Abstract
BMP signaling plays crucial roles in the development of many organs, including the tooth. Equally important is BMP signaling homeostasis, as demonstrated by multiple organ defects in mice lacking the extracellular BMP antagonist Noggin. Here, we show that Noggin is initially expressed in the maxillary mesenchyme adjunct to the upper incisor at the initiation stage, and then in the developing teeth, including incisors and molars, from the bud stage. Noggin mutants develop normal molars and mandibular incisors, but form a single, medially located upper incisor that is arrested at the late bud stage. Histological and molecular marker analyses demonstrated that two distinct upper incisor placodes initiate independently at E11.5, but begin to fuse at E12.5, coupling with elevated cell proliferation rates in the developing tooth germs. We further found that Chordin and Gremlin, two other BMP antagonists, are co-expressed with Noggin in the developing lower incisor and molar teeth. These observations indicate the importance of BMP signaling homeostasis, and suggest a functional redundancy between BMP antagonists during tooth development.
Keywords: BMP antagonist, BMP homeostasis, Chordin, Gremlin, tooth development, tooth fusion
Introduction
BMP signaling is introduced into cells via transmembrane serine/threonine kinase of type I and type II BMP receptors (Sieber et al., 2009). The activated type I receptor phosphorylates the receptor-regulated Smads (R-Smad), which then bind to the common Smad (Smad4) and move into the nucleus to regulate gene expression. BMP signaling is modulated by several proteins at different levels (Gazzerro and Canalis, 2006). In the extracellular compartment, secreted BMP antagonists, such as Noggin, Chordin, and Gremlin, and other molecules, such as Wise, modulate BMP signaling activity by binding to selective BMP ligands, thus preventing binding of ligands to their receptors. In the intracellular compartment, BMP signal can be modulated by inhibitory Smads that compete with Smad4 or mediate R-Smad degradation.
While BMP signaling is essential for embryonic development, overactive BMP activities are detrimental, as exemplified in Noggin mutant mice. Noggin binds preferentially to BMP2, BMP4, and BMP7 to prevent their signaling (Zimmerman et al., 1996; Groppe et al., 2002). Deletion of the Noggin (Nog) gene in mice results in a series of defects in organogenesis, including a spectrum of craniofacial defects (Brunet et al., 1998; McMahon et al., 1998; Bachiller et al., 2000; Stottmann et al., 2001; Anderson et al., 2006; He et al., 2010). In addition, mice deficient for both Noggin and Chordin exhibit more severe defects in several organs, suggesting a functional redundancy among the BMP antagonists (Bachiller et al., 2000; Stottmann et al., 2001; Anderson et al., 2006).
Several Bmp genes are expressed in the developing tooth and are implicated in regulating many aspects of tooth development, including the determination of tooth-forming sites and tooth type (Neubüser et al., 1997; Tucker et al., 1998; Zhang et al., 2005), progression and growth (Chen et al., 1996; Zhang et al., 2000; Zhao et al., 2000), terminal differentiation, and tooth eruption (Gluhak-Heinrich et al., 2010; Yao et al., 2010). Disruption of BMP signaling results in an arrest of tooth development (Andl et al., 2004; Liu et al., 2005; Li et al., 2011). Given the importance of BMP signaling in tooth development and the absolute requirement of BMP homeostasis in the development of many other organs, we investigated Noggin expression patterns in developing teeth and analyzed tooth phenotypes in mice lacking Noggin.
Materials & Methods
Animals
The generation and genotyping of Noggin mutant mice (Nog+/−) have been described previously (McMahon et al., 1998). Embryos were collected from timed-mated pregnant mice, and embryonic ages were determined by the day when the vaginal plug was discovered, which was designated as embryonic day 0.5 (E0.5). Embryonic tails were used for PCR-based genotyping.
Histology, in situ Hybridization, Immunostaining, X-Gal Staining, BrdU Labeling, and TUNEL Assay
Standard hematoxylin/eosin staining and non-radioactive in situ hybridization were conducted on paraffin sections as described previously (St. Amand et al., 2000). For immunostaining, samples were embedded in O.C.T. compound, cryo-sectioned, and subjected to antibodies against pSmad1/5/8 (from Cell Signaling Technology, Danvers, MA, USA) as described previously (He et al., 2010). For whole-mount X-Gal staining coupled with whole-mount in situ hybridization, samples were fixed in 4% PFA fixative, and subjected to X-Gal staining first, followed by whole-mount in situ hybridization without proteinase K treatment. X-Gal staining, BrdU labeling, and TUNEL assay were performed following protocols described previously (He et al., 2010).
