Abstract
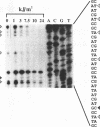
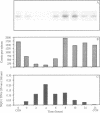
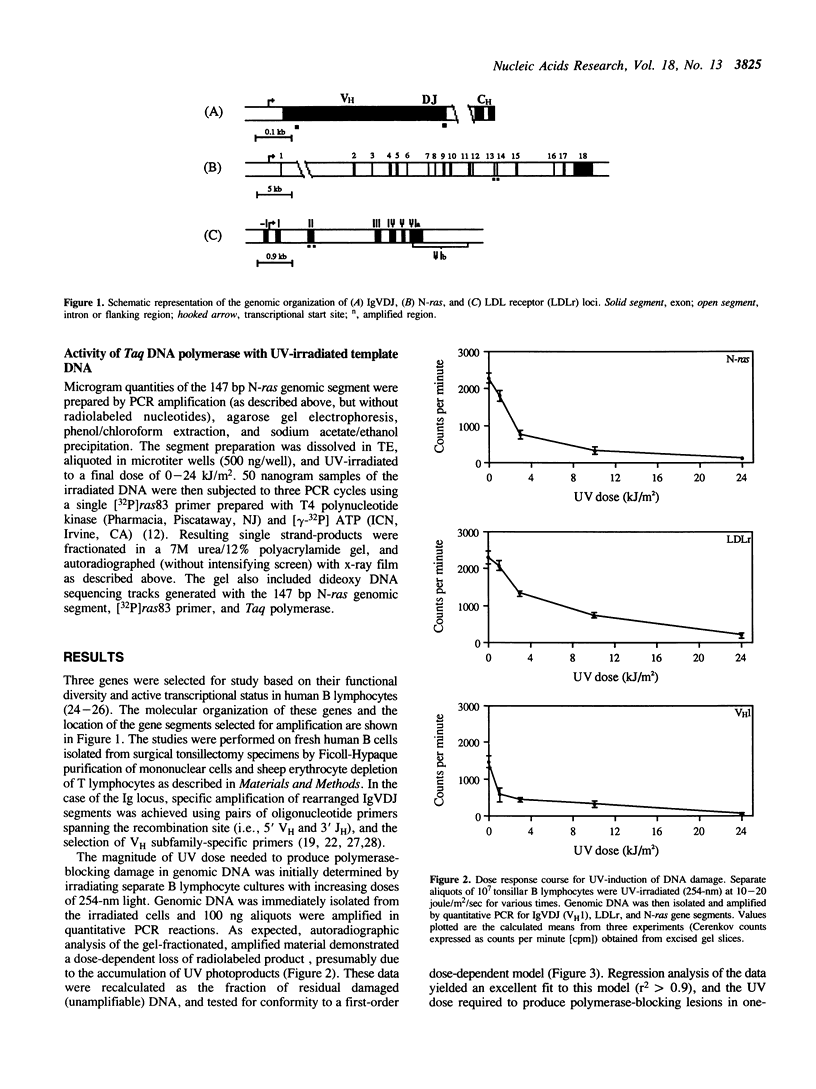
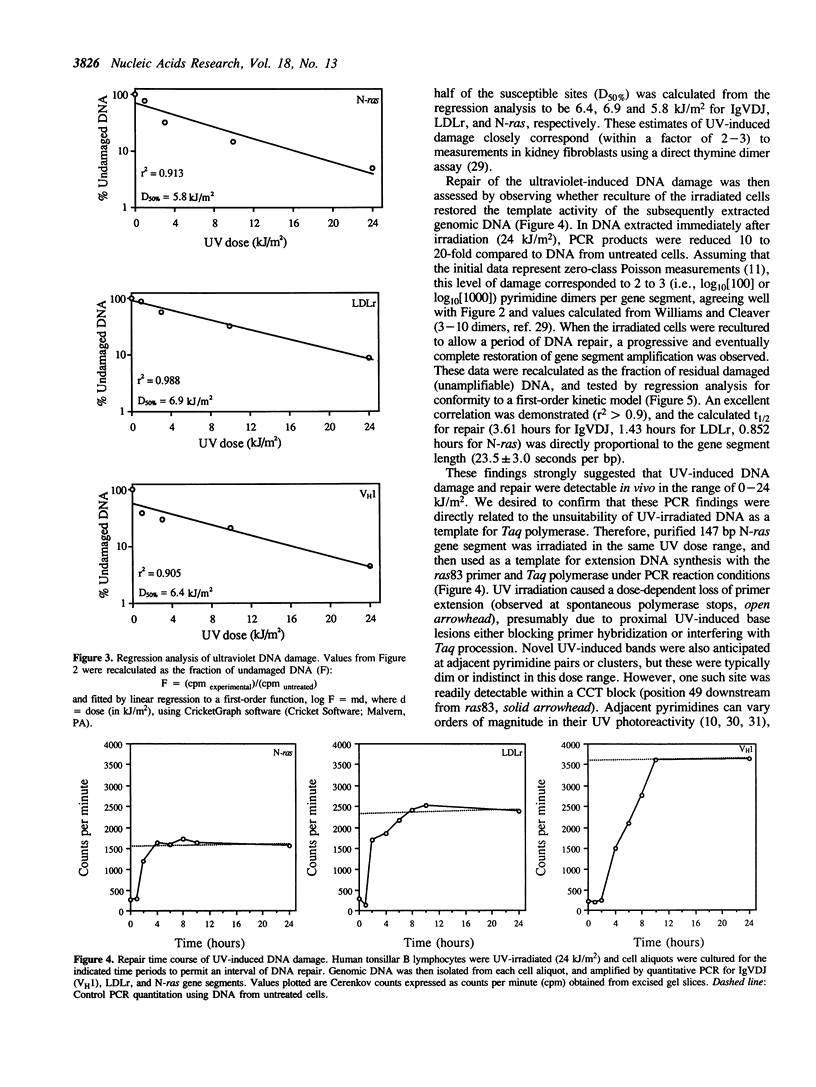
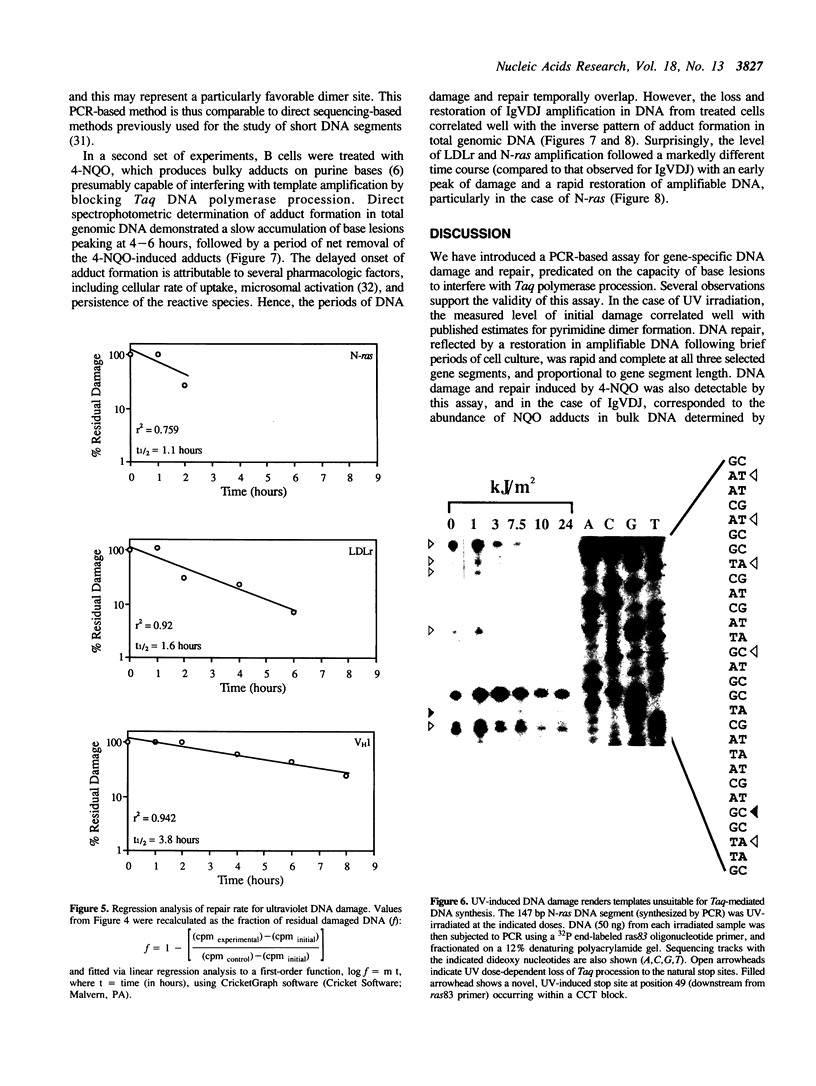
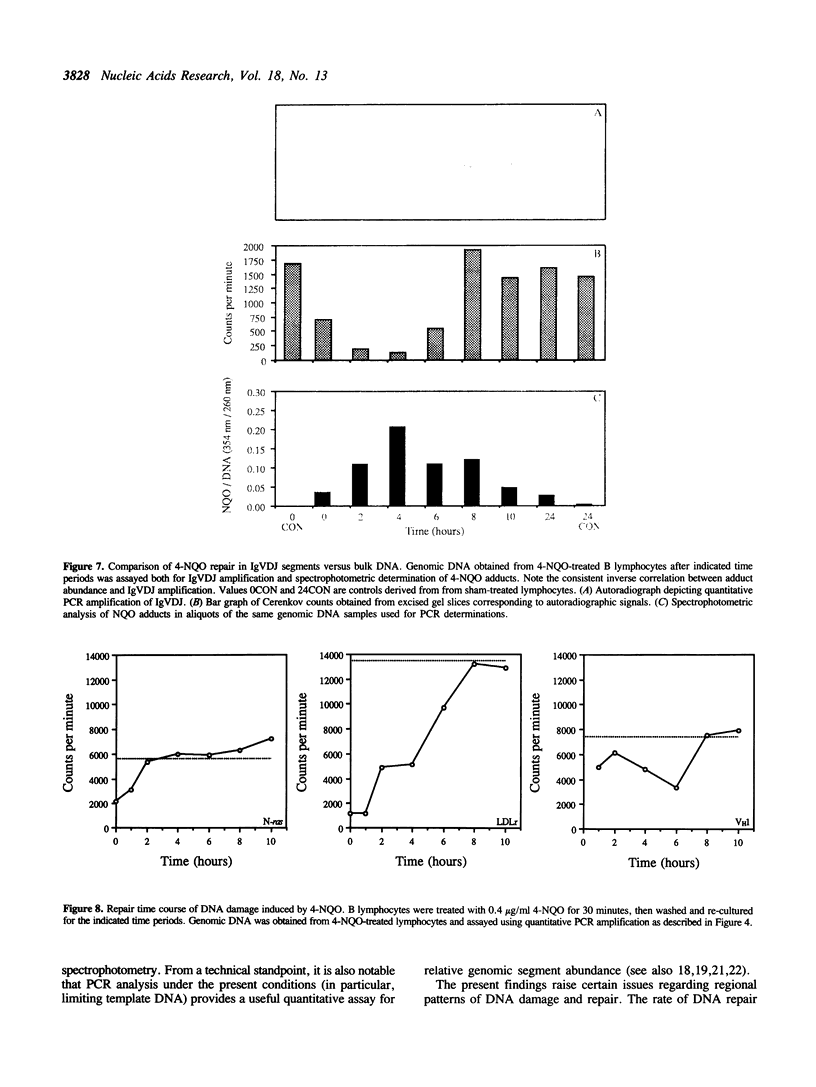
The susceptibility of various genomic regions to DNA damage and repair is heterogeneous. While this can be related to factors such as primary sequence, physical conformation, and functional status, the exact mechanisms involved remain unclear. To more precisely define the key features of a genomic region targeted for these processes, a useful tool would be a method for fine-mapping gene-specific DNA damage and repair in vivo. Here, a polymerase chain reaction-based assay is described for measuring DNA damage and repair in small (less than 500 bp) genomic segments of three transcriptionally active but functionally distinct loci (rearranged immunoglobulin heavy chain variable region [Ig VDJ], low-density lipoprotein receptor gene, and N-ras proto-oncogene) in human tonsillar B lymphocytes. Analysis of ultraviolet (254 nm)-induced DNA damage revealed single-hit kinetics and a similar level of sensitivity (D50% approximately 6000 joule/m2) in all three regions, indicating that a single photoproduct was sufficient to fully block PCR amplification. A similar time period per unit length was required for repair of this DNA damage (average t1/2 per fragment length = 23.5 seconds per bp). DNA damage and repair was also detectable with the base adducting agent, 4-nitroquinoline-1-oxide. However, in this