
- 2′-amino-3′-methoxyflavone
- ELK
- E twenty-six like transcription factor.
Authorship Contributions
Participated in research design: Kandala, Wright, and Srivastava.
Conducted experiments: Kandala.
Performed data analysis: Kandala and Srivastava.
Wrote or contributed to the writing of the manuscript: Kandala, Wright, and Srivastava.
References
- Ahmed N, Pansino F, Baker M, Rice G, Quinn M. (2002) Association between alphavbeta6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer. J Cell Biochem 84:675–686 [DOI] [PubMed] [Google Scholar]
- Ali S, Banerjee S, Ahmad A, El-Rayes BF, Philip PA, Sarkar FH. (2008) Apoptosis-inducing effect of erlotinib is potentiated by 3,3′-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol Cancer Ther 7:1708–1719 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alper O, Bergmann-Leitner ES, Bennett TA, Hacker NF, Stromberg K, Stetler-Stevenson WG. (2001) Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst 93:1375–1384 [DOI] [PubMed] [Google Scholar]
- Barrena Medel NI, Wright JD, Herzog TJ. (2010) Targeted therapies in epithelial ovarian cancer. J Oncol 2010:314326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast RC, Jr, Hennessy B, Mills GB. (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9:415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E. (1998) Localized activation of RTK/MAPK pathways during Drosophila development. Bioessays 20:189–194 [DOI] [PubMed] [Google Scholar]
- Boreddy SR, Pramanik KC, Srivastava SK. (2011) Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res 17:1784–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova ML, Larcher F, Casanova B, Murillas R, Fernández-Aceñero MJ, Villanueva C, Martínez-Palacio J, Ullrich A, Conti CJ, Jorcano JL. (2002) A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res 62:3402–3407 [PubMed] [Google Scholar]
- Ciska E, Verkerk R, Honke J. (2009) Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3′-diindolylmethane in fermented cabbage. J Agric Food Chem 57:2334–2338 [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77:841–852 [DOI] [PubMed] [Google Scholar]
- Crews CM, Alessandrini A, Erikson RL. (1992) The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 258:478–480 [DOI] [PubMed] [Google Scholar]
- Del Priore G, Gudipudi DK, Montemarano N, Restivo AM, Malanowska-Stega J, Arslan AA. (2010) Oral diindolylmethane (DIM): pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol 116:464–467 [DOI] [PubMed] [Google Scholar]
- Grose KR, Bjeldanes LF. (1992) Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol 5:188–193 [DOI] [PubMed] [Google Scholar]
- Hall JP, Davis RJ. (2002) Inhibition of the p38 pathway upregulates macrophage JNK and ERK activities, and the ERK, JNK, and p38 MAP kinase pathways are reprogrammed during differentiation of the murine myeloid M1 cell line. J Cell Biochem 86:1–11 [DOI] [PubMed] [Google Scholar]
- Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. (2010) A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res 2:402–411 [PMC free article] [PubMed] [Google Scholar]
- Jang K, Kim M, Seo HS, Shin I. (2010) PTEN sensitizes MDA-MB-468 cells to inhibition of MEK/Erk signaling for the blockade of cell proliferation. Oncol Rep 24:787–793 [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. (2011) Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- Kandala PK, Srivastava SK. (2010) Activation of checkpoint kinase 2 by 3,3′-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells. Mol Pharmacol 78:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Janz I, Wintzer HO, Wagner E, Bauknecht T. (1989) The expression of EGF receptors, EGF-like factors and c-myc in ovarian and cervical carcinomas and their potential clinical significance. Anticancer Res 9:1537–1547 [PubMed] [Google Scholar]
- Leung PC, Choi JH. (2007) Endocrine signaling in ovarian surface epithelium and cancer. Hum Reprod Update 13:143–162 [DOI] [PubMed] [Google Scholar]
- Li Y, Chinni SR, Sarkar FH. (2005) Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci 10:236–243 [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Takeshita A, Taniguchi A, Komatsu N, Togitani K, Koeffler HP, Yokoyama A. (2008) Blockade of MEK/ERK signaling enhances sunitinib-induced growth inhibition and apoptosis of leukemia cells possessing activating mutations of the FLT3 gene. Leuk Res 32:865–872 [DOI] [PubMed] [Google Scholar]
- Ozols RF, Bookman MA, du Bois A, Pfisterer J, Reuss A, Young RC. (2006) Intraperitoneal cisplatin therapy in ovarian cancer: comparison with standard intravenous carboplatin and paclitaxel. Gynecol Oncol 103:1–6 [DOI] [PubMed] [Google Scholar]
- Pramanik KC, Boreddy SR, Srivastava SK. (2011) Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One 6:e20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman KW, Sarkar FH. (2005) Inhibition of nuclear translocation of nuclear factor-{kappa}B contributes to 3,3′-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res 65:364–371 [PubMed] [Google Scholar]
- Rajoria S, Suriano R, Wilson YL, Schantz SP, Moscatello A, Geliebter J, Tiwari RK. (2011) 3,3′-diindolylmethane inhibits migration and invasion of human cancer cells through combined suppression of ERK and AKT pathways. Oncol Rep 25:491–497 [DOI] [PubMed] [Google Scholar]
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A. (2008) Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev 17:2619–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JK, Choi YJ, Jeon BS, Choi SJ, Cheon GJ, Woo SK, Kim HR, Kim CH, Choi CM, Lee JC. (2010) Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790M mutation. Mol Cancer Ther 9:3233–3243 [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sahu RP, Srivastava SK. (2008) Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer 8:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. (2007) The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 75:770–787 [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL, Pescovitz OH. (2004) Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol 18:2570–2582 [DOI] [PubMed] [Google Scholar]
- Stewart JR, O'Brian CA. (2004) Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs 22:107–117 [DOI] [PubMed] [Google Scholar]
- Thoennissen NH, O'Kelly J, Lu D, Iwanski GB, La DT, Abbassi S, Leiter A, Karlan B, Mehta R, Koeffler HP. (2010) Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 29:285–296 [DOI] [PubMed] [Google Scholar]
- Turner T, Chen P, Goodly LJ, Wells A. (1996) EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metastasis 14:409–418 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kristensen GB, Helland A, Nesland JM, Børresen-Dale AL, Holm R. (2005) Protein expression and prognostic value of genes in the erb-b signaling pathway in advanced ovarian carcinomas. Am J Clin Pathol 124:392–401 [DOI] [PubMed] [Google Scholar]
- Wieduwilt MJ, Moasser MM. (2008) The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 65:1566–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137 [DOI] [PubMed] [Google Scholar]
- Zeineldin R, Muller CY, Stack MS, Hudson LG. (2010) Targeting the EGF receptor for ovarian cancer therapy. J Oncol 2010:414676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yang ZY, Binns CW, Lee AH. (2002) Diet and ovarian cancer risk: a case-control study in China. Br J Cancer 86:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.