Abstract
The response properties of tooth pulp neurons that respond to noxious thermal stimulation of the dental pulp have been not well-studied. The present study was designed to characterize the response properties of tooth pulp neurons to noxious thermal stimulation of the dental pulp. Experiments were conducted on 25 male ferrets, and heat stimulation was applied by a computer-controlled thermode. Only 15% of tooth pulp neurons (n = 39) responded to noxious thermal stimulation of the teeth. Tooth pulp neurons were found in both the superficial and deep nuclear regions of the subnucleus caudalis (Vc) and in the interface between the nucleus caudalis and interpolaris (Vc/Vi). Thirty-seven neurons had cutaneous receptive fields and were classified as either NS (16) or WDR (21) neurons. Repeated heat stimulation of the dental pulp sensitized and increased the number of electrically evoked potentials of tooth pulp neurons. These results provide evidence that both the Vc and Vc/Vi regions contain neurons that respond to noxious thermal stimulation of the dental pulp, and that these cells may contribute to the sensitization process associated with symptomatic pulpitis.
Keywords: tooth pulp neuron, trigeminal, sensitization, thermal stimulation, dental pain, tooth
Introduction
The trigeminal brainstem sensory nuclear complex relays somatosensory afferent information from the face, including the oral cavity. Several previous studies demonstrated that the trigeminal subnucleus caudalis relays orofacial nociceptive information, including input from the tooth pulp. Transganglionic tracers applied to the tooth pulp have revealed afferent projections to various rostro-caudal subdivisions of the trigeminal complex (Arvidsson and Gobel, 1981; Marfurt and Turner, 1984). Electrophysiological studies have also demonstrated that a large number of neurons in the medullary dorsal horn responded to electrical stimulation of the tooth pulp (Nord and Young, 1975; Yokota, 1975; Nord, 1976). However, most electrophysiological studies that have examined tooth pulp input to trigeminal brain stem neurons have been performed with non-natural electrical stimulation.
Very little is known about the response properties of tooth pulp neurons that respond to natural stimulation of the tooth pulp which evokes dental pain, although a few studies reported that tooth-pulp-driven neurons respond to thermal stimulation of the tooth pulp (Hu and Sessle, 1984). Therefore, the present study was designed to characterize the response properties of tooth pulp neurons that respond only to natural stimulation of the tooth pulp in ferrets. The natural stimulus we utilized was noxious heat stimulation by the computer-controlled device and cold stimulation by Endoice® application. We also investigated the sensitization of tooth pulp neurons evoked by repeated noxious heat stimulation.
Materials & Methods
Animal Preparation
All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Experiments were carried out on 25 adult male ferrets (Mustela putorius furo) weighing between 0.9 and 1.4 kg. The animals were anesthetized with 3% halothane with oxygen for induction, followed by a mixture of chloral hydrate (110 mg/kg) and pentobarbital sodium (20 mg/kg) intraperitoneally. Anesthesia was maintained with periodic intravenous injections of a mixture of chloral hydrate (22 mg/kg) and pentobarbital sodium (4 mg/kg). Arterial blood pressure and body temperature were maintained at 90 ~ 120 mm Hg and 37°C ~ 38°C, respectively. The neck muscles and the dura were removed to expose the medulla. The exposed surface of the medulla was covered with warm saline. During the recording sessions, animals were immobilized with vecuronium bromide (0.4 mg/kg) and artificially ventilated. End tidal CO2 level was kept at 3.5 ~ 4.5%.
Tooth Preparation for Stimulation
The right upper and lower canine teeth were prepared for electrical and heat stimulation. We isolated the teeth by placing a dental rubber dam around them, to prevent unintentional stimulation of other intra-oral tissues. In a recent study, we introduced a stimulator system that allows for both electrical and thermal stimulation of the intact tooth by a computer-controlled device (Ahn et al., 2011). The computer-controlled device system delivers a precisely controlled heat stimulus for thermal stimulation of the canines in ferrets. A custom-designed, computer-controlled probe was placed over the exposed tooth. This device was able to deliver either constant electrical current or noxious heat stimuli through the same probe. The cathode electrode was connected to the canines, and the anode electrode was positioned on the animal’s neck muscle. The stimulating probe was secured to each canine with colloidal silver liquid (No. 16031, TED PELLA Inc., Redding, CA, USA).
