Abstract
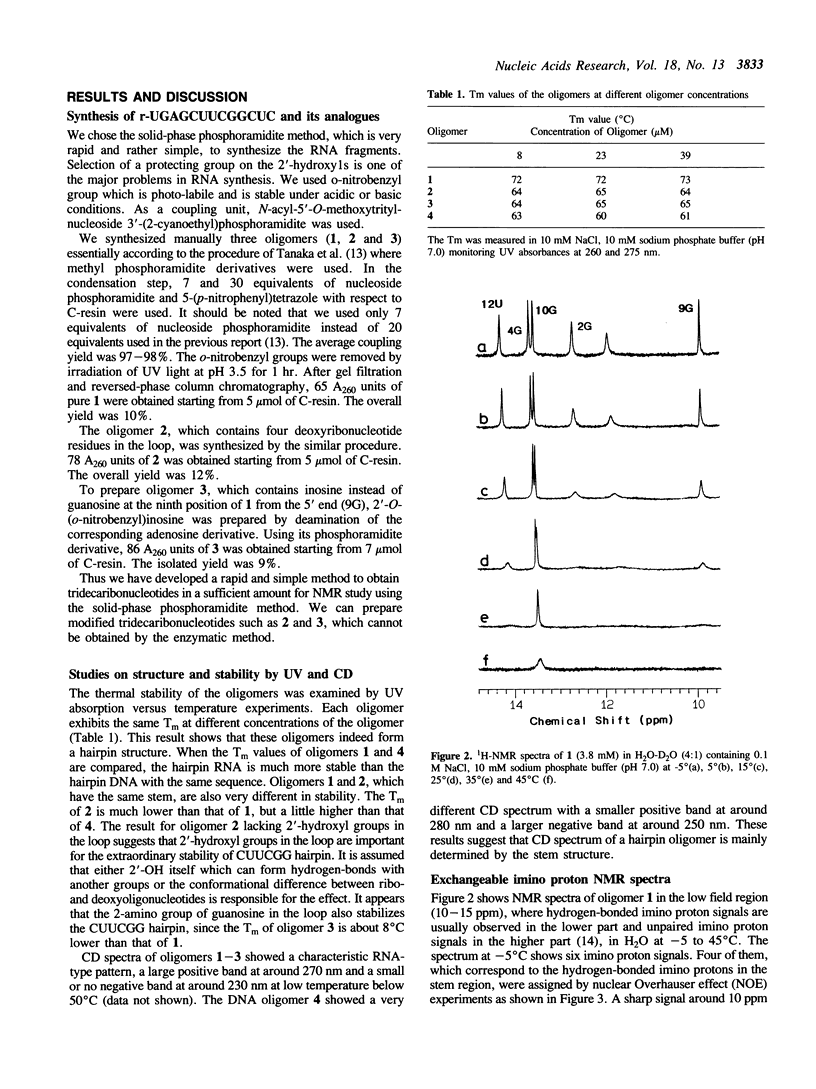
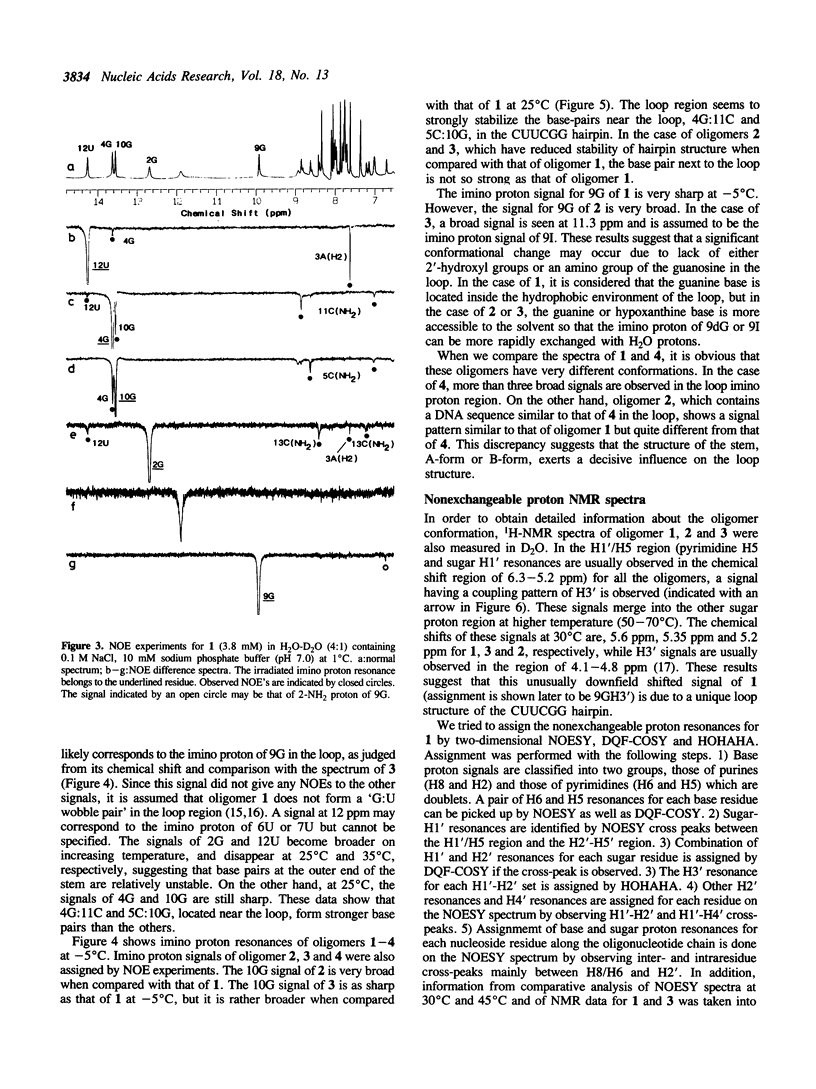
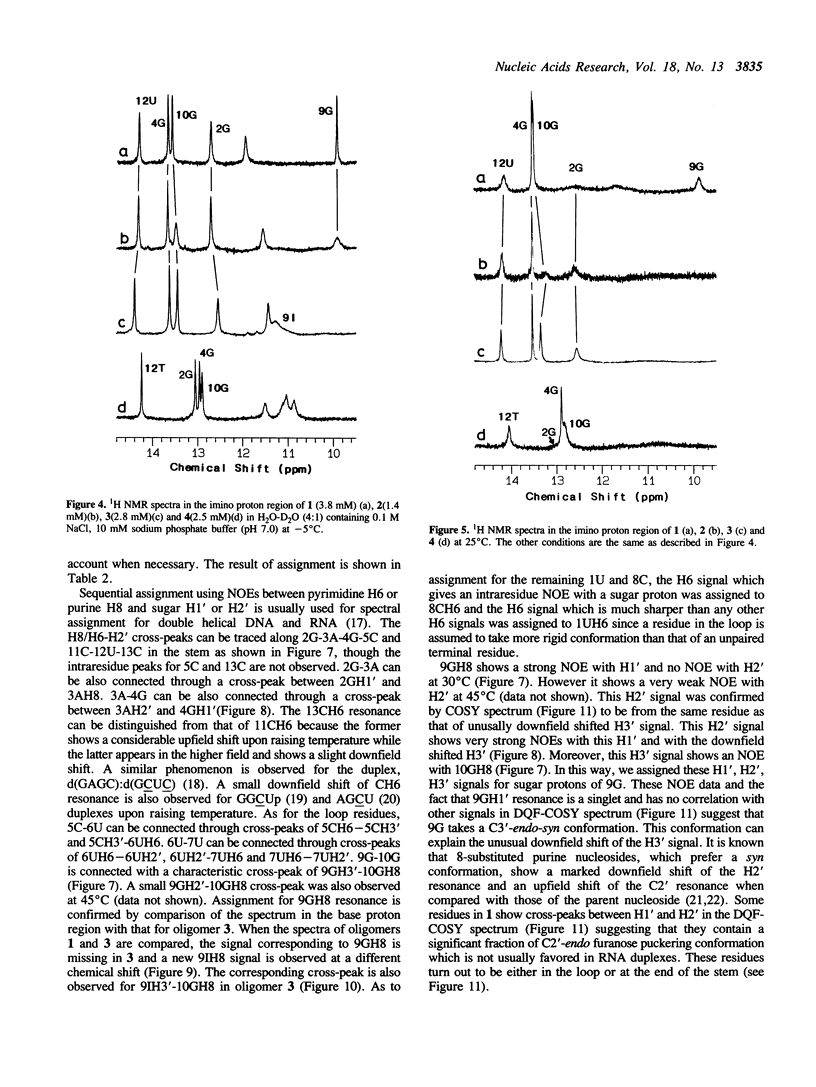
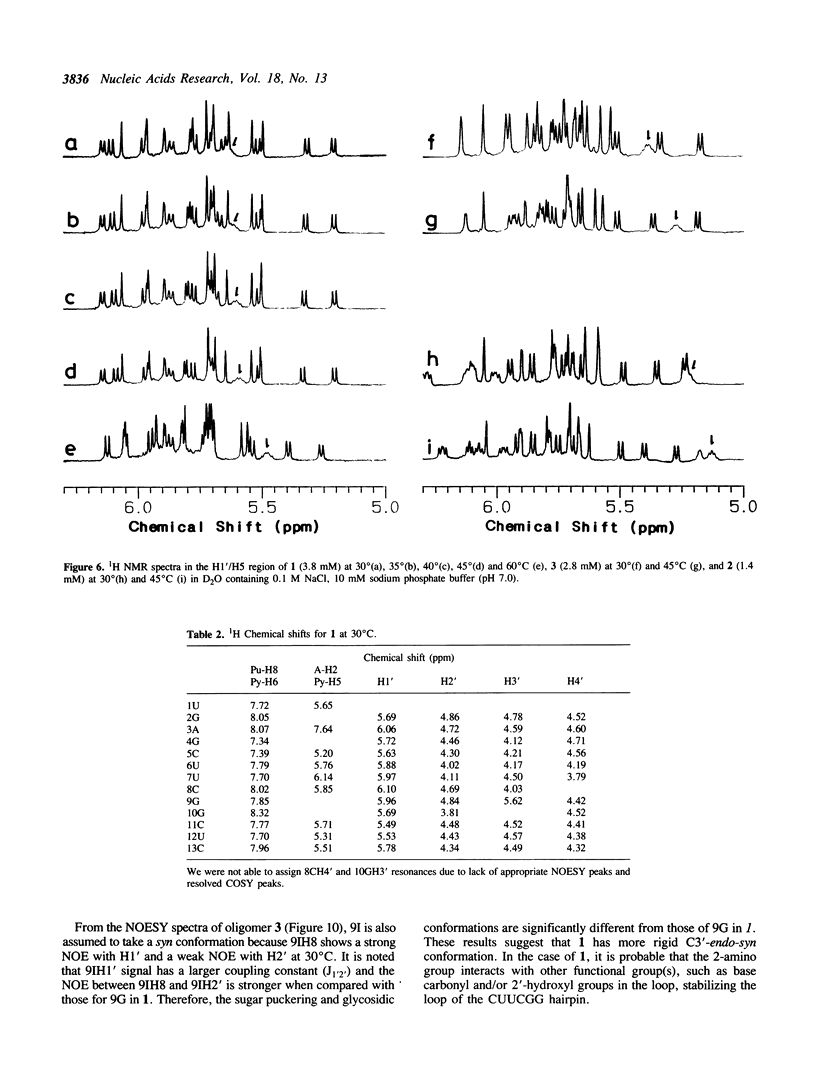
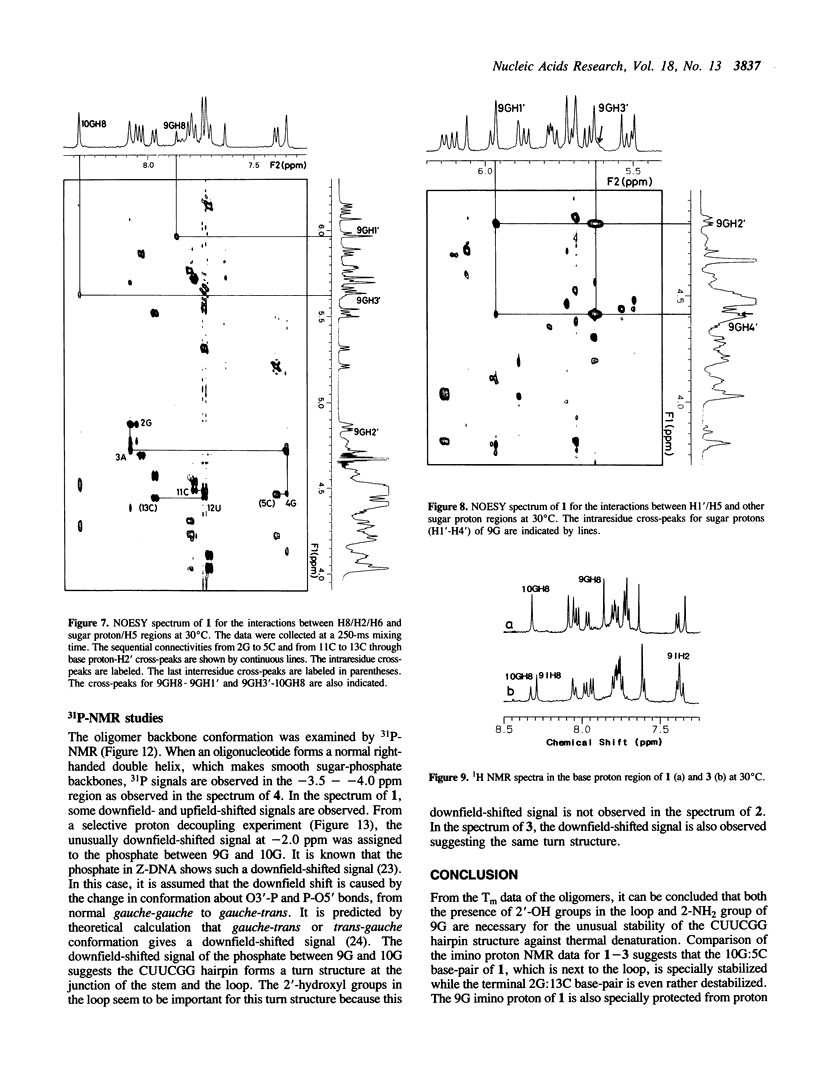
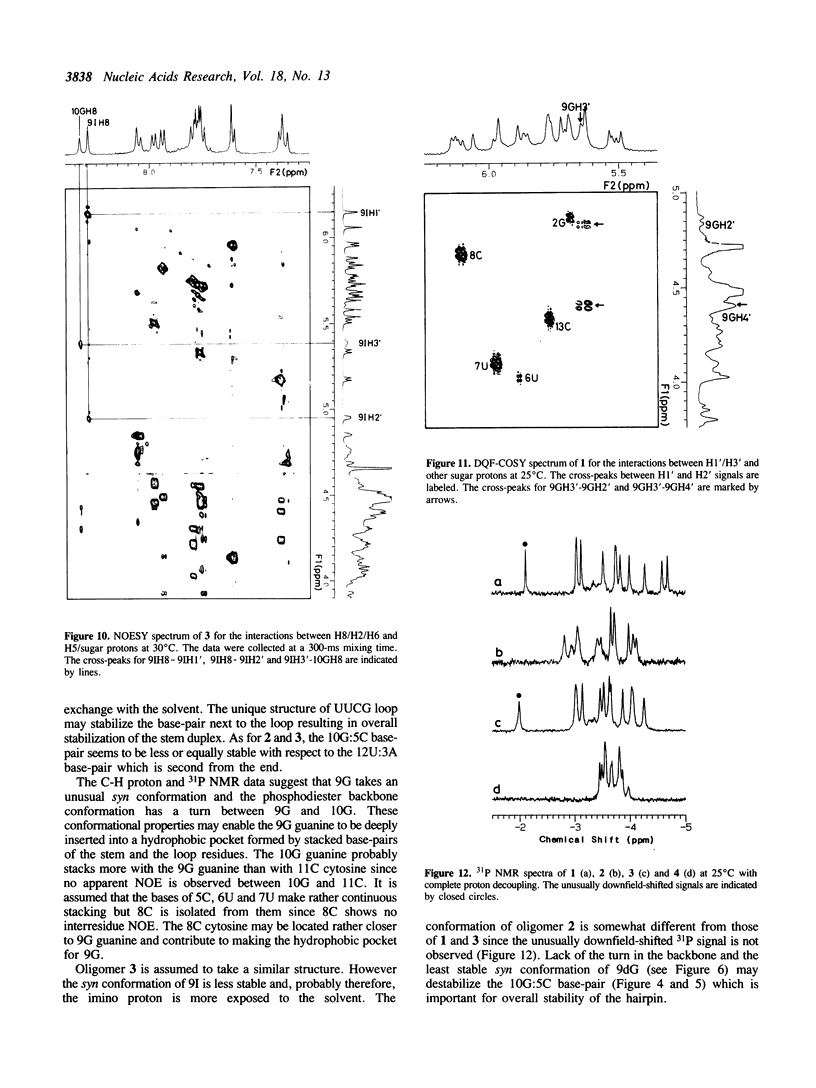
A tridecaribonucleotide, r-UGAGCUUCGGCUC, and two analogues r(UGAGC)d(UUCG)r(GCUC) and r-UGAGCUUCIGCUC, which form a hairpin structure with a four-base-paired stem and a UUCG loop, were synthesized by the solid-phase phosphoramidite method. Properties of these three oligomers and d-TGAGCTTCGGCTC, the DNA analogue, were studied by UV, CD and NMR spectroscopy. The melting temperature (Tm) data suggest that the 2'-hydroxy1 groups and the 2-amino group of guanosine in the loop (9G) stabilize the CUUCGG hairpin which is known to have an unusually high Tm. NMR studies show that this 9G takes a syn conformation and the phosphodiester backbone has a turn at 9G-10G which is a junction of the stem and loop.