In vitro Organ Culture and Bead Implantation
E11.5 embryos were collected from intercrosses of Nog+/− mice. Each embryo from one litter was labeled and the embryonic tail collected for genotyping. The embryonic head was dissected; the mandible was removed and placed in a Trowell-type organ culture with the oral side facing up. Agarose beads soaked with BSA or Noggin protein (from R&D Systems, Minneapolis, MN, USA) at a concentration of 50 ng/mL was implanted into the midline of, but anterior to, the 2 forming upper incisor germs. Samples were cultured in DMEM supplemented with 10% FCS for 24 hrs prior to being harvested for whole-mount in situ hybridization.
Results and Discussion
Expression of Noggin in the Developing Tooth
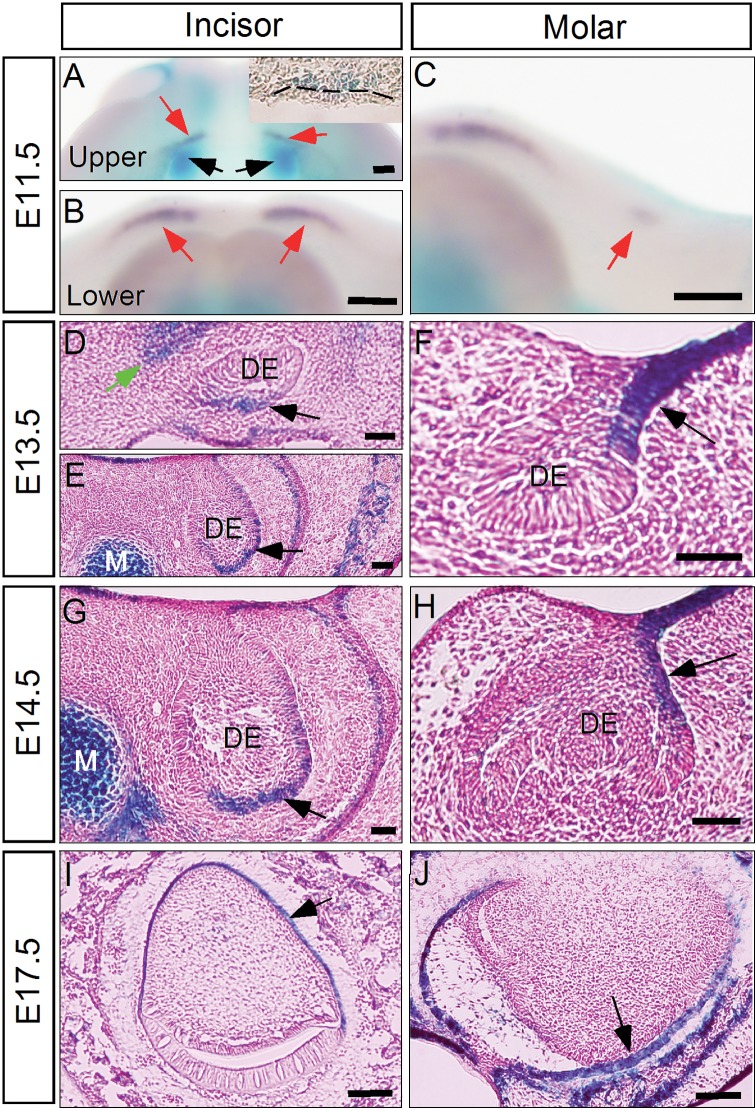
To investigate the role of Noggin in tooth development, we first examined Noggin expression in the developing mouse tooth at several critical stages. We took advantage of the Nog+/− mice in which the Noggin coding sequences were replaced by the LacZ gene (McMahon et al., 1998). Utilizing whole-mount X-Gal staining on Nog+/− embryos, followed by whole-mount in situ hybridization on Shh, which serves as a molecular marker for the dental epithelium, we failed to detect Noggin expression in and around the lower incisor and molar placodes at E11.5 (Figs. 1B, 1C). However, strong Noggin expression was seen in the maxillary mesenchyme immediately posterior to the upper incisor placodes (Fig. 1A). Noggin expression was also found in some cells in the dental mesenchyme immediately underneath the placode epithelium (insert in Fig. 1A). At the E13.5 bud and E14.5 cap stages, Noggin expression was detected in the dental epithelium of the incisors and molars and was also observed in the maxillary mesenchyme adjacent to the upper incisor at the bud stage (Figs. 1D-1H). In the developing molars at both stages (Figs. 1F, 1H), Noggin expression was restricted in the dental epithelium on the buccal side, where Bmp4 is preferentially expressed in the mesenchyme (Zhang et al., 2009). At the E17.5 bell stage, in the incisor, Noggin expression became localized to the dental epithelium on the lingual side (Fig. 1I), and was seen in the external enamel epithelium of the molar (Fig. 1J). This spatiotemporal Noggin expression profile prompted us to examine potential tooth phenotypes in Nog−/− mice.