Extracellular Recording of Tooth Pulp Neurons
Extracellular recordings were conducted with glass electrodes with a resistance of 2 to 10 MΩ. In general, the first penetration was made 1.8 to 2.3 mm lateral to the midline at the level of the obex. In ferrets, this area shows dense c-Fos expression following noxious thermal stimulation of the maxillary and mandibular canines (Chattipakorn et al., 1999). Recording of single-unit activity and measuring latency followed the conventional method, described in the Appendix. Minimum conduction velocity was determined by a post-stimulus histogram constructed from 50 electrical pulses delivered to the tooth pulp by standard techniques (Price et al., 1976; Hu et al., 1981; Bossut and Maixner, 1996).
Electrical and Thermal Stimulation of Teeth
Constant-current square wave pulses (2-ms duration) were delivered to the tooth once every sec, as a search stimulus. Delivered current was monitored by assessment of the voltage drop across a 100-Ω resistance in series with the preparation. To prevent stimulation of adjacent tissues, the intensity of the applied current did not exceed 150 to 200 µA (Matthews and Searle, 1976). A computer-controlled probe also delivered heat stimuli applied either in a staircase or single-pulse manner, as described previously (Ahn et al., 2011). Endoice® (The Hygenic Co., Akron, OH, USA) was applied to the labial surface of the tooth. The cold stimulation was applied by soaked cotton pellets attached to a non-conducting material, such as a gutta-percha rod, for 10 sec. Thus, this stimulation did not produce electrical interferences during the recording of tooth pulp neurons, as described previously (Jyväsjärvi and Kniffki, 1987). In an in vitro experiment with the extracted canines, the rapid cooling of the tooth decreased the pulp temperature to between 5 and 10°C, as described previously (Hu and Sessle, 1984; Jyväsjärvi and Kniffki, 1987).
Stimulation of the Skin and Oral Mucosa
Only the tooth pulp neurons responding to thermal stimulation of teeth were further characterized by the testing of their responses to both non-noxious tactile and noxious stimuli, to assess convergent connections from the overlying facial skin and surrounding oral mucosa. Tooth pulp neurons with receptive fields in surrounding tissues were classified as low-threshold mechano-receptive neurons (LTM), wide dynamic range neurons (WDR), or nociceptive-specific neurons (NS), as previously described (Hu and Sessle, 1984; Bossut and Maixner, 1996).
Statistical Analysis
Sensitization of neuronal activities by repeated thermal stimulation was presented as mean ± SE for arithmetic averaging and compared statistically by Student’s t test. In all statistical comparisons, p < 0.05 was used as the criterion for statistical significance.
Results
Only 39 tooth pulp neurons activated by noxious heat and/or cold applied to the canine teeth were recorded in the present study. Responding tooth pulp neurons were found in both the superficial and deep nuclear regions of the subnucleus caudalis (Vc) and in the interface between the nucleus caudalis and interpolaris (Vc/Vi). Seven responding neurons showed spontaneous background activity.
Responses to Natural Noxious Stimulation of the Tooth
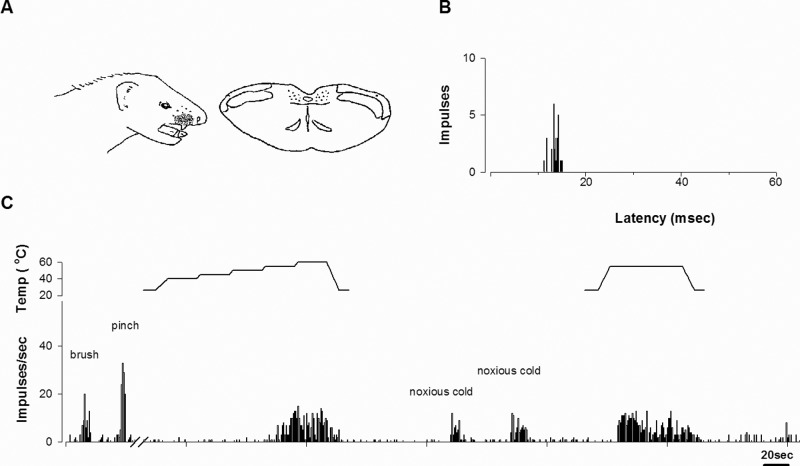
Fig. 1 shows the typical response of a tooth pulp neuron activated by stimulation of the right upper canine. This tooth pulp neuron had a cutaneous receptive field in the face and was located in lamina II (Fig. 1A). Electrical stimulation of the tooth with 50 successive pulses evoked responses with 10- to 15-msec delay, consistent with the Aδ fiber input (Fig. 1B). The tooth pulp neuron responded to both noxious (pinch) and non-noxious (brush) stimulation of the skin and was classified as a WDR neuron. The neuron was excited by both noxious heat and cold stimulation of the canine (Fig. 1C).
