Abstract
Objectives
Previous studies report a survival advantage in ovarian cancer patients with Ashkenazi Jewish (AJ) breast cancer gene (BRCA) founder mutations. The purpose of this study was to determine if this association exists in patients with non-Ashkenazi Jewish (non-AJ) BRCA mutations. We also sought to account for “survival bias” by minimizing lead time that may exist between diagnosis and genetic testing.
Methods
Patients with stage III/IV ovarian cancer and a non-AJ BRCA mutation, seen between January 1996 and July 2007, were identified from eight institutions. Patients with sporadic ovarian cancer were compared to similar cases, matched by age, stage, year of diagnosis, and vital status at time interval to BRCA testing. Progression-free (PFS) and overall survival (OS) were calculated by the Kaplan–Meier method. Multivariate Cox proportional hazards models were calculated for variables of interest. Fisher’s exact test and chi-square were also used for analysis.
Results
Ninety-five advanced stage ovarian cancer patients with non-AJ BRCA mutations and 183 sporadic controls were analyzed. Compared to sporadic ovarian cancer patients, non-AJ BRCA patients had longer PFS (27.9 months vs. 17.9 months, HR 0.61 [95% CI 0.43–0.86]) and OS (101.7 months vs. 54.3 months, HR 0.43 [95% CI 0.27–0.68]). BRCA status was an independent predictor of PFS and OS.
Conclusions
This multicenter study demonstrates a significant survival advantage in advanced stage ovarian cancer patients with non-AJ BRCA mutations, confirming the previous studies in the Jewish population. This improved survival was evident when accounting for the “survival bias” that coincides with genetic testing.
Keywords: Ovarian cancer, Survival, BRCA, Non-Askenazi Jewish, Survival bias
Introduction
Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in the United States [1]. It is estimated that there will be 21,880 new cases of ovarian cancer in the United States in 2010 with 13,850 deaths from ovarian cancer this year [2]. Ovarian cancer is most often diagnosed at an advanced stage and the prognosis is dismal [1]. Although prognosis is poor, studies have shown that ovarian cancer patients with breast cancer (BRCA) gene mutations are afforded a survival advantage. However, due to the relatively high background prevalence of these mutations within the Jewish population compared to other populations and the relative ease of identifying mutations in this population, most studies on this topic have focused on patients of Ashkenazi Jewish heritage. These patients typically have one of three founder mutations in the BRCA genes, 185delAG or 5382insC in BRCA1 or 6174delT in BRCA2.
A women’s lifetime risk for developing ovarian cancer is 1.7% [3]. A woman with a BRCA1 mutation has a 20–53% lifetime risk of developing ovarian, fallopian tube or primary peritoneal cancer and with BRCA2 the risk is 20–30% [4–10]. The prevalence of BRCA1 mutations is 1 in 800 in the general population [11], yet in the Jewish population the frequency of BRCA mutations is 2.3% [10].
Three studies in Jewish populations have shown that ovarian cancer patients who are carriers of BRCA founder mutations have improved survival, compared to ovarian cancer patients in the same populations without BRCA founder mutations (Table 1) [12–14]. Studies outside of the Jewish population have been inconclusive. These studies have either been small; based on high-risk families [15–17] or tumor registries lacking in relevant clinical information (Table 1) [18]. Thus, there is conflicting data regarding the impact of BRCA mutations on survival in ovarian, fallopian tube and primary peritoneal cancer patients in women who are not of Ashkenazi Jewish heritage [15,18]. Given that U.S. population data estimates that less than 2% of the U.S. population is of Ashkenazi Jewish heritage, the current study is designed to compare progression-free and overall survival in advanced-stage ovarian, fallopian tube and primary peritoneal cancer patients with non-Ashkenazi Jewish (non-AJ) BRCA mutations to sporadic (non-hereditary) advanced-stage ovarian, fallopian tube and primary peritoneal cancer patients without a BRCA mutation or a family history of breast or ovarian cancer.
Table 1.
