Abstract
Primary care is understudied as a re-entry drug and alcohol treatment setting. This study compared treatment retention and opioid misuse among opioid dependent adults seeking buprenorphine/naloxone maintenance in an urban primary care clinic following release from jail vs. community referrals. Post-release patients were either; a) induced to buprenorphine in-jail as part of a clinical trial, or, b) seeking buprenorphine induction post-release. From 2007–2008, N=142 patients were new to primary care buprenorphine: n=32 post-release; n=110 induced after community referral and without recent incarceration. Jail-released patients were more likely African American or Hispanic and uninsured. Treatment retention rates for post-release (37%) vs. community (30%) referrals were similar at 48 weeks. Rates of opioid positive urines and self-reported opioid misuse were also similar between groups. Post-release patients in primary care buprenorphine treatment had equal treatment retention and rates of opioid abstinence vs. community-referred patients.
INTRODUCTION
An estimated 2–3 million U.S. persons age >12 years reported non-prescription opioid or heroin use in 2006.(NSDUH) Buprenorphine, a partial µ opioid receptor agonist, was approved by the Food and Drug Administration in 2002 as office-based pharmacotherapy for opioid dependence.(1,2) Buprenorphine maintenance for opioid treatment in jail and prison settings and as prisoners re-enter community treatment has recently been shown feasible and effective.(3–5) This follows many years of the effective use of methadone maintenance at re-entry.(6–9)
Methadone and buprenorphine are both highly effective (vs. placebo) as daily opioid agonist treatment medications.(1) Differences between the two medications are logistic and regulatory; buprenorphine may be prescribed in less restrictive general medical settings and dispensed by community pharmacies, whereas methadone treatment follows a more tightly regulated paradigm of observed treatment. However, the current U.S. availability of either buprenorphine or methadone at re-entry is limited.(J Rich, cite needed)
Expansion of re-entry buprenorphine treatment will be most effective if a broad range of office-based providers are engaged, including those in primary care and related medical specialty clinics (i.e., HIV care settings). Public hospital and community health center primary care settings are typically designed to see large volumes of Medicaid and uninsured adults, common demographics of heroin-using and post-release individuals. In New York City and in many other areas, public hospitals currently serve as the tertiary care centers for the local inmate populations and may include dedicated forensic and hospital secure units.(10) Further, diverse models of primary care have been shown to feasibly deliver buprenorphine treatment, including within academic and community health centers, public hospitals, and among the homeless.(11–15) Primary care buprenorphine treatment, compared to MTP or intensive outpatient models, may involve less stringent visit schedules or fewer ancillary treatment requirements, all of which may appeal to former inmates eager to address employment or housing needs, or those ambivalent towards more intensive treatment.
This study evaluated treatment retention and on-going opioid use among a cohort of jail-released patients receiving primary care-based buprenorphine treatment at a large public hospital and compared their retention and opioid use outcomes to those of new community-referred patients. A recent randomized trial comparing buprenorphine and methadone in NYC jails found higher rates of in-jail and post-release treatment retention among buprenorphine patients.(4) In this study, 90% of the buprenorphine patients were referred to Bellevue Hospital Center’s (BHC) adult primary care buprenorphine program. While the prior study’s main results suggested buprenorphine is a viable route of opioid treatment at re-entry, important questions for primary care providers considering re-entry buprenorphine were not addressed, including post-release treatment outcomes in primary care.
METHODS
Population and recruitment
All patients were opioid-dependent adults seeking office-based buprenorphine treatment. Post-release patients were either referred directly from the NYC Department of Correction’s Key Extended Entry Program (KEEP), the opioid treatment program within Rikers Island jail facilities, as part of the randomized comparison of buprenorphine to methadone, or self-referred after learning of the BHC primary care buprenorphine program while incarcerated.(4) Community-recruited patients not currently prescribed buprenorphine were referred from a variety of sources: the BHC inpatient detoxification unit, other BHC addiction and medicine clinics, area drug treatment providers, internet locators, and patient word-of-mouth.