Figure 1.
A representative example of a tooth pulp neuron that responded to noxious heat and cold stimulation. (A) The cutaneous receptive field and camera lucida drawing of the cell’s location within the trigeminal nucleus. The receptive field was located in the right facial area and the upper lip. The cell was classified as a WDR neuron and was located in lamina II. (B) Peristimulus histogram of electrical stimulation of the tooth. The neuron received Aδ afferent input. (C) Responses to noxious cold and heat stimulation of the upper canine. A computer-controlled probe delivered heat stimuli that were applied in a staircase manner, from an adapting temperature of 40°C, increasing to 60°C in 5°C increments every 25 sec, or as a single pulse for 60 sec. This particular neuron was excited by both noxious heat and cold stimulation of the tooth.
Responses of Tooth Pulp Neurons to Cutaneous Stimulation
Almost all of the tooth pulp neurons (37/39) activated by noxious heat and/or cold stimulation of tooth had a cutaneous receptive field located in the facial skin or in the oral cavity (Appendix Table). Two neurons, however, did not have identifiable receptive fields. Both these neurons were activated by both noxious heat and cold stimulation of the mandibular canine. Sixteen tooth pulp neurons that responded only to noxious stimulation of the cutaneous receptive fields were classified as NS neurons, whereas 21 tooth pulp neurons that responded to both noxious and non-noxious stimulation of the cutaneous receptive fields were classified as WDR neurons.
Tooth pulp neurons were also classified by their noxious heat and/or cold stimulation of the tooth. Three tooth pulp neurons were excited only by noxious heat stimulation of the tooth pulp. Fourteen neurons were excited only by noxious cold stimulation. Twenty neurons were excited by both types of stimuli to the tooth pulp. Nineteen tooth pulp neurons were located in the superficial lamina and 18 in the deep lamina. Sixteen tooth pulp neurons were activated by stimulation of the maxillary canines and 18 by stimulation of the mandibular canines. Three neurons responded to electrical stimulation of both maxillary and mandibular teeth.
Response Latencies of Tooth Pulp Neurons to Electrical Stimulation
We determined minimum conduction velocity to identify the latency distribution of tooth pulp neurons that responded to thermal stimulation. Almost all the tooth pulp neurons (36/39) received only Aδ-fiber input, and 3 neurons received C-fiber input as well. The latency distribution of afferent input to the individual tooth pulp neurons is illustrated in Appendix Fig. 1.
Sensitization of Tooth Pulp Neurons by Repeated Noxious Heat Stimuli
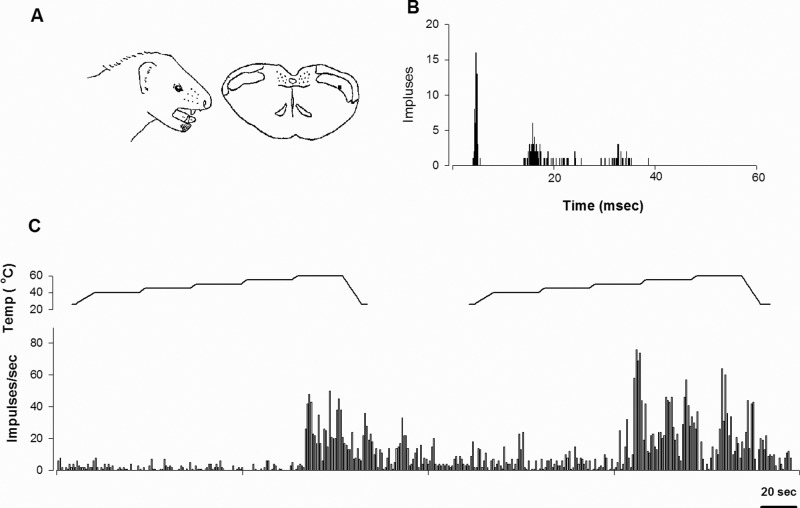
Fig. 2 illustrates a typical example of sensitization by repeated noxious heat stimuli to the tooth pulp. This tooth pulp neuron was activated by noxious heat applied to the mandibular canine and was classified as a WDR neuron. The neuron was located in the lamina V, and afferent input from the tooth pulp was in the Aδ range. Heat stimuli were applied to the canine with 1-minute inter-trial intervals. During the first heat trial, the neuron responded at 60°C. The impulses evoked by heat stimulus were increased and the threshold temperature was lowered during the second heat trial. This tooth pulp neuron had spontaneous activity which was enhanced by repeated heat stimuli. Four (3 WDR and 1 NS) of the 7 examined neurons (4 WDR and 3 NS) were sensitized by repeated noxious heat stimulation. In the 4 sensitized neurons, the second noxious heat stimulus significantly increased the number of evoked impulses of tooth pulp neurons by 190%, compared with their response to the first noxious heat stimulus (p < 0.05).