Figure 1.
Expression of Noggin in the developing tooth. (A-C) At E11.5, X-Gal staining of the LacZ-Nog knock-in allele combined with whole-mount in situ hybridization reveals absent Noggin expression in the upper incisor (A), lower incisor (B), and lower molar (C), as localized by Shh expression (red arrows). However, strong Noggin expression, indicated by LacZ staining, is seen in the maxillary mesenchyme (black arrows), and in the upper incisor mesenchyme (insert in A). (D-F) At E13.5, Noggin expression, as detected by LacZ expression, is observed in the epithelium in upper (D) and lower (E) incisors and molars (F). Black arrows point to the expression sites. In the upper incisor at this stage, Noggin is also expressed in the adjacent maxillary mesenchyme (green arrow in D). (G, H) Noggin is continuously expressed in the epithelium of incisors (G) and molars (H) at E14.5. (I, J) At E17.5, Noggin expression is detected in the ameloblasts of the incisor (I) and molar (J). Dashed lines outline the dental epithelium. DE, dental epithelium. Scale bar: 100 µm.
Phenotypic Analysis of Developing Teeth in Noggin Mutants
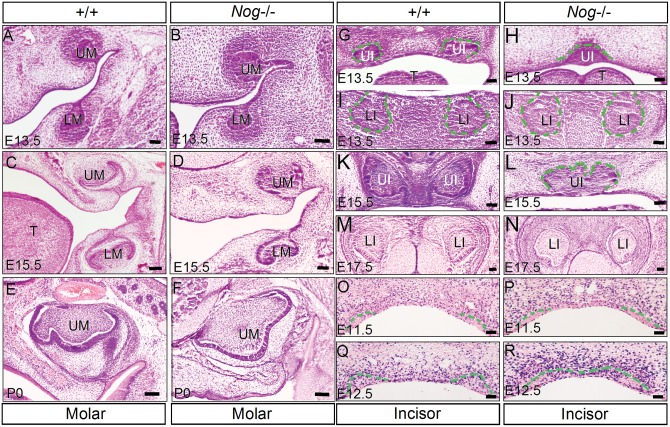
At E13.5, the mutant molars developed to the bud stage, morphologically comparable with the wild-type controls (Figs. 2A, 2B). While the Nog−/− molars at E15.5 appeared slightly delayed in development (Figs. 2C, 2D), at post-natal day 0 (P0), we observed almost identically patterned and grown molars in both Nog−/− and wild-type mice (Figs. 2E, 2F), indicating that Noggin is dispensable for early molar development, consistent with a previous report (Stottmann et al., 2001).
Figure 2.