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bubienko E., Uniack M. A., Borer P. N. Sequence and structure in double-stranded ribonucleic acid: (A-G-C-U)2 and (A-C-G-U)2. Biochemistry. 1981 Nov 24;20(24):6987–6994. doi: 10.1021/bi00527a032. [DOI] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M., McLaughlin L. W. The structure of the double-stranded RNA pentamer 5'(CACAG) . 5'(CUGUG) determined by nuclear Overhauser enhancement measurements: interproton distance determination and structure refinement on the basis of X-ray coordinates. Eur J Biochem. 1985 Aug 15;151(1):153–165. doi: 10.1111/j.1432-1033.1985.tb09080.x. [DOI] [PubMed] [Google Scholar]
- Dock-Bregeon A. C., Chevrier B., Podjarny A., Moras D., deBear J. S., Gough G. R., Gilham P. T., Johnson J. E. High resolution structure of the RNA duplex [U(U-A)6A]2. Nature. 1988 Sep 22;335(6188):375–378. doi: 10.1038/335375a0. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Ribas Prado F., Cheng D. M., Iuorno V., Ts'o P. O. Contributions of the PO ester and CO torsion angles of the phosphate group to 31P-nuclear magnetic shielding constant in nucleic acids: theoretical and experimental study of model compounds. Biopolymers. 