Figure 2.
Response of a tooth pulp neuron to repeated noxious heat stimulation. (A) Location of the cutaneous receptive field and camera lucida drawings of the cell’s location. The receptive field was located in the surrounding gingiva and right lower lip. The cell was classified as a WDR neuron and was located in lamina V. (B) Peristimulus histogram to electrical stimulation of the tooth (50 presentations) revealed that this cell received Aδ afferent input. (C) Responses of the cell to repeated noxious heat stimulation of the lower canine. The computer-controlled probe delivered repeated heat stimuli which were gradually applied from an adapting temperature of 40°C, increasing up to 60°C in 5°C increments every 25 sec. The second noxious heat stimuli significantly increased the firing rate of the tooth pulp neurons.
We also examined the effects of noxious heat conditioning on responses to electrical stimulation of the tooth in 7 tooth pulp neurons. Fig. 3 shows that noxious heat conditioning increased evoked responses produced by electrical tooth pulp stimulation. Ten consecutive electrical pulses were applied to the canine before and after a 2-minute period of noxious heat conditioning. After heat stimulation, the responses evoked by electrical tooth pulp stimulation increased from 4.3 ± 0.2 to 6.6 ± 0.4 action potentials per test pulse (p < 0.01).
Figure 3.
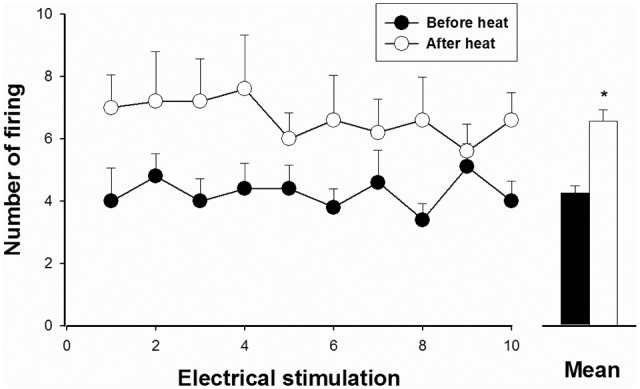
Evoked responses produced by electrical stimulation before and after noxious heat stimulation of the tooth pulp neurons, performed to evaluate sensitization of the tooth pulp neurons. The evoked action potentials in 7 neurons that responded to 10 electrical stimuli applied to the dental pulp are presented. The responses after heat conditioning were significantly increased compared with those before heating (*p < 0.05). Results are mean ± SEM at each time-point tested.
Discussion
The present study demonstrated the response properties of tooth pulp neurons that responded to noxious thermal stimulation of the dental pulp. Although a few previous studies reported that tooth pulp neurons respond to thermal stimulation (Hu et al., 1981; Hu and Sessle, 1984), their response properties in noxious thermoreception of teeth have not been extensively investigated. The present study demonstrated the characterization of the response properties of trigeminal neurons that responded to noxious heat and/or cold stimuli applied to the canines, using a computer-controlled stimulator system (Ahn et al., 2011) that allows for both electrical and thermal stimulation. Only 15% of the neurons that responded to electrical stimulation of the tooth pulp also responded to noxious thermal stimulation of the tooth. These findings are consistent with results of previous studies which reported that less than 20% of the neurons were excited by natural tooth pulp stimulation (Hu and Sessle, 1984). These authors also reported that heat stimulation was more effective than cold stimulation in evoking neuronal responses. However, our findings showed that a large proportion of tooth pulp neurons responded to both noxious heat and cold stimulation of the tooth pulp. These contrasting findings may have resulted from variations in stimulation modalities and procedures. Moreover, our computer-controlled stimulator system, which accurately controls the target temperature, provides experimental conditions for identifying tooth pulp neurons that can respond to noxious heat.