Tooth phenotype in Noggin mutants. (A, C, E) Coronal sections show molar development in the wild-type controls at E13.5 (A), E15.5 (C), and P0 (E). (B, D, F) Coronal sections show comparable molar development in Noggin mutants at each corresponding stage. (G) A coronal section shows the formation of 2 maxillary incisor buds in a wild-type embryo at E13.5. (H) A coronal section through a Nog−/− embryo at E13.5 shows a single maxillary incisor bud located at the midline. (I, J) Coronal sections show comparable lower incisors in both wild-type (I) and Nog−/− (J) embryos at E13.5. (K, L) At E15.5, the wild-type upper incisors have developed to the late cap stage (K), but the single upper incisor in the mutant remained at the late bud stage (L). (M, N) Coronal sections show comparable development of the lower incisor in both wild-type (M) and Nog−/− (N) mice at E17.5. (O-R) BrdU labeling assay shows BrdU-positive cells in the upper incisor regions of both wild-type controls and Noggin mutant at E11.5 and E12.5. We measured cell proliferation rates by counting BrdU-positive cells and total cells in defined areas, including dental epithelium, the mesenchyme between the dental placodes, and dental mesenchyme. Three continuous sections from each of 2 individual samples of each genotype were counted, and the outcome was expressed as a percentage of BrdU-positive cells among total cells in each defined area. Student’s t test was used to determine the significance of difference, and results are presented in the text and detailed in the Appendix Fig. Dashed lines outline the dental epithelium. T, tongue; LI, lower incisor; LM, lower molar; UI, upper incisor, UM, upper molar. Scale bar: 100 µm.
Like the molar, the lower incisors developed indistinguishably from their wild-type counterparts (Figs. 2I, 2J, 2M, 2N). However, we found the presence of a single upper (maxillary) incisor bud at the midline at the E13.5 bud stage in 13 out of 14 samples examined (Fig. 2H). At E15.5, the single maxillary incisor developed slightly further, but was arrested at the late bud stage, when the control developed to the late cap stage (Figs. 2K, 2L). This residual tooth bud regressed thereafter, because it was not observed in embryos at E17.5 and P0 (data not shown). To investigate cellular alterations that may contribute to defective upper incisor development, we conducted TUNEL and BrdU assays on the Nog−/− upper incisor region at E11.5 and E12.5. While no obviously altered level of cell apoptosis was observed (data not shown), significantly increased levels of cell proliferation rates were found in the dental placode epithelium and in the intervening mesenchyme between the placodes of mutants at E11.5, as compared with controls (p < 0.01 in both sites) (Figs. 2O, 2P, Appendix Fig.). However, cell proliferation rates appeared similar in the oral epithelium between the dental placodes in both wild-type and mutants at this stage (p > 0.1). At E12.5, the cell proliferation rate in the mutant dental mesenchyme was elevated, as compared with that in controls (p < 0.05), but the mutant dental epithelium exhibited a decreased level of cell proliferation (p < 0.05) (Figs. 2Q, 2R, Appendix Fig.). These observations indicate that the lack of Noggin has a direct impact on the developing upper incisor. As shown in Figs. 2P and 2R, 2 separate upper incisor placodes were distinct at E11.5, but began to fuse at the midline at E12.5 of Noggin mutants. While this single upper incisor phenotype might have been an oversight in the previous study (Stottmann et al., 2001), this result was reported in a recent study (Lana-Elola et al., 2011). This formation of a single large incisor phenotype in the Noggin mutant is consistent with the proposed function of BMP signaling in the patterning of incisor placodes (Munne et al., 2010).
We next examined the expression of Shh, a molecular marker for the dental epithelium. Whole-mount in situ hybridization clearly revealed 2 separate upper incisor placodes in the Nog−/− embryo at E11.5 (N = 4; Figs. 3A, 3B), which was confirmed by in situ section hybridization (Figs. 3C, 3D), despite the lower level of expression. At E12.5, these 2 dental placodes began to fuse at the midline (N = 3; Fig. 3F), consistent with morphological observations. Our results appear to contradict those in the studies by Lana-Elola et al. (2011), who reported a single Shh expression domain in the upper-incisor-forming region of Noggin mutants at E11.5. This difference in results may be due to the transient nature of the separate Shh expression domains. The embryos in the other study may have been slightly older, and thus the Shh expression domain had already fused. These observations indicate that, in the absence of Noggin, the upper incisors were initially positioned correctly, but fused subsequently. To support this conclusion, we performed a rescue experiment. We implanted beads soaked with either BSA or Noggin protein close to the forming upper incisors of E11.5 Nog−/− heads that were placed in organ culture. In the samples implanted with BSA beads after 24 hrs in culture, Shh expression had extended from the incisor placodes toward the midline (N = 3; Fig. 3H), indicating the progression of placode fusion. However, this fusion process was prevented by exogenously applied Noggin protein, as evidenced by 2 separate Shh expression domains (N = 3; Fig. 3J). Consistent with the defective upper incisor is the elevation of BMP signaling activity at E12.5, as determined by immunostaining on phosphorylated Smad1/5/8 (pSmad1/5/8), an indicator of BMP-activated Smad signaling (Figs. 3K, 3L). However, the activity of BMP signaling appeared unaltered in the Nog−/− molars, as compared with the controls (Figs. 3M, 3N).
