Abstract
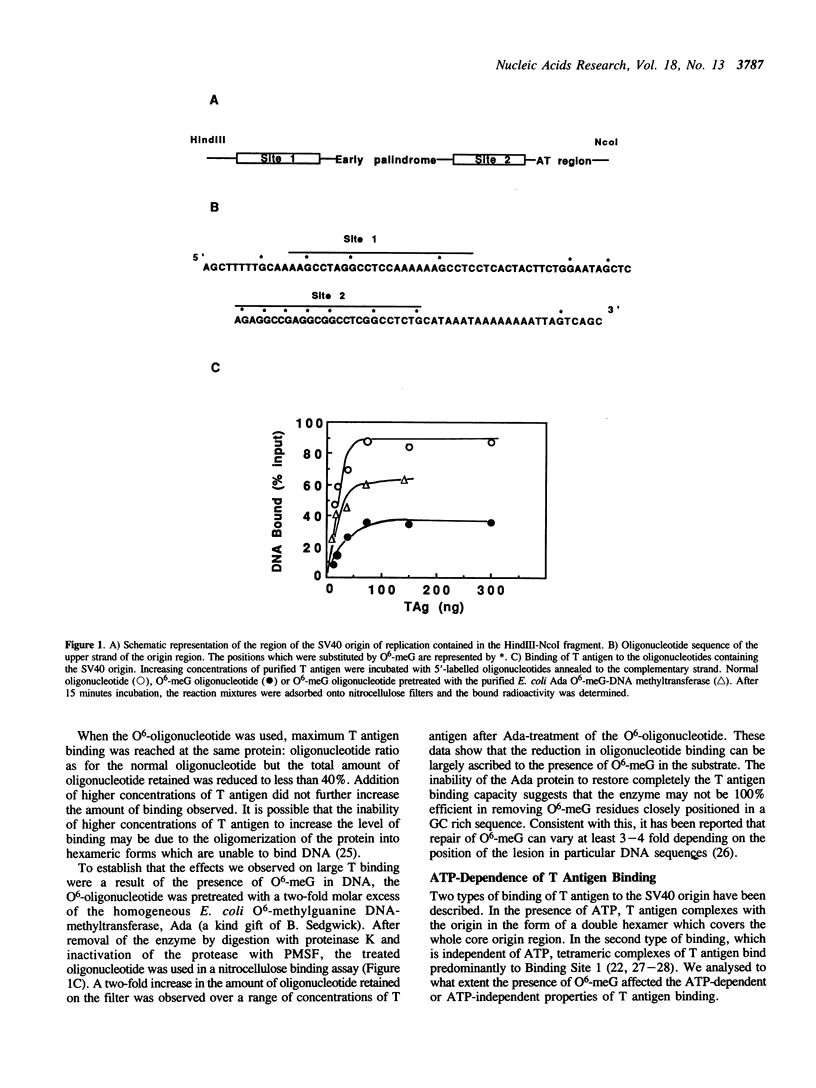
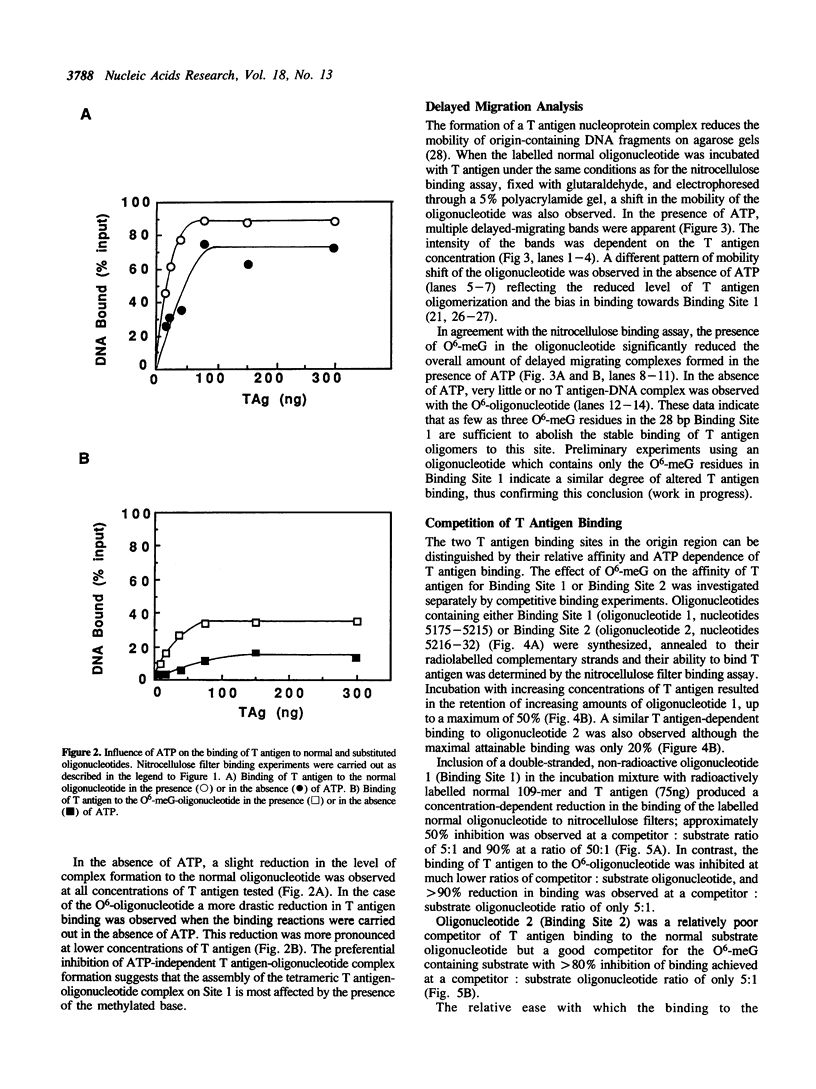
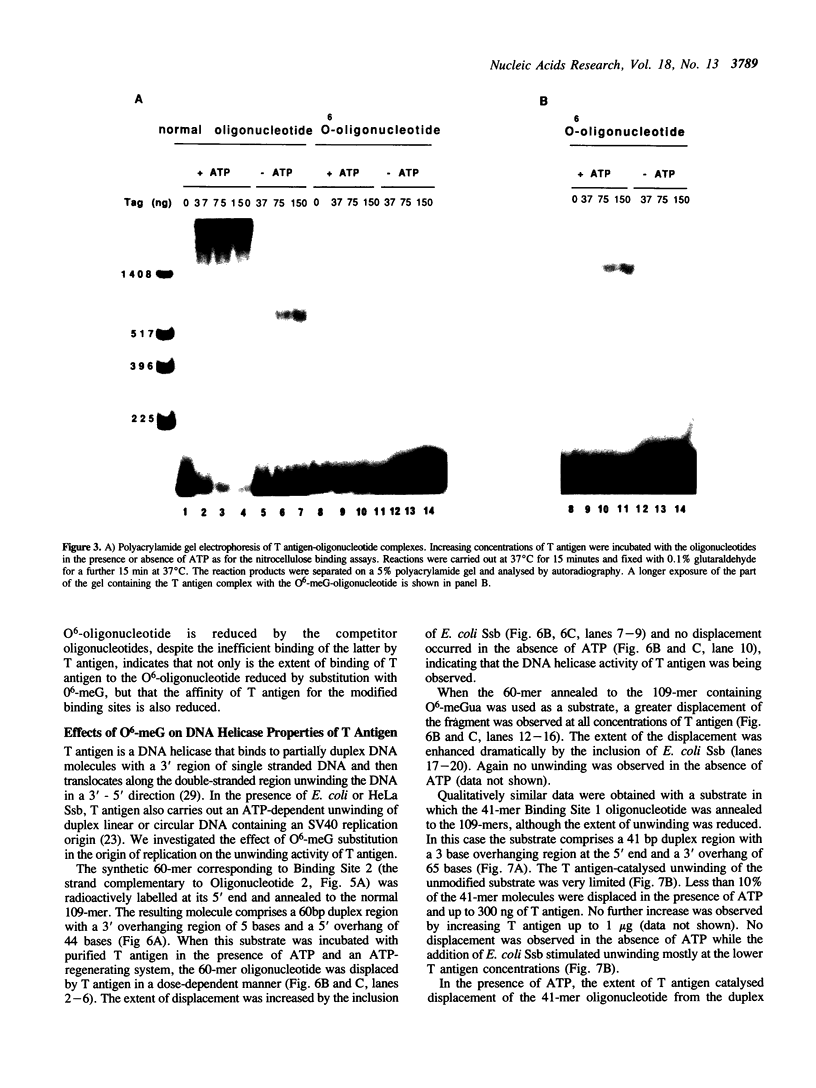
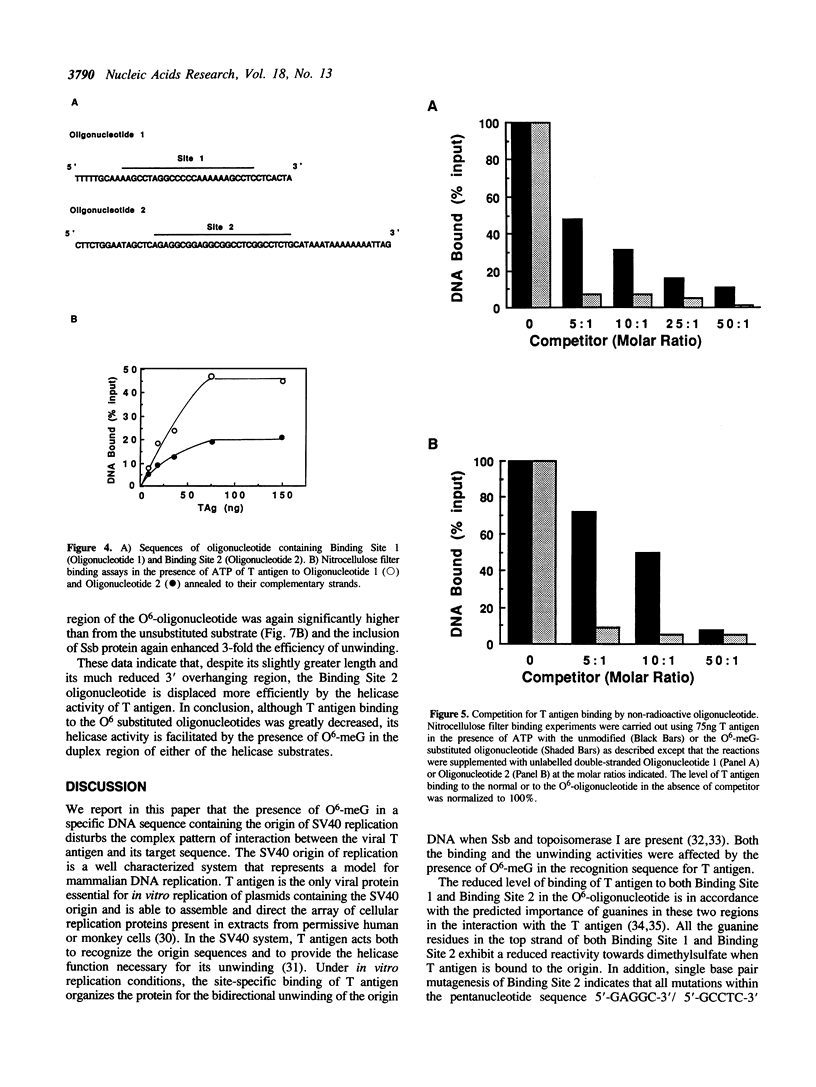
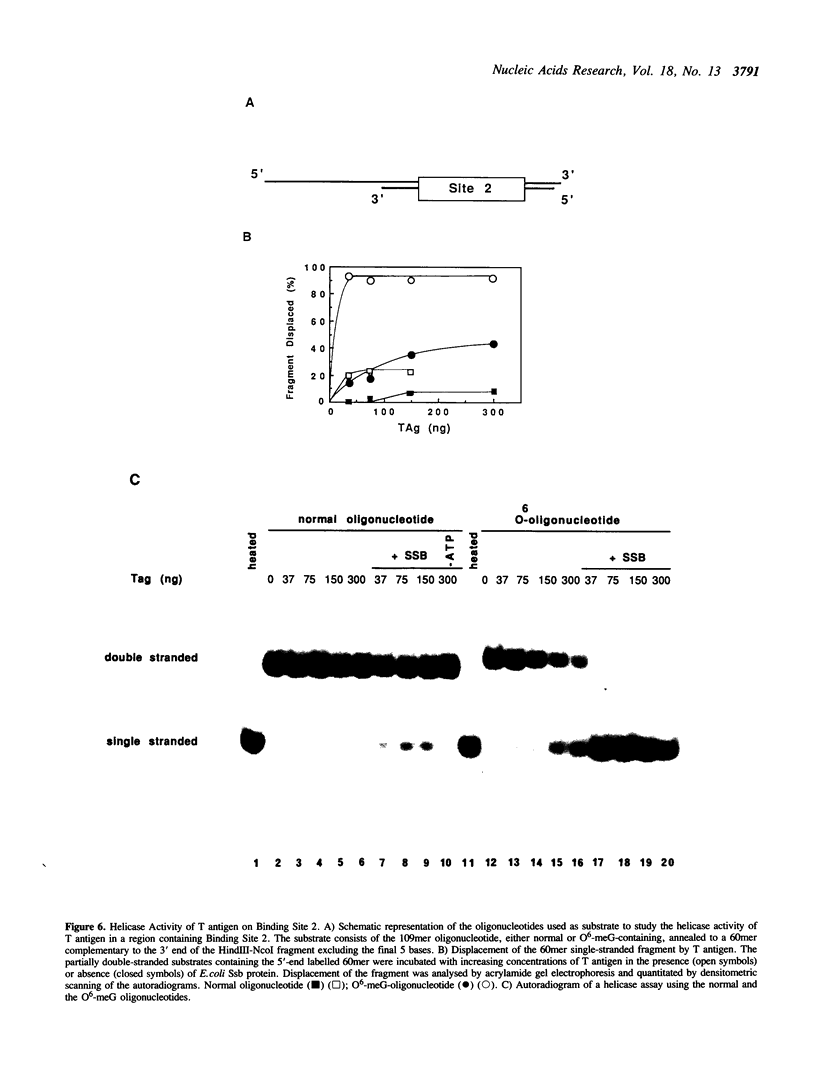
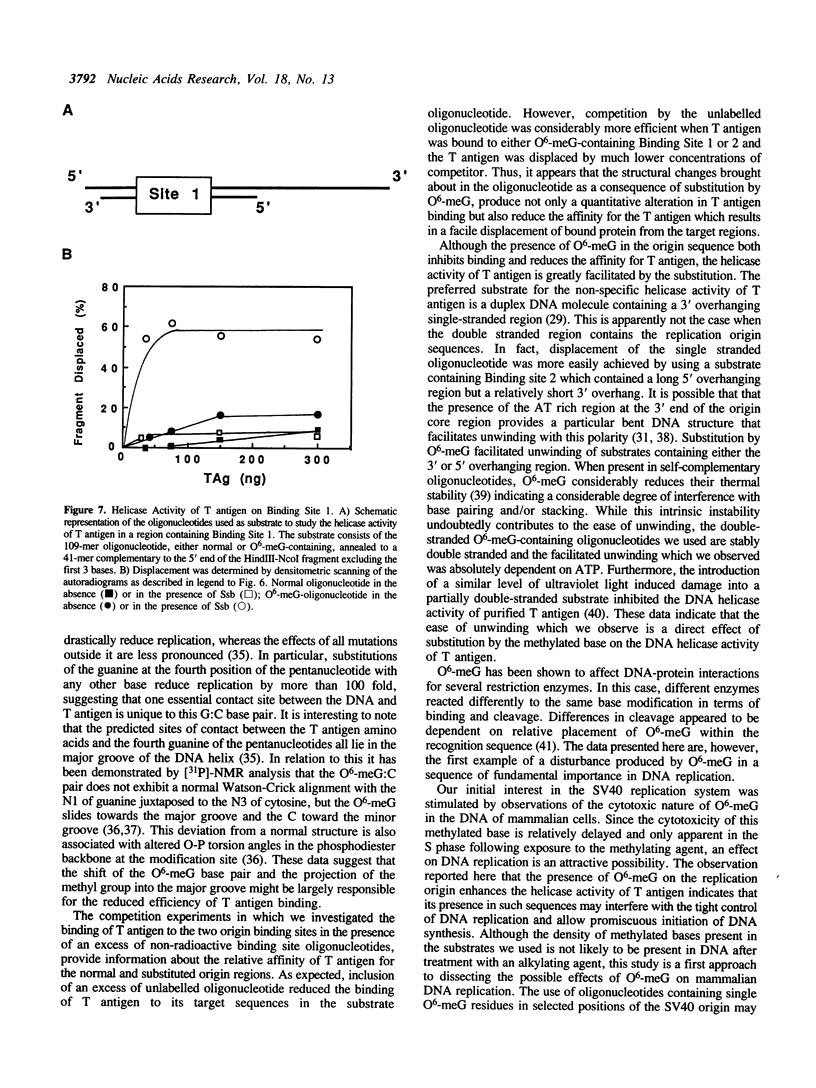
To study the effect of the potentially cytotoxic base O6-methylguanine (O6-meG) on the initiation of DNA replication, double-stranded oligonucleotides corresponding to the SV40 origin of replication were constructed in which O6-meG replaced guanine in one strand. Out of 14 methylated residues, 10 were present in the Binding sites for T antigen (3 in Binding Site 1 and 7 in Binding Site 2). Binding of purified T antigen to the substituted oligonucleotide was considerably reduced in comparison to the unsubstituted one, as measured by nitrocellulose filter binding. Both the ATP-dependent and ATP-independent binding of T antigen were affected by the presence of the methylated base. Band shift analysis revealed an altered pattern of delayed-migrating complexes of T antigen with the O6-meG-containing oligonucleotide. Competition experiments, in which unmodified oligonucleotides containing Binding Site 1 or 2 were included in the binding assays, indicated that the affinity of T antigen for the O6-meG modified sites was reduced. When partially duplex oligonucleotides containing either Binding Site 1 or Site 2 of the origin of replication were used as