case IgVDJ differed from segments within the other two loci by its relative inaccessibility to alkylation. This assay thus permits high-resolution mapping of DNA damage and repair activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. D., Majors J. An improved method for photofootprinting yeast genes in vivo using Taq polymerase. Nucleic Acids Res. 1989 Jan 11;17(1):171–183. doi: 10.1093/nar/17.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Ho Y. K., Brown M. S., Anderson R. G., Goldstein J. L. Genetics of the low density lipoprotein receptor. Diminished receptor activity in lymphocytes from heterozygotes with familial hypercholesterolemia. J Clin Invest. 1978 Mar;61(3):678–696. doi: 10.1172/JCI108980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G. L., Doetsch P. W., Haseltine W. A. Cyclobutane pyrimidine dimers and (6-4) photoproducts block polymerization by DNA polymerase I. Biochemistry. 1985 Oct 8;24(21):5723–5728. doi: 10.1021/bi00342a006. [DOI] [PubMed] [Google Scholar]
- Chiang Y. L., Sheng-Dong R., Brow M. A., Larrick J. W. Direct cDNA cloning of the rearranged immunoglobulin variable region. Biotechniques. 1989 Apr;7(4):360–366. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Crescenzi M., Seto M., Herzig G. P., Weiss P. D., Griffith R. C., Korsmeyer S. J. Thermostable DNA polymerase chain amplification of t(14;18) chromosome breakpoints and detection of minimal residual disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Cook K. H., Friedberg E. C. The kinetics of thymine dimer excision in ultraviolet-irradiated