Figure 3.
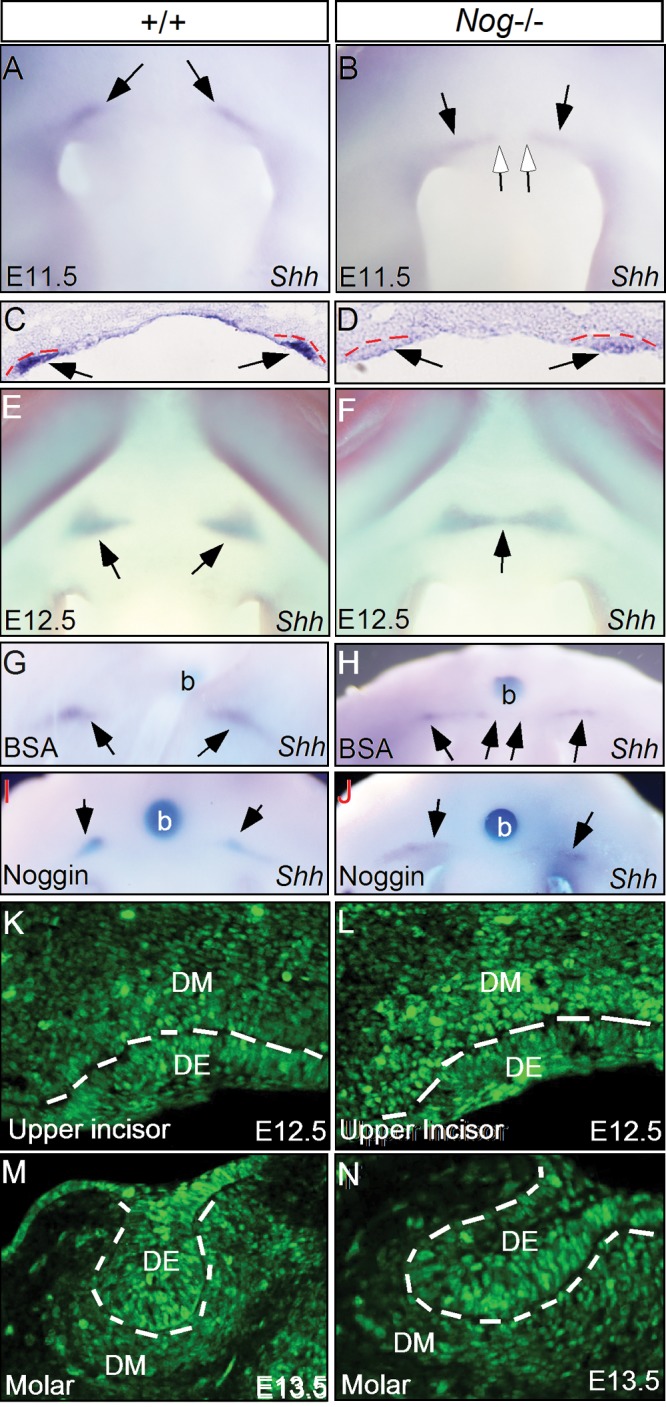
Lack of Noggin causes fusion of upper incisor placodes. (A-F) Shh expression indicates upper incisor placodes at E11.5 and E12.5. At E11.5, 2 separate Shh expression domains were seen in the maxillary region of control (A, C) and Nog mutant (B, D). At E12.5, the 2 separate Shh expression domains remain in the control (E). However, in the mutant, the 2 Shh expression domains have fused (F). Black arrows points to Shh expression domains and open arrows in (B) point to the medial boundary of Shh expression domains. (G) An E11.5 wild type head implanted with a BSA bead shows 2 separate Shh expression domains after 24 hrs in organ culture. (H) An E11.5 Nog−/− head implanted with a BSA bead shows extension of Shh expression from the upper incisor placodes towards the midline after 24 hrs in organ culture. (I) An E11.5 wild type head implanted with a bead soaked with Noggin protein shows 2 separate Shh expression domains after 24 hrs in organ culture. (J) An E11.5 Nog−/− head implanted with a bead soaked with Noggin protein shows wild type like Shh expression domains after 24 hrs in organ culture. Black arrows point to Shh expression domains. (K, L) Immunohistochemical staining shows an enhanced level of pSmad1/5/8 staining in the mesenchyme and epithelium of the Nog−/− upper incisor (L), as compared to the wild type control (K). (M, N) Immunostaining shows comparable level of pSmad1/5/8 in the molar of control (M) and Noggin mutant (N). Dash lines demarcate the dental epithelium. b, bead; DE, dental epithelium; DM, dental mesenchyme.