substrates for the helicase activity of T antigen, the presence of O6-meG increased the extent of T antigen catalysed displacement of single-stranded DNA fragments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquilina G., Frosina G., Zijno A., Di Muccio A., Dogliotti E., Abbondandolo A., Bignami M. Isolation of clones displaying enhanced resistance to methylating agents in O6-methylguanine-DNA methyltransferase-proficient CHO cells. Carcinogenesis. 1988 Jul;9(7):1217–1222. doi: 10.1093/carcin/9.7.1217. [DOI] [PubMed] [Google Scholar]
- Aquilina G., Zijno A., Moscufo N., Dogliotti E., Bignami M. Tolerance to methylnitrosourea-induced DNA damage is associated with 6-thioguanine resistance in CHO cells. Carcinogenesis. 1989 Jul;10(7):1219–1223. doi: 10.1093/carcin/10.7.1219. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. ATP stimulates the binding of simian virus 40 (SV40) large tumor antigen to the SV40 origin of replication. Proc Natl Acad Sci U S A. 1988 Jan;85(1):64–68. doi: 10.1073/pnas.85.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Dodson M., Echols H., Hurwitz J. ATP-dependent formation of a specialized nucleoprotein structure by simian virus 40 (SV40) large tumor antigen at the SV40 replication origin. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8981–8985. doi: 10.1073/pnas.84.24.