human cells. Biophys J. 1978 May;22(2):249–264. doi: 10.1016/S0006-3495(78)85487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Frye R. A., Benz C. C., Liu E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene. 1989 Sep;4(9):1153–1157. [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H. Adducts from in vivo action of the carcinogen 4-hydroxyaminoquinoline 1-oxide in rats and from in vitro reaction of 4-acetoxyaminoquinoline 1-oxide with DNA and polynucleotides. Cancer Res. 1985 Feb;45(2):520–525. [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Quantitation of cyclobutane pyrimidine dimer formation in double- and single-stranded DNA fragments of defined sequence. Radiat Res. 1982 Jan;89(1):99–112. [PubMed] [Google Scholar]
- Gorski J., Rollini P., Mach B. Somatic mutations of immunoglobulin variable genes are restricted to the rearranged V gene. Science. 1983 Jun 10;220(4602):1179–1181. doi: 10.1126/science.6857243. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Loss of the normal N-ras allele in a mouse thymic lymphoma induced by a chemical carcinogen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7810–7814. doi: 10.1073/pnas.82.23.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L., Bohr V. A., Hanawalt P. C. Demethylation enhances removal of pyrimidine dimers from the overall genome and from specific DNA sequences in Chinese hamster ovary cells. Mol Cell Biol. 1989 Apr;9(4):1594–1603. doi: 10.1128/mcb.9.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Rapidly occurring DNA excision repair events determine the biological expression of u.v.