Expression of Chordin and Gremlin in the Developing Tooth
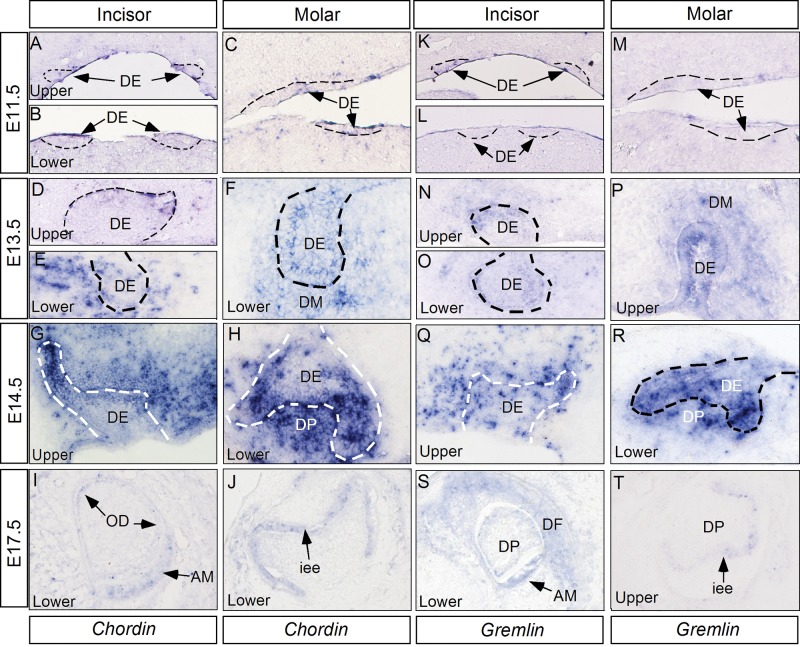
Since Noggin is expressed in the developing molar and lower incisor from the bud stage on (Fig. 1), we wondered why these teeth develop normally in the absence of Noggin. There exist several other secreted BMP antagonists. While these BMP antagonists bind preferentially to selective BMPs and with different affinities, Chordin has been demonstrated to have a redundant role with Noggin in the development of several organs (Bachiller et al., 2000; Stottmann et al., 2001; Anderson et al., 2006). It is possible that other BMP antagonists have redundant functions to compensate for the loss of Noggin in the development of molars and mandibular incisors. To address this possibility, we examined the expression of Chordin and Gremlin, because Chordin and Noggin are classified in the same family of BMP antagonists (Sieber et al., 2009). While Gremlin belongs to the Cerberus/Dan family of modulators for TGF-β/BMP proteins and for Wnt proteins, it also has a role similar to that of Noggin in the inhibition of BMP activity, as demonstrated by the fact that overexpression of Gremlin or Noggin in the chick limbs exhibits similar effects in inhibiting chondrogenesis (Capdevila and Johnson, 1998; Merino et al., 1999). Almost identical expression patterns of both genes were found in the developing teeth (Fig. 4). The expression was barely detectable in all types of tooth germs at E11.5 (Figs. 4A-4C, 4K-4M), but was observed in both the epithelial and mesenchymal components of all tooth germs at E13.5 (Figs. 4D-4F, 4N-4P). At E14.5, the expression remained in the dental epithelium and mesenchyme, particularly in the epithelial tips (Figs. 4G, 4H, 4Q, 4R). The expression became down-regulated at E17.5, but was detectable in the enamel epithelium of both molars and incisors (Figs. 4I, 4J, 4S, 4T).