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Baur C. P., Koff A., Tegtmeyer P. Domain structure of the simian virus 40 core origin of replication. Mol Cell Biol. 1986 May;6(5):1663–1670. doi: 10.1128/mcb.6.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Tsui S., Koff A., DeLucia A. L., Parsons R., Tegtmeyer P. The T-antigen-binding domain of the simian virus 40 core origin of replication. J Virol. 1987 Jul;61(7):2143–2149. doi: 10.1128/jvi.61.7.2143-2149.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., Dean F. B., Bullock P., Echols H., Hurwitz J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science. 1987 Nov 13;238(4829):964–967. doi: 10.1126/science.2823389. [DOI] [PubMed] [Google Scholar]
- Ellison K. S., Dogliotti E., Connors T. D., Basu A. K., Essigmann J. M. Site-specific mutagenesis by O6-alkylguanines located in the chromosomes of mammalian cells: influence of the mammalian O6-alkylguanine-DNA alkyltransferase. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8620–8624. doi: 10.1073/pnas.86.22.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. L., Jones R. A. Thermodynamic comparison of the base pairs formed by the carcinogenic lesion O6-methylguanine with reference both to Watson-Crick pairs and to mismatched pairs. Biochemistry. 1989 Jul 11;28(14):5881–5889. doi: 10.1021/bi00440a026. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R., Hughes M. Characterization of a CHO variant in respect to alkylating agent-induced biological effects and DNA repair. Mutat Res. 1987 Sep;184(2):139–146. doi: 10.1016/0167-8817(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Gough G., Wood R. D. Inhibition of in vitro SV40 DNA replication by ultraviolet light. Mutat Res. 1989 Nov;227(3):193–197. doi: 10.1016/0165-7992(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Green M. H., Lowe J. E., Petit-Frère C., Karran P., Hall J., Kataoka H. Properties of N-methyl-N-nitrosourea-resistant, Mex- derivatives of an SV40-immortalized human fibroblast cell line. Carcinogenesis. 1989 May;10(5):893–898. doi: 10.1093/carcin/10.5.893. [DOI] [PubMed] [Google Scholar]
- Hill-Perkins M., Jones M. D., Karran P. Site-specific mutagenesis in vivo by single methylated or deaminated purine bases. Mutat Res. 1986 Sep;162(2):153–163. doi: 10.1016/0027-5107(86)90081-3. [DOI] [PubMed] [Google Scholar]
- Ishida R., Takahashi T. N-methyl-N'-nitro-N-nitrosoguanidine-resistant HeLa S3 cells still have little O6-methylguanine-DNA methyltransferase activity and are hypermutable by alkylating agents. Carcinogenesis. 1987 Aug;8(8):1109–1113. doi: 10.1093/carcin/8.8.1109. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Hall J., Karran P. Complementation of sensitivity to alkylating agents in Escherichia coli and Chinese hamster ovary cells by expression of a cloned bacterial DNA repair gene. EMBO J. 1986 Dec 1;5(12):3195–3200. doi: 