-induced damage in human cells. Carcinogenesis. 1987 Sep;8(9):1251–1256. doi: 10.1093/carcin/8.9.1251. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Mushinski J. F., Honda M., Ishigatsubo Y., Mountz J. D., Raveche E. S., Steinberg A. D. Oncogene expression in autoimmune and normal peripheral blood mononuclear cells. J Exp Med. 1986 May 1;163(5):1292–1307. doi: 10.1084/jem.163.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Snowden M. M. Differential repair of DNA damage in the human metallothionein gene family. Mol Cell Biol. 1988 Dec;8(12):5331–5338. doi: 10.1128/mcb.8.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. S., Malipiero U. V., Lebecque S. G., Gearhart P. J. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J Exp Med. 1989 Jun 1;169(6):2007–2019. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Bengtström M., Tenhunen J., Söderlund H. Quantification of polymerase chain reaction products by affinity-based hybrid collection. Nucleic Acids Res. 1988 Dec 9;16(23):11327–11338. doi: 10.1093/nar/16.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Th'ng J. P., Walker I. G. Excision repair of DNA in the presence of aphidicolin. Mutat Res. 1986 May;165(3):139–150. doi: 10.1016/0167-8817(86)90048-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Morton A. G., Bohr V. A., Sancar A. General method for quantifying base adducts in specific mammalian genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3723–3727. doi: 10.1073/pnas.85.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]