Figure 4.
Expression of Chordin and Gremlin in the developing tooth. (A-C) Chordin expression is not seen in the tooth germs of upper incisors (A), lower incisors (B), and molars (C) at E11.5. (D-F) Chordin expression becomes detectable in the epithelium of upper incisors (D), and the epithelium and mesenchyme of lower incisors (E) and molars (F) at E13.5. (G, H) At E14.5, strong Chordin expression appears in the dental epithelium and mesenchyme of incisors (G) and molars (H). (I, J) Weak Chordin expression is observed in the enamel epithelium of incisors (I) and molars (J) at E17.5. A lower level of Chordin expression is also seen in the odontoblasts. (K-M) In situ hybridization failed to detect Gremlin expression in the upper (K) and lower incisors (L), and in molars (M) at E11.5. (N-P) Gremlin expression is detected in the epithelium and mesenchyme of upper (N) and lower incisors (O), as well as molars (P) at E13.5. (Q, R) At E14.5, Gremlin expression remains in the epithelium and mesenchyme of incisors (Q) and molars (R). (S, T) Weak Gremlin expression is detected in the enamel epithelium of both incisors (S) and molars (T). Dashed lines demarcate the dental epithelium. AM, ameloblasts; DE, dental epithelium; DM, dental mesenchyme; DP, dental papilla; OD, odontoblast; iee, inner enamel epithelium.
The strength of BMP activity, which is crucial for biological responses, depends on the concentration of BMP ligands and the availability of receptors and intracellular effectors, and is regulated by extracellular and intracellular modulators. The presence of BMP antagonists is essential for the maintenance of BMP homeostasis. This explains why BMPs and their antagonists are often co-expressed in developing organs or tissues and the existence of a Noggin-BMP autoregulatory loop, as evidenced by the fact that BMP and Noggin induce their mutual expression (Kameda et al., 1999; Nifuji and Noda, 1999; Stottmann et al., 2001; Ashique et al., 2002). Loss of a BMP antagonist in a developing embryo would disrupt the finely tuned BMP activity, leading to aberrant responses of tissues/cells to overactive BMP signaling.
The fact that Noggin is expressed in the developing tooth suggests a role for Noggin in modulating BMP activity during tooth development. However, despite the fact that loss of Noggin causes a series of defects in many organs, the early development of teeth (except the maxillary incisor) appears normal. One possibility is that the developing tooth has a higher tolerance to an enhanced level of BMP activity, but the fusion and an early developmental arrest of the maxillary incisor would argue against it. Alternatively, a plausible interpretation is the functional redundancy by other BMP antagonists. In fact, we showed that both Chordin and Gremlin are co-expressed with Noggin in the developing tooth from the bud stage until at least the E17.5 bell stage. In contrast to Noggin expression in the dental mesenchyme and the maxillary mesenchyme immediately adjacent to the upper incisors at the initiation stage (E11.5), expression of Chordin and Gremlin was detected neither in the developing tooth nor in the surrounding tissues at this stage. Noggin expression appears to have a direct impact on upper incisor development by regulating BMP activity. The absence of Noggin leads to overactive BMP signaling, which may induce an odontogenic fate in the epithelium between the dental placodes, as manifested by ectopic Shh expression. Meanwhile, the increased cell proliferation rate in the dental epithelium at E11.5 could cause encroachment of dental epithelium on the midline. These events together would contribute to the fusion phenotype. The application of exogenous Noggin to the mutants could have reversed these altered molecular and cellular events in the tissues between the placodes, leading to the increased spacing between the incisors. The expression of Chordin and Gremlin in the developing tooth from the bud stage could compensate for the absence of Noggin, allowing for normal development of molars and lower incisors in Nog−/− mice. The lack of potentially functional compensation from Chordin and Gremlin at the tooth initiation stage could contribute to the defectively developed maxillary incisor in Nog−/− mice, providing additional evidence for a requirement of BMP homeostasis in tooth development. Noggin is not required for the initial positioning of the upper incisor placodes, but is essential for the maintenance of their position and subsequent development. Taken together, our results support the idea that Noggin, Chordin, and Gremlin have functionally redundant roles in mouse tooth development.