1984 Feb;23(2):377–388. doi: 10.1002/bip.360230215. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Luxon B. A., Goldfield E. M., Lai K., Vegeais D. Phosphorus-31 nuclear magnetic resonance of double- and triple-helical nucleic acids. Phosphorus-31 chemical shifts as a probe of phosphorus-oxygen ester bond torsional angles. Biochemistry. 1982 Feb 2;21(3):580–589. doi: 10.1021/bi00532a026. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., de Bruin S. H., Berendsen R. G., Janssen H. G., Binnendijk T. J., Hilbers C. W., van der Marel G. A., van Boom J. H. Structure, kinetics and thermodynamics of DNA hairpin fragments in solution. J Biomol Struct Dyn. 1983 Oct;1(1):115–129. doi: 10.1080/07391102.1983.10507429. [DOI] [PubMed] [Google Scholar]
- Happ C. S., Happ E., Nilges M., Gronenborn A. M., Clore G. M. Refinement of the solution structure of the ribonucleotide 5'r(GCAUGC)2: combined use of nuclear magnetic resonance and restrained molecular dynamics. Biochemistry. 1988 Mar 8;27(5):1735–1743. doi: 10.1021/bi00405a053. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Uesugi S., Yoshida K. Studies on the conformation of purine nucleosides and their 5'-phosphates. Biochemistry. 1972 Feb 29;11(5):830–836. doi: 10.1021/bi00755a023. [DOI] [PubMed] [Google Scholar]
- Kyogoku Y., Watanabe M., Kobayashi Y., Kainosho M., Uesugi S., Nakagawa E., Ohtsuka E., Ikehara M. NMR studies of base-pairing in 15N-labeled ribotetranucleotide GGCUp. Nucleic Acids Symp Ser. 1982;(11):273–276. [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Dahl O. Improved synthesis of (Pri2 N)2POCH2CH2CN. Nucleic Acids Res. 1987 Apr 24;15(8):3626–3626. doi: 10.1093/nar/15.8.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. R. NMR studies on RNA structure and dynamics. Annu Rev Biochem. 1981;50:969–996. doi: 10.1146/annurev.bi.50.070181.004541. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using o-nitrobenzyl protection of 2'-hydroxyl via a phosphite triester approach. Nucleic Acids Res. 1986 Aug 11;14(15):6265–6279. doi: 10.1093/nar/14.15.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using the o-nitrobenzyl group for 2'-hydroxyl protection and H-phosphonate chemistry. Nucleic Acids Res. 1987 Sep 25;15(18):7235–7248. doi: 10.1093/nar/15.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Gauss P., Thermes C., Groebe D. R., Gayle M., Guild N., Stormo G., d'Aubenton-Carafa Y., Uhlenbeck O. C., Tinoco I., Jr CUUCGG hairpins: extraordinarily stable RNA secondary structures associated with various biochemical processes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1364–1368. doi: 10.1073/pnas.85.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi S., Ikehara M. Carbon-13 magnetic resonance spectra of 8-substituted purine nucleosides. Characteristic shifts for the syn conformation. J Am Chem Soc. 1977 May 11;99(10):3250–3253. doi: 10.1021/ja00452a008. [DOI] [PubMed] [Google Scholar]