Previous studies of survival in ovarian cancer patients with BRCA mutations.
| Author | Ovarian cancer cases | Ovarian cancer controls | Survival results |
|---|---|---|---|
| Rubin (US), 1996 | n=53 BRCA1 | n=53 (sporadic) | Overall Survival. 77 months vs. 29 months (p<0.001) |
| Aida (Japan), 1998 | n=25 BRCA 1 | n=29 (sporadic) | Overall Survival, 115 months vs 53 months 5-year survival, 78.6% vs 30.3% (p<0.05) Disease-free interval, 91 months vs 41 months (p<0.05) |
| Johannsson (Sweden), 1998 | n=38 BRCA 1 | n=97 (from registry) | No difference in long-term ovarian cancer survival. |
| Pharoah (UK), 1999 | n=159, BRCA 1/2+n= 139, “Familial” (not tested) | n=552 | No difference in survival b/w BRCA and controls. |
| Boyd (NY), 2000 | n=88 Jewish BRCA+ | n=101 Jewish BRCA- | BRCA cases had longer cumulative survival (p=0.004) Time to recurrence, 14 months vs. 7 months |
| Ben David (Israel), 2002 Chetrit (Israel), 2008 | n=n=229 Jewish BRCA + | n=662 Jewish BRCA- | Overall Survival, 51.2 months vs. 33.1 months 3-year survival, 65.8% vs. 51.9% (p<0.001) Overall Survival, 53.7 months vs. 37.9 months (p=0.002) 5- year survival, 38.1% vs. 24.5% (p<0.001) |
| Cass (LA), 2003 | n=34 Jewish BRCA+ | n=37 Jewish BRCA- | Overall Survival, 91 months vs 54 months (p=0.046) 2-year survival, 100% vs. 83% (ns) 5-year survival, 65% vs. 48% (ns). Disease- free interval, 49 months vs. 19 months (ns) |
| Tan (UK), 2008 | n=22 BRCA 1/2 | n=44 “Non-hereditary” | Overall Survival, 100.8 months vs. 34.8 months (p<0.002) |
| Lacour/Lu (Current Study), 2009 | n=95 BRCA 1/2 (“Non-Jewish”) | n=183 (sporadic) | Overall Survival, 83 months vs. 55 months (p<0.01) Progression- Free Survival, 25.4 months vs. 18.3 months (p<0.01) |
Methods
After obtaining approval from the Institutional Review Boards of the University of Texas M.D. Anderson Cancer Center, University of Texas Southwestern Medical Center, Washington University, Moffitt Cancer Center, New York University, University of Alabama-Birmingham, University of Southern California, University of California-San Francisco, a retrospective cohort study of advanced-stage ovarian, fallopian tube or primary peritoneal cancer patients with a non-AJ BRCA founder mutation was performed. Those advanced-stage ovarian, fallopian tube or primary peritoneal cancer patients with a non-AJ BRCA mutation, seen for genetic testing at the participating institutions between January 1, 1996 and July 31, 2007, were compared to sporadic ovarian, fallopian tube or primary peritoneal cancer patients during the same time period.
Study subjects and data collection
The study population included women with stage III or IV ovarian, fallopian tube or primary peritoneal cancer. Patients with stage I and II disease were excluded. Tumors of low malignant potential (“border-line”) tumors were excluded. The exposure in this study was been defined as all BRCA mutations other than the three Ashkenazi Jewish founder mutations, specifically, BRCA1: 185delAG and 5382insC and BRCA2: 6174delT, regardless of family history. Patients in the exposure group were identified through genetic counseling records at the respective institution. Any patient tested during the time period or seen for counseling secondary to a known non-Ashkenazi Jewish BRCA mutation was selected for inclusion. All included mutations were confirmed deleterious; variants of uncertain significance were excluded. The sporadic group for this study consists of ovarian, fallopian tube or primary peritoneal cancer patients who have not been tested for a BRCA mutation, have no family history of breast or ovarian cancer, and have no history of Ashkenazi Jewish ancestry. Family history was obtained from the medical record. Any patient with incomplete family history was not included as a sporadic control.
The matched cohort was included to control for confounders that may be associated with both BRCA status and survival, including year of diagnosis, stage at diagnosis, age at diagnosis within 5 years, and institution. Matching for year of diagnosis was performed to account for changes in treatment regimens over time. The cohort was also matched to sporadic patients for the “interval to testing.” This technique was used to minimize lead in survival that a patient may acquire by living to be tested for a BRCA mutation. Specifically, a non-AJ BRCA patient was matched to a sporadic patient that lived at least the same number of months that the BRCA patient lived in order to undergo genetic testing. By addressing this “survival bias,” non-AJ BRCA patients were not afforded an advantage by surviving longer than the sporadic patients before they were identified as being a non-AJ BRCA mutation carrier (at the time of genetic testing). When possible, two sporadic ovarian cancer patients were matched to each BRCA patient. If at least one match was not identified, the non-AJ BRCA patient was excluded.