Acknowledgments
We thank Dr. John Klingensmith of Duke University for providing Nog+/− mice.
Footnotes
Y.P.C. was funded by the NIH (DE12329 and DE15123). Y.Z. was funded by the Ministry of Science and Technology of China (“973” Program Project: 2010CB944800), the National Natural Science Foundation of China (30771132), and the Ministry of Education of China (20093503110001). X.H. was funded by the National Natural Science Foundation of China (81100730).
The authors declare no conflicts of interest.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Anderson RM, Stottmann RW, Choi M, Klingensmith J. (2006). Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev Dyn 235:2507-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. 2004. Epithelial BmprIa regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131:2257-2268 [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. (2002). Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development 129:4647-4660 [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, et al. (2000). The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403:658-661 [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. (1998). Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Nature 280:1455-1457 [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. (1998). Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol 197:205-217 [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. (1996). Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122:3035-3044 [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Canalis E. (2006). Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord 7:51-65 [DOI] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, Guo D, Yang W, Harris MA, Lichtler A, Kream B, et al. (2010). New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 46:1533-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W, et al. (2002). Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420:636-642 [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, et al. (2010). Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol 347:109-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda T, Koike C, Saitoh K, Kuroiwa A, Iba H. (1999). Developmental patterning in chondrocytic cultures by morphogenetic gradients: BMP induces expression of Indian hedgehog and noggin. Genes Cells 4:175-184 [DOI] [PubMed] [Google Scholar]
- Lana-Elola E, Tylzanowski P, Takatalo M, Alakurtti K, Veistinen L, Mitsiadis TA, et al. (2011). Noggin null allele mice exhibit a microform of holoprosencephaly. Hum Mol Genet 20:4005-4015 [DOI] [PubMed] [Google Scholar]
- Li L, Lin M, Wang Y, Cserjesi P, Chen Z, Chen Y. (2011). BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev Biol 349:451-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, et al. (2005). Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132:1453-1461 [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. (1998). Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12:1438-1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R, Rodriguez-Leon J, Macias D, Gañan Y, Economides AN, Hurle JM. (1999). The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 126:5515-5522 [DOI] [PubMed] [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, Suomalainen M, Thesleff I, Jernvall J. (2010). Splitting placodes: effects of bone morphogenetic protein and Activin on the patterning and identity of mouse incisors. Evol Dev 12:383-392 [DOI] [PubMed] [Google Scholar]
- Neubüser A, Peters H, Ballings R, Martin GR. (1997). Antagonistic interactions between FGF and BMP4 signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90:247-255 [DOI] [PubMed] [Google Scholar]
- Nifuji A, Noda M. (1999). Coordinated expression of noggin and bone morphogenetic proteins (BMPs) during early skeletogenesis and induction of noggin expression by BMP-7. J Bone Miner Res 14:2057-2066 [DOI] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. (2009). Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20:343-355 [DOI] [PubMed] [Google Scholar]
- St. Amand TR, Zhang YD, Semina EV, Zhao X, Hu YP, Nguyen L, et al. (2000). Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol 217:323-332 [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. (2001). The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol 240:457-473 [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. (1998). Transformation of tooth type induced by inhibition of BMP signaling. Science 282:1136-1138 [DOI] [PubMed] [Google Scholar]
- Yao S, Prpic V, Pan F, Wise GE. (2010). TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle—implications for tooth eruption. Connect Tissue Res 51:59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, et al. (2000). A new function of BMP4: dual role of BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127:1431-1443 [DOI] [PubMed] [Google Scholar]
- Zhang YD, Chen Z, Song Y, Liu C, Chen YP. (2005). Making a tooth: growth factors, transcription factors, and stem cells. Cell Res 15:301-316 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. (2009). Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science 323:1232-1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, Hu Y, et al. (2000). Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech Dev 99:29-38 [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. (1996). The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86:599-606 [DOI] [PubMed] [Google Scholar]