In the present study, similar to the trigeminal neurons with cutaneous receptive fields (Beitel and Dubner, 1976), tooth pulp neurons with thermal input were sensitized by repeated heat stimuli applied to the tooth. Previous studies have also shown that repeated heat stimulation of the dental pulp causes sensitization of pulpal primary afferents (Matthews, 1977; Ahlberg, 1978). In contrast, the finding of an apparent lack of sensitization of the tooth pulp neurons in a previous study (Hu and Sessle, 1984) is not in agreement with our findings. The present study demonstrated that the second trial of thermal stimulation evoked tooth pulp neurons with a low threshold of thermal stimuli and increased the thermally evoked responses, compared with that of the first trial of thermal stimulation. Several previous studies on central sensitization of trigeminal nociceptive neurons in the Vc demonstrated the involvement of P2X7 receptors (Itoh et al., 2011), NMDA receptor subunits, and opioid-related inhibitory mechanisms (Kaneko et al., 2011), astroglial glutamate-glutamine shuttle (Tsuboi et al., 2011), and glia (Xie et al., 2007).
We recorded 39 tooth pulp neurons that responded to local noxious heat and/or cold stimulation of the dentition, and they were found in both the Vc and the Vc/Vi interface. Similar to previous studies with electrical stimulation (Nord and Young, 1975; Yokota, 1975), neurons that responded to electrical tooth pulp stimulation fell into 2 groups. One group was located in the superficial layers and the other group in the deeper layers of both the Vc and the Vc/Vi interface. These results are consistent with our previous findings, that these regions contain neurons that express the immediate early gene c-Fos in response to noxious heat stimulation of the canine teeth (Chattipakorn et al., 1999). The largest cluster of Fos-positive neurons was found in the superficial laminae and deeper regions of the medullary dorsal horn near the level of the obex.
The present study demonstrated the tooth pulp neurons that received Aδ input from the tooth but very few neurons with C-fiber input. Only 3 tooth pulp neurons that responded to thermal stimulation received C-fiber input. In contrast to our findings, previous histological studies of the dental pulp have shown C-fibers to constitute a considerable proportion of the pulpal afferent nerve fibers in anatomical studies (Fried and Hildebrand, 1981; Holland and Robinson, 1983) and electrophysiological studies (Jyväsjärvi and Kniffki, 1987, 1989). Several previous studies demonstrated that conduction velocity was slower in the pulp compared with that outside of the tooth pulp (Cadden et al., 1983; Dong et al., 1985; Jyväsjärvi and Kniffki, 1989). These findings might partially explain why electrophysiological studies did not reveal a large number of central trigeminal cells with C-fiber input. It is also possible that C-fiber input to trigeminal neurons is suppressed by electrical stimuli that cause a concomitant activation of myelinated A-β fibers, which may inhibit synaptic transmission via C-fibers.
It has been established that the central trigeminal neurons receive convergent inputs from the dentition and the nearby skin and mucosa (Robinson, 1979; Sessle et al., 1986). The extensive convergence of afferent input onto central trigeminal neurons is probably one of the central mechanisms responsible for the poor localization of pulpal pain. Therefore, patients often have difficulties in localizing an inflamed painful tooth (Friend and Glenwright, 1968). In the present study, almost all the tooth pulp neurons that responded to thermal stimuli received convergent input from the nearby skin or mucosa. Thus, the tooth pulp neurons were classified as either NS or WDR neurons based on their response to stimuli applied to their cutaneous or mucosal receptive fields.
In conclusion, the present study was designed to characterize the response properties of tooth pulp neurons that respond to noxious thermal stimulation of the dental pulp. The present results provide evidence that both the Vc and Vc/Vi interface contain neurons that respond to natural noxious stimulation of the dental pulp, and that these cells may contribute to the sensitization process associated with symptomatic pulpitis.
Footnotes
This study was supported by DE 11661 from NIDCR to Dr. W. Maixner.