The primary outcome in this study was survival. Secondary outcomes of interest were response to initial chemotherapy and recurrence. Overall survival was defined as the time from date of cancer diagnosis to date of death (or date of last follow-up if the patient remains living). Response to initial chemotherapy was a dichotomous variable (complete versus incomplete). A complete response (no evidence of disease after the initial course of chemotherapy) was defined as no clinical or radiographic evidence of disease and normalization of the serum CA-125 biomarker. Recurrence was defined as clinical or radiographic presence of new areas of disease or increase in size of previously stable disease. Progression-free interval was defined as the time from the date of cancer diagnosis to the date of diagnosis of recurrence, as defined above.
The medical records were obtained and reviewed for age, race, diagnosis, BRCA status, symptoms, treatment, follow-up, recurrence and survival. Additional variables of interest included in the data collection and analysis included platinum-based versus non-platinum based chemotherapy and completeness of primary surgical debulking procedure as documented by the medical record (optimal versus suboptimal, as defined by GOG standards at the time of each individual patient’s surgical procedure).
Data supplied by the Gynecologic Oncology Group (GOG) Statistical and Data Center (Buffalo, NY), from the Gynecologic Cancer InterGroup/GOG protocol 182 [19] was used as a historical control group to provide a second, large group of advanced-stage ovarian cancer patients for comparison and to validate the results obtained with our sporadic-matched patients. The protocol allowed for inclusion of patients with stage III or IV, epithelial ovarian or primary peritoneal cancer. Patients could have had either optimal or suboptimal debulking surgery. The GOG data includes survival information on 775 U.S. ovarian cancer patients treated in the carboplatin and paclitaxel “reference arm” (Arm I) of the study. International patients that were enrolled in the study were not included in this comparison.
Data analysis
Data was analyzed to compare response to chemotherapy, progression-free and overall survival between the non-AJ BRC-Aassociated and sporadic ovarian, fallopian tube and primary peritoneal cancer patients. Odds ratios were calculated to determine the risk of incomplete response to initial chemotherapy and recurrence in women with sporadic ovarian, fallopian tube and primary peritoneal cancers compared to women with non-AJ BRCA-associated ovarian, fallopian tube and primary peritoneal cancers. Differences between cohorts were evaluated using a t-test for continuous variables and Fisher’s exact test for categorical variables.
The method of Kaplan–Meier was used to compare progression-free and overall survival between both groups of sporadic ovarian, fallopian tube and primary peritoneal cancer patients and the non-AJ BRCA mutation carriers. Multivariate Cox proportional hazard models were used to test the independence of variables that could be associated with the outcome in each group. Matching was accounted for by treating the matched sets as clusters. Stratified log rank test was used to calculate survival data for matched pairs using paired event times for the Kaplan–Meier analysis and the Cox proportional hazards model used a sandwich covariance matrix estimate to account for the intracluster dependence. A 95% confidence interval was used for testing the study hypothesis. A p-value of less than 0.05 is considered statistically significant for all tests.
Results
Patients
Ninety-five advanced stage ovarian, fallopian tube or primary peritoneal cancer patients with non-AJ (founder) BRCA mutations were identified as being seen for genetic testing at the eight participating institutions between January 1, 1996 and July 31, 2007. One hundred eighty-three matched sporadic patients were available for comparison. Median follow-up was 42.6 months for the non-AJ BRCA patients and 37.5 months for the sporadic patients. Age, race and stage did not significantly differ between the two groups (Table 2). BRCA-associated tumors were more likely to be of higher grade (p=0.01). The distribution of histologies was somewhat different (p=0.04) between groups, with more endometrioid tumors in the sporadic cohort. Serous histologies include “serous,” “papillary serous” and “mixed” histologies that included a serous component. The proportion of patients that had an optimal cytoreduction and those that received platinum-based chemotherapy did not differ between groups (Table 2).
Table 2.