Setting
The BHC primary care-based buprenorphine treatment program and visit protocols have been detailed elsewhere.(14) Briefly, general internists certified in buprenorphine prescribing and an MPH-level clinical coordinator (DD) staffed a weekly half-day primary care addiction medicine clinic with occasional visits at other times. Participants were encouraged to present for buprenorphine treatment with active New York State Medicaid or commercial insurance that covered buprenorphine prescriptions but were seen regardless of insurance status or ability to pay. There was no free or discounted buprenorphine medication for uninsured community patients (12%), while uninsured post-release patients (47%) received a free supply of buprenorphine-naloxone (Suboxone®) donated by the manufacturer (Reckitt-Benkiser) to the jail-based clinical trial. Extramural funds from the NYC Department of Health and Mental Hygiene (DOHMH) and the NYC Health and Hospitals Corporation provided partial support for clinical effort and the development of written patient education materials.
Treatment
An initial visit included a medical, psychiatric, and substance use assessment and confirmed DSM-IV criteria for opioid dependence. Patients were required to provide urine samples for later analysis by laboratory toxicology assays (point-of-care urine dip tests were not used). Burpenorphine induction followed home induction protocols described previously.(14) Observed, on-site buprenorphine induction was not available. Patients new to the practice and previously induced onto buprenorphine, including the majority of persons presenting immediately after jail release, were continued on previously established buprenorphine maintenance doses. Insured patients filled their prescriptions at community pharmacies. Uninsured, post-release patients received free medication from the hospital pharmacy. Follow-up visits assessed on-going drug use through self-report and urine toxicologies and occurred at varying intervals, with patients typically seen every 1–4 weeks during the first few months, and at 4–8 week intervals when on a stable buprenorphine dose and without on-going other opioid use.
Assessments
Standardized baseline and follow-up visit data collection forms were completed by physicians or coordinator. Baseline data included opioid and other drug use, general health history, and demographic information. Follow-up data included self-report of adverse events, drug use, buprenorphine dosing patterns, and other addiction and medical or psychiatric treatment involvement. The New York University School of Medicine Institutional Review Board (IRB) approved a protocol of observational data collection and at Bellevue; jail-based buprenorphine treatment was initiated under a protocol approved by the New York City Department of Health and Mental Hygiene IRB.
Analysis
Primary outcomes were treatment retention and rates of opioid positive urine toxicologies and self-reported opioid use. Retention was analyzed as a continuous variable (weeks-in-treatment) and was defined as beginning with the initial primary care visit and extending until the final week of the last active buprenorphine prescription. Urine toxicologies were considered opioid positive if morphine (opiate) or methadone metabolites were detected; extended synthetic opioid assays (i.e., oxycodone, hydrocodone) were not available. Patient self-report data was dichotomized as any use (Yes/No) between visits and analyzed in 4-week intervals. Analysis consisted of descriptive statistics, Fishers exact tests for significant differences between groups, and linear regression including odds ratios and 95% CIs measuring baseline patient characteristics associated with treatment retention. All analysis was performed using Stata IC 10.0 (Stata Corp., College Station, TX).
RESULTS
Referral Sources and Demographics
One-hundred forty consecutive patients were offered buprenorphine treatment from August 2006 to January 2008, with complete 48 week data available on all patients. Referral sources are shown (Table 1). Thirty-two patients presented for buprenorphine treatment following release from jail; 27 of these were RCT participants. A greater proportion of post-release patients were male, Hispanic (vs. white), unemployed, uninsured, and heroin (vs. prescription opioid or methadone transfers) and cocaine users (Table 2).
Table 1.
Primary Care Buprenorphine Treatment, Jail- vs. Community-Referrals
| n (% of sub-sample) | |
|---|---|
| Jail referrals | 32 (100) |
| Jail-based clinical trial, buprenorphine arm | 22 (69) |
| Jail-based clinical trial, methadone arm | 5 (16) |
| Jail referral, non-trial participant, not