10.1002/j.1460-2075.1986.tb04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson K., Sahm J., Shenkar R., Strauss B. Methylation-induced blocks to in vitro DNA replication. Mutat Res. 1985 Jun-Jul;150(1-2):77–84. doi: 10.1016/0027-5107(85)90103-4. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Miller J. H., Calos M. P. Determination of DNA sequence changes induced by ethyl methanesulfonate in human cells, using a shuttle vector system. Mol Cell Biol. 1986 May;6(5):1838–1842. doi: 10.1128/mcb.6.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Maybaum J., Bainnson A. N., Roethel W. M., Ajmera S., Iwaniec L. M., TerBush D. R., Kroll J. J. Effects of incorporation of 6-thioguanine into SV40 DNA. Mol Pharmacol. 1987 Nov;32(5):606–614. [PubMed] [Google Scholar]
- Maybaum J., Mandel H. G. Unilateral chromatid damage: a new basis for 6-thioguanine cytotoxicity. Cancer Res. 1983 Aug;43(8):3852–3856. [PubMed] [Google Scholar]
- Parsons R., Anderson M. E., Tegtmeyer P. Three domains in the simian virus 40 core origin orchestrate the binding, melting, and DNA helicase activities of T antigen. J Virol. 1990 Feb;64(2):509–518. doi: 10.1128/jvi.64.2.509-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Kozlowski S. A., Gaffney B. L., Jones R. A. Structural studies of the O6meG.C interaction in the d(C-G-C-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986 Mar 11;25(5):1027–1036. doi: 10.1021/bi00353a012. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero D. A., Meyer S. A., Clatterbuck B. E., Mattern M. R., Ziolkowski C. H., Day R. S., 3rd Sensitivity of human cell strains having different abilities to repair O6-methylguanine in DNA to inactivation by alkylating agents including chloroethylnitrosoureas. Cancer Res. 1984 Jun;44(6):2467–2474. [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Lewton B. A., DeLucia A. L., Wilson V. G., Ryder K. Topography of simian virus 40 A protein-DNA complexes: arrangement of protein bound to the origin of replication. J Virol. 1983 Apr;46(1):151–161. doi: 10.1128/jvi.46.1.151-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M. D., Eadie J. S., Conrad M. O6-methylguanine mutation and repair is nonuniform. Selection for DNA most interactive with O6-methylguanine. J Biol Chem. 1986 Jul 25;261(21):9879–9885. [PubMed] [Google Scholar]
- Voigt J. M., Topal M. D. O6-methylguanine in place of guanine causes asymmetric single-strand cleavage of DNA by some restriction enzymes. Biochemistry. 1990 Feb 13;29(6):1632–1637. doi: 10.1021/bi00458a039. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Schwarz M. W., Stahl H. Simian virus 40 large T antigen DNA helicase. Characterization of the ATPase-dependent DNA unwinding activity and its substrate requirements. J Biol Chem. 1988 Jan 5;263(1):436–442. [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]