Demographic and clinical data.
| Non-AJ BRCA n=95 (%) | Sporadic-Matched n=183 (%) | |
|---|---|---|
| Institution | ||
| MDACC | 44 (46.3) | 86 (47.0) |
| WU | 17 (17.9) | 34 (18.6) |
| Moffitt | 14 (14.7) | 27 (14.8) |
| UTSW | 9 (9.5) | 18 (9.8) |
| UAB | 5 (5.3) | 9 (4.9) |
| UCSF | 3 (3.2) | 6 (3.3) |
| NYU | 2 (2.1) | 2 (1.1) |
| USC | 1 (1.1) | 1 (0.5) |
| Age | ||
| Mean (SD) | 54.6 (9.8) | 55.0 (9.6) |
| Median | 54.0 | 55.0 |
| Race | ||
| Caucasian | 80 (84.2) | 144 (78.7) |
| African American | 5 (5.2) | 12 (6.6) |
| Hispanic | 6 (6.3) | 17 (9.3) |
| Other | 4 (4.2) | 10 (5.5) |
| Stage | ||
| III | 84 (88.4) | 163 (88.1) |
| IV | 11 (11.6) | 20 (10.9) |
| Tumor Grade | ||
| 1 | 1 (1.1) | 14 (7.7) |
| 2 | 3 (3.2) | 14 (7.7) |
| 3 | 87 (91.6) | 147 (80.3) |
| Unknown | 4 (4.4) | 8 (4.4) |
| Histology | ||
| Serous | 59 (62.1) | 130 (71.0) |
| Endometrioid | 4 (4.2) | 14 (7.7) |
| MMMT | 3 (3.2) | 6 (3.3) |
| Undifferentiated/Mixed | 25 (26.3) | 20 (10.9) |
| Mucinous | 0 (0.0) | 4 (2.2) |
| Clear Cell | 0 (0.0) | 3 (1.6) |
| Unknown | 4 (4.2) | 6 (3.3) |
| BRCA Mutation | ||
| BRCA 1 | 62 (65.2) | N/A |
| BRCA 2 | 33 (34.8) | N/A |
| Debulking Status | ||
| Optimal | 67 (70.5) | 128 (70.0) |
| Suboptimal | 14 (14.7) | 37 (20.2) |
| Unknown | 14 (14.7) | 18 (9.8) |
| Response to Initial Chemotherapy | ||
| Complete | 67 (70.5) | 113 (61.7) |
| Incomplete | 18 (19.0) | 50 (27.3) |
| Unknown | 10 (10.5) | 20 (11.0) |
University of Texas M.D. Anderson Cancer Center, MDACC; Washington University, WU; Moffitt Cancer Center, Moffitt; University of Texas Southwestern Medical Center, UTSW; University of Alabama Birmingham, UAB; University of California San Francisco, UCSF; New York University, NYU; University of Southern California, USC.
Response to chemotherapy
There was no difference in proportion of patients with complete response to initial chemotherapy between the two groups. Furthermore, when controlling for diagnosis, age, stage, and interval to testing, the odds of complete response to chemotherapy was not significantly different between the non-AJ BRCA mutation carriers and the sporadic cohort (OR 1.65, 95% CI 0.89–3.05).
Progression-free and overall survival
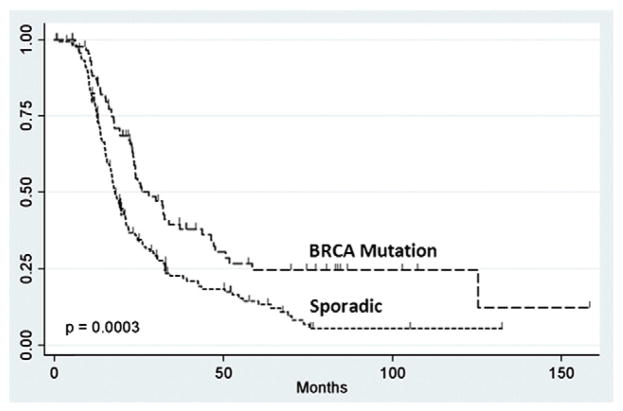
Median progression-free (27.9 months vs. 17.9 months, p=0.0003) and overall survival (101.7 months vs. 54.3 months, p<0.0001) were significantly longer in the non-AJ BRCA mutation carriers, compared to their sporadic-matched counterparts. As demonstrated by the method of Kaplan–Meier, progression-free survival (HR 0.61, 95% CI 0.43–0.86, p=0.0043) (Fig. 1) and overall survival (HR 0.43, 95% CI 0.27–0.68, p=0.0003) (Fig. 2) were significantly improved in the non-AJ BRCA patients, compared to the sporadic-matched cohort.
Fig. 1.
Progression free- survival of non-Ashkenazi Jewish BRCA and sporadic patients.