The authors have no conflicts of interests to declare related to this study.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ahlberg KF. (1978). Influence of local noxious heat stimulation on sensory nerve activity in the feline dental pulp. Acta Physiol Scand 103:71-80 [DOI] [PubMed] [Google Scholar]
- Ahn DK, Monbureau O, Närhi M, Maixner W. (2011). A novel computerized system for thermal stimulation of tooth in ferrets. J Neurosci Methods 203:305-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson J, Gobel S. (1981). An HRP study of central projections of primary trigeminal neurons which innervate tooth pulps in the cat. Brain Res 210:1-16 [DOI] [PubMed] [Google Scholar]
- Beitel RE, Dubner R. (1976). Response of unmyelinated (C) polymodal nociceptors to thermal stimuli applied to monkey’s face. J Neurophysiol 39:1160-1175 [DOI] [PubMed] [Google Scholar]
- Bossut DF, Maixner W. (1996). Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain 65:101-109 [DOI] [PubMed] [Google Scholar]
- Cadden SW, Lisney SJ, Matthews B. (1983). Thresholds to electrical stimulation of nerves in cat canine tooth-pulp with A beta-, A delta- and C-fibre conduction velocities. Brain Res 261:31-41 [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, Light AR, Willcockson HH, Närhi M, Maixner W. (1999). The effect of fentanyl on c-fos expression in the trigeminal brainstem complex produced by pulpal heat stimulation in the ferret. Pain 82:207-215 [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH, Martin RF. (1985). Physiological properties of intradental mechanoreceptors. Brain Res 334:389-395 [DOI] [PubMed] [Google Scholar]
- Fried K, Hildebrand C. (1981). Pulpal axons in developing, mature, and aging feline permanent incisors. A study by electron microscopy. J Comp Neurol 203:23-36 [DOI] [PubMed] [Google Scholar]
- Friend LA, Glenwright HD. (1968). An experimental investigation into the localization of pain from the dental pulp. Oral Surg Oral Med Oral Pathol 25:765-774 [DOI] [PubMed] [Google Scholar]
- Holland GR, Robinson PP. (1983). The number and size of axons at the apex of the cat’s canine tooth. Anat Rec 205:215-222 [DOI] [PubMed] [Google Scholar]
- Hu JW, Sessle BJ. (1984). Comparison of responses of cutaneous nociceptive and nonnociceptive brain stem neurons in trigeminal subnucleus caudalis (medullary dorsal horn) and subnucleus oralis to natural and electrical stimulation of tooth pulp. J Neurophysiol 52:39-53 [DOI] [PubMed] [Google Scholar]
- Hu JW, Dostrovsky JO, Sessle BJ. (1981). Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J Neurophysiol 45:173-192 [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiang CY, Li Z, Lee JC, Dostrovsky JO, Sessle BJ. (2011). Central sensitization of nociceptive neurons in rat medullary dorsal horn involves purinergic P2X7 receptors. Neuroscience 192:721-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyväsjärvi E, Kniffki KD. (1987). Cold stimulation of teeth: a comparison between the responses of cat intradental A delta and C fibres and human sensation. J Physiol 391:193-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyväsjärvi E, Kniffki KD. (1989). Afferent C fibre innervation of cat tooth pulp: confirmation by electrophysiological methods. J Physiol 411:663-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Kaneko T, Kaneko R, Chokechanachaisakul U, Kawamura J, Sunakawa M, et al. (2011). The role of N-methyl-D-aspartate receptor subunits in the rat thalamic mediodorsal nucleus during central sensitization. Brain Res 1371:16-22 [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Turner DF. (1984). The central projections of tooth pulp afferent neurons in the rat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol 223:535-547 [DOI] [PubMed] [Google Scholar]
- Matthews B. (1977). Responses of intradental nerves to electrical and thermal stimulation of teeth in dogs. J Physiol 264:641-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B, Searle BN. (1976). Electrical stimulation of teeth. Pain 2:245-251 [DOI] [PubMed] [Google Scholar]
- Nord SG. (1976). Electrical stimulation of the tooth pulp in the study of pain. Brain Res Bull 1:251-254 [DOI] [PubMed] [Google Scholar]
- Nord SG, Young RF. (1975). Projection of tooth pulp afferents to the cat trigeminal nucleus caudalis. Brain Res 90:195-204 [DOI] [PubMed] [Google Scholar]
- Price DD, Dubner R, Hu JW. (1976). Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal, and nociceptive stimulation of monkey’s face. J Neurophysiol 39:936-953 [DOI] [PubMed] [Google Scholar]
- Robinson PP. (1979). The course, relations and distribution of the inferior alveolar nerve and its branches in the cat. Anat Rec 195:265-271 [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW, Amano N, Zhong G. (1986). Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurons in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain 27:219-235 [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Iwata K, Dostrovsky JO, Chiang CY, Sessle BJ, Hu JW. (2011). Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci 34:292-302 [DOI] [PubMed] [Google Scholar]
- Xie YF, Zhang S, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. (2007). Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn). Brain Behav Immun 21:634-641 [DOI] [PubMed] [Google Scholar]
- Yokota T. (1975). Excitation of units in marginal rim of trigeminal subnucleus caudalis elicited by tooth pulp stimulation. Brain Res 95:154-158 [DOI] [PubMed] [Google Scholar]