Abstract
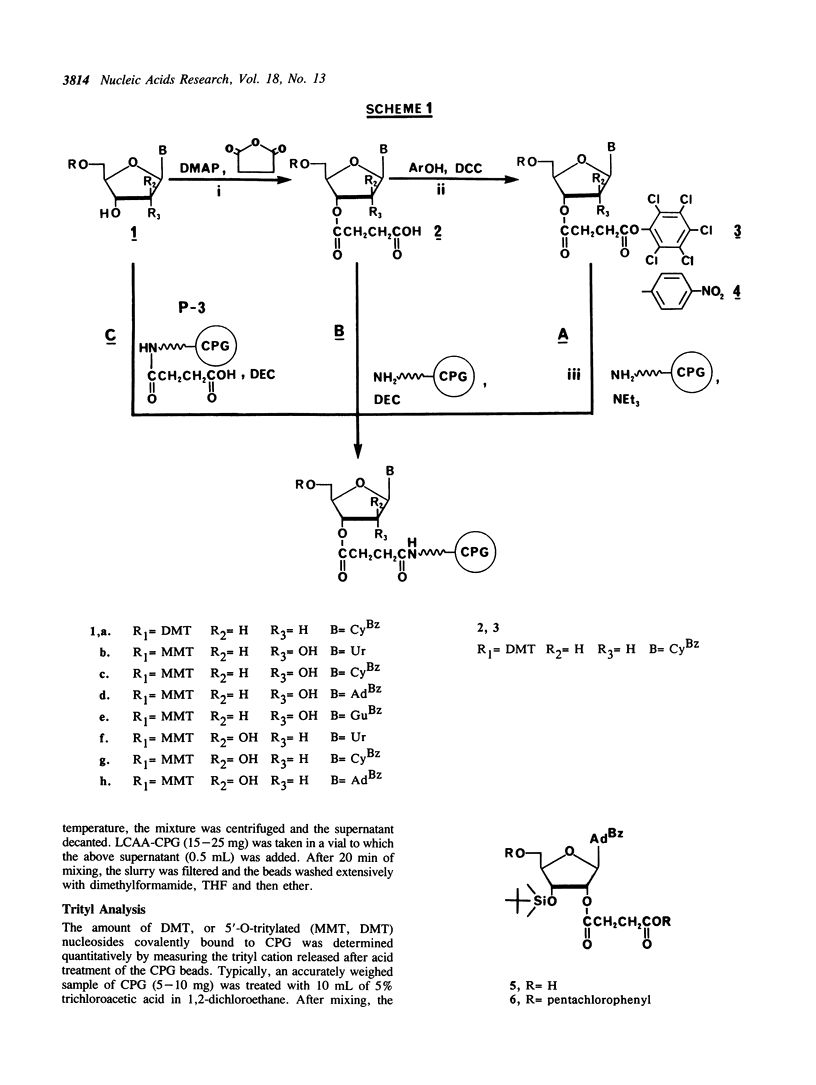
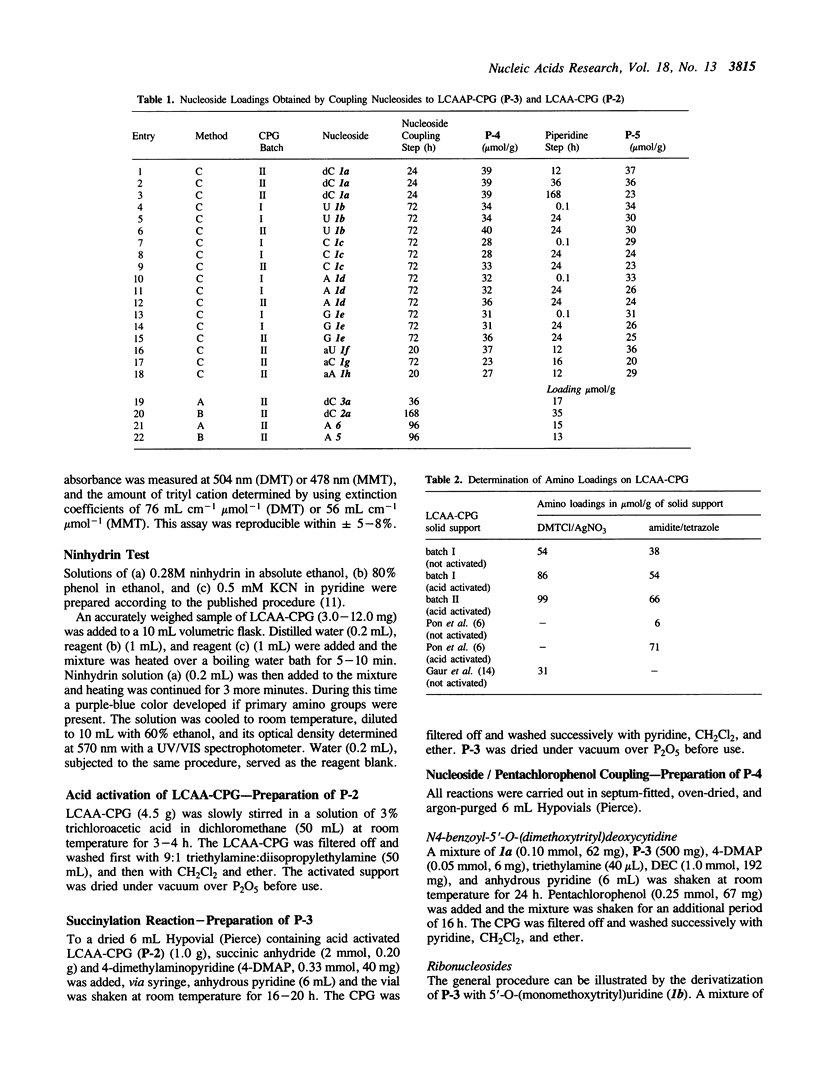
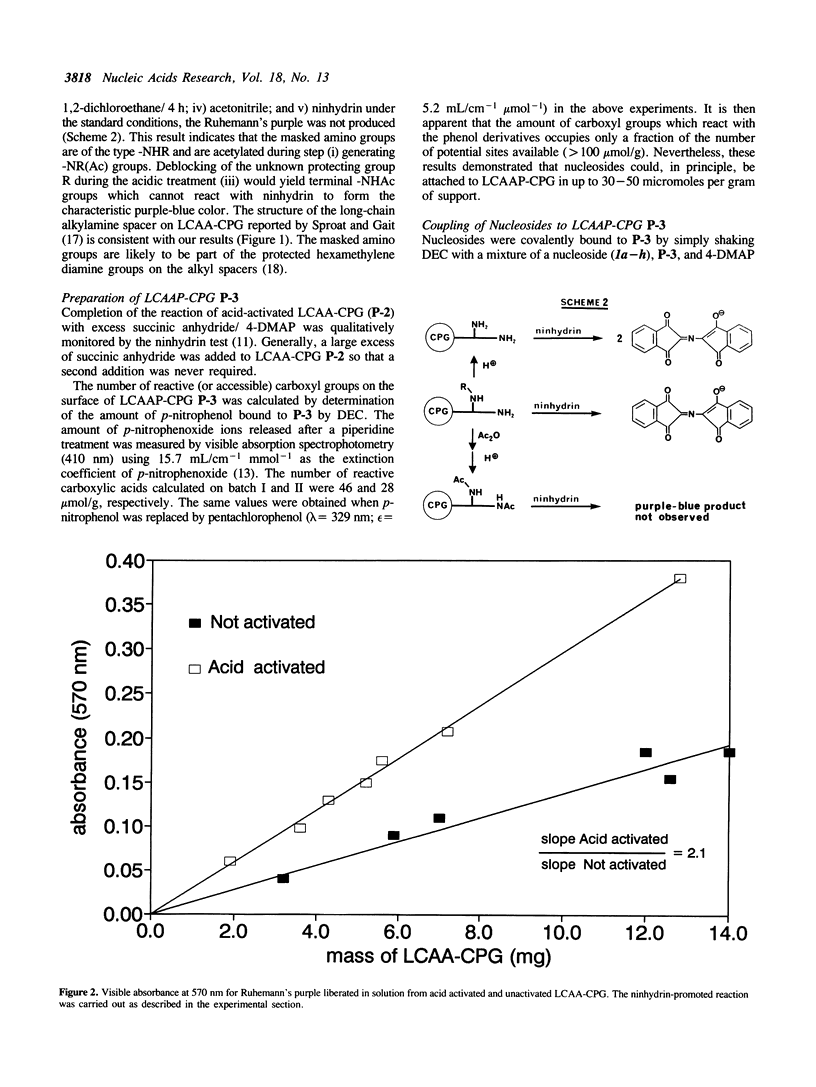
A simplified and economical method for the attachment of 2'-deoxyribo, ribo and arabinonucleosides onto long-chain alkylamidopropanoic acid controlled-pore glass (LCAAP-CPG, P-3) is described. In this procedure, 5'-O-tritylated nucleosides are coupled directly to LCAAP-CPG in excellent yields using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (DEC) as coupling reagent. The conventional and time-consuming preparation of nucleoside-3'-O-succinates is no longer required.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon R. T., Usman N., Ogilvie K. K. Derivatization of controlled pore glass beads for solid phase oligonucleotide synthesis. Biotechniques. 1988 Sep;6(8):768–775. [PubMed] [Google Scholar]