Fig. 2.
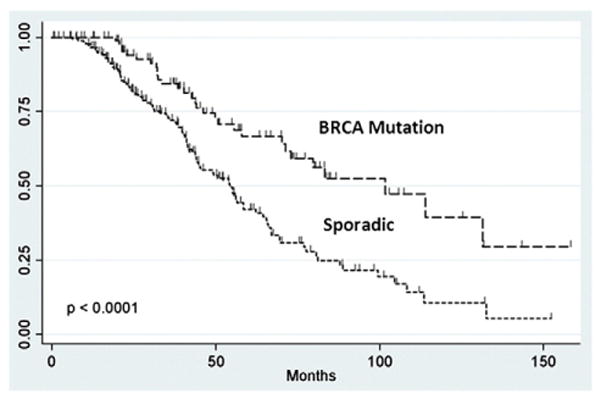
Overall survival of non-Ashkenazi Jewish BRCA and sporadic patients.
Multivariate Cox proportional hazards models
Tumor grade and debulking status were not found to be significantly associated with progression-free or overall survival by a multivariate Cox proportional hazards model (Table 3). However, there was a trend toward improved progression-free and overall survival in those who underwent and optimal tumor debulking. BRCA status (having a non-AJ BRCA mutation) was an independent predictor of progression– free (p=0.004) and overall survival (p=0.003), while complete response to initial chemotherapy was an independent predictor of progression-free survival (p=0.02; Table 3).
Table 3.
Cox proportional hazards model: factors associated with progression- free and overall survival.
| Variable | Progression-Free Survival
|
Overall Survival
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | p | 95% CI | Hazard ratio | p | 95% CI | |
| Non-AJ BRCA Mutation | 0.59 | 0.002 | 0.42–0.82 | 0.52 | 0.003 | 0.34–0.80 |
| Grade 2/3 | 0.74 | 0.339 | 0.40–1.37 | 0.85 | 0.681 | 0.39–1.84 |
| Optimal Debulking | 0.87 | 0.188 | 0.71–1.07 | 0.91 | 0.433 | 0.73–1.15 |
| Response to Chemotherapy | 0.70 | 0.036 | 0.50–0.98 | 0.72 | 0.080 | 0.49 – 1.04 |
Matched for year of diagnosis, age, stage and interval to testing
GOG control group
When compared to U.S. advanced-stage ovarian cancer patients treated with carboplatin and paclitaxel on GOG 182, the BRCA mutation carriers in our study cohort had improved progression-free survival (27.9 vs. 16.8 months, p=0.0006) (Fig. 3). To analyze differences in overall survival, events were censored at 5 years, due to the longer follow-up data available for the cohort of patients in our study. During the 5-year period, there were 419 events among the 775 patients (46% 5-year overall survival) in the GOG control and 22 events among the 96 BRCA mutation carriers (77% 5-year overall survival) (HR=0.36, 95% CI [0.14–0.91], p<0.0001) (Fig. 4).
Fig. 3.
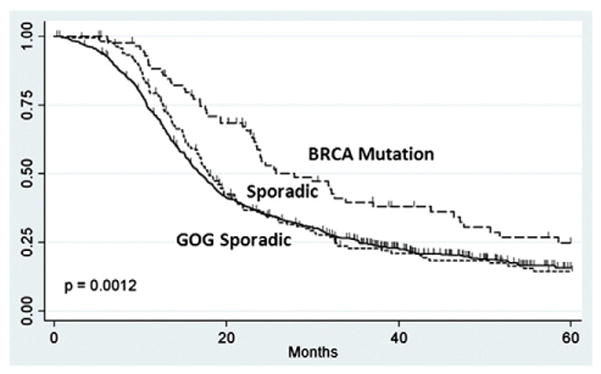
Progression free-survival of Non-Ashkenazi Jewish BRCA and GOG controls.
Fig. 4.
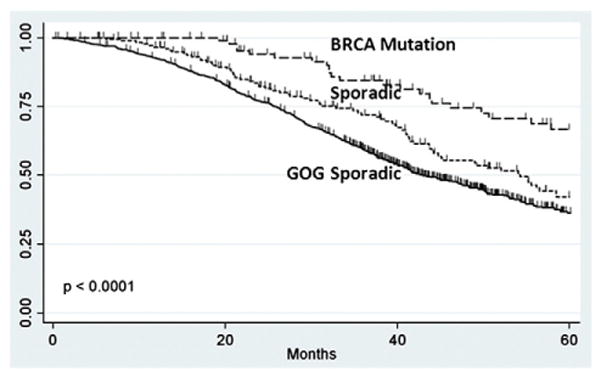
Overall survival of Non-Ashkenazi Jewish BRCA and GOG Controls.
Discussion
From this analysis, we report a progression-free and overall survival advantage for ovarian cancer patients with germline BRCA1 or BRCA2 mutations not associated with Ashkenazi Jewish heritage. Our data are consistent with reports of the survival advantage afforded patients with Ashkenazi Jewish BRCA founder mutations [12–14,19]. Boyd et al. showed that eighty-one Jewish patients with BRCA-associated ovarian cancer had a significantly longer disease-free interval after primary chemotherapy (p < 0.001), and improved overall survival (p = 0.004) compared to those patients without a BRCA mutation [12]. Ben David et al. demonstrated that 234 Israeli ovarian cancer patients carrying Ashkenazi Jewish BRCA mutations had an improved survival of 3 years (p < 0.001) over non-carriers [13]. Our study demonstrates that, compared to matched, ovarian cancer patients, non-AJ BRCA mutation carriers had longer PFS (p<0.001) and OS (p<0.001). In addition, BRCA status was an independent predictor of both progression-free and overall survival. A recent retrospective-cohort study by Tan et al., reported similar finding regarding differences in overall survival (p<0.002) and time to first relapse (p<0.001), using matching criteria similar to that of the current study [17]. However, the Tan study included only 22 BRCA-positive patients, which included patients with both BRCA Jewish founder mutations and non-Jewish BRCA mutations. Our study included a large number of ovarian cancer patients with non-Jewish BRCA mutations and was consistent in finding a survival advantage for women with BRCA mutations. In addition, this report clarifies previous earlier reports of conflicting data regarding BRCA-positive ovarian cancer patients not of Jewish heritage [15,18].
The control group from GOG 182 provides a second, large group of advanced-stage ovarian cancer patients for comparison and validates the results obtained with our sporadic-matched patients. The survival advantage afforded to BRCA mutation carriers is evident, even though the GOG control group may include BRCA mutation carriers. In fact, the inclusion of BRCA mutation carriers would tend to shift the results toward the null hypothesis of no difference in survival. The GOG control group is similar to our control group, in that it includes only advanced-stage epithelial ovarian cancer patients. Also, both control groups include a variety of patients that may have had optimal or suboptimal tumor reduction/cytoreductive surgery, as well as possible interval cytoreductive surgery.
It is noted that overall survival is shorter in the GOG control group than our sporadic-matched group (42.9 months vs. 54.3 months). The difference could be attributed to younger age of the matched-sporadic cohort (as a result of matching). In addition, because the sporadic patients had to survive for a certain period of time in order to be matched for the “interval to testing,” their survival times may be longer than that of a random group of advanced-stage epithelial ovarian cancer patients.
Our study failed to demonstrate a significant difference in odds of complete response to initial chemotherapy between the BRCA mutation carriers and their sporadic counterparts. This may be due to the fact that data on response to initial chemotherapy was unknown in 10.8% of patients.
There is an inherent dilemma in performing a study based on a genetic test, when that particular test is not “standard of care” for all patients with ovarian cancer. This dilemma is easily overcome in the Jewish population because a majority of Jewish mutation carriers carry one of only three founder mutations. Genetic testing is not only warranted, but is less expensive, in that specific population. When not all patients in both cohorts are tested, there is concern for possible “survival bias,” a criticism of the Rubin study [20] and others [21]. Our goal was to overcome these confounding factors in study design in order to address the question of a possible survival advantage afforded by non-AJ BRCA mutations. In order to be included in this matched group, a sporadic patient had to have survived at least as long as the corresponding BRCA mutation carrier lived to undergo genetic testing. Thus, the date of genetic testing was the starting point for any difference in survival. Matching for “interval to testing” may have actually underestimated the survival advantage, because sporadic patients who died early of their disease would have been excluded from the sporadic-matched group and would not have been included in the analysis. This specific bias would potentially affect the results by shifting the survival of the sporadic group toward the null, lessening the reported difference in both progression-free and overall survival in the matched comparison. Therefore, the difference in survival, between the non-AJ BRCA patients and the sporadic patients, may have been underestimated in our study.
As defined, even patients in the sporadic group have up to a 35% risk of carrying a deleterious BRCA mutation, with a negative personal and family history [22,23], leading to possible misclassification of BRCA-associated cancers within the sporadic group. In addition, age-matching within in the sporadic group led to a younger median age of diagnosis then the “typical” sporadic patients. Although these patients have no other risk factors for BRCA mutation, young age alone may increase risk of BRCA mutation. The possibility of undetected BRCA mutations in the sporadic group may have lead to a bias toward the null hypothesis, underestimating the survival difference attributable to BRCA mutations.
This study gives insight into the natural history of ovarian cancer in BRCA mutations carriers. This is the largest evaluation of survival in a group of non-Ashkenazi Jewish BRCA mutation carriers. Given that U.S. population data estimates that less than two percent of the U.S. population is of Ashkenazi Jewish heritage, this study provides information on survival of BRCA mutations carriers that is more generalizable than information provided in previous studies of Jewish BRCA mutation carriers alone.
Acknowledgments
The authors would like to acknowledge Sheri Babb, Lynda Roman and Jacob Estes for their assistance with this project. In addition, we would like to recognize the Lynne Cohen Foundation for Ovarian Cancer Research for their support.
Footnotes
Funding: R01 CA106414 (RS).
Conflict of interest
None of the authors have any conflicts of interest in relation to this manuscript.
References
- 1.Ozols RF, Rubin SC, Thomas GM, Robboy SJ. Epithelial ovarian cancer. In: Hoskins WJ, Young RC, Markman M, Perez CA, Barakat RR, Randall ME, editors. Principles and practice of gynecologic oncology. 4. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 895–987. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Ovarian Cancer Control Initiative from the National Center for Chronic Disease Prevention and Health Promotion. 2010 [updated 2010 May 26; cited 2010 Aug 4]. Available from: http://www.cdc.gov/cancer/ovarian/index.htm.
- 4.Boyd J. Specific keynote: hereditary ovarian cancer: what we know. Gynecol Oncol. 2003;88:S8–S10. doi: 10.1006/gyno.2002.6674. [DOI] [PubMed] [Google Scholar]
- 5.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Easton DF, Steele L, Fields P, Ormiston W, Averill D, Daly PA, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12–13. Am J Hum Genet. 1997;61:120–8. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–8. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 8.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moslehi R, Chu W, Karlan B, Fishman D, Risch H, Fields A, et al. BRCA1 and BRCA2 mutation analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am J Hum Genet. 2000;66:1259–72. doi: 10.1086/302853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King MC, Marks JH, Mandell JB The New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Genetics of Breast and Ovarian Cancer. 2010 [updated 2010 July 22; cited 2010 Aug 4]. Available from: http://www.cancer.gov/cancertopics/pdq/genetics/breast-and-ovarian.
- 12.Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–5. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 13.Bendavid Y, Chetrit A, Hirsh-Yechezkel G, Friedman E, Beck BD, Beller U, et al. Effect of BRCA mutations n length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20:463–6. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 14.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effects of BRCA 1/2 mutations on long- term survival of patients with invasive ovarian cancer: The National Israeli Study of Ovarian Cancer. J Clin Oncol. 2008;26:20–5. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 15.Pharoah PD, Easton DF, Stockton DL, Gayther S, Ponder BA. Survival in familial, BRCA1-associated epithelial ovarian cancer. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. Cancer Res. 1999;59:868–71. [PubMed] [Google Scholar]
- 16.Zweemer RP, Verheijen RHM, Coebergh JW, Jacobs IJ, van Diest PJ, Gille JJ, et al. Survival analysis in familial ovarian cancer, a case control study. Eur J Obstet Gynecol Reprod Biol. 2001;98:219–23. doi: 10.1016/s0301-2115(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 17.Tan DSP, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” Syndrome in Ovarian Cancer: a control- control study describing the clinical features and outcomes of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–6. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 18.Johannsson OT, Ranstam J, Borg A, Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from Southern Sweden. J Clin Oncol. 1998;16:397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 19.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum- based treatment regimens in advanced- stage ovarian cancer: A phase III trial of the Gynecologic Cancer InterGroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, et al. Clinical and pathologic features of ovarian cancer in women with germline mutations of BRCA1. N Engl J Med. 1996;335:1413–6. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 21.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian cancer. Cancer. 2003;97:2187–95. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 22.Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10, 000 individuals. J Clin Oncol. 2002;20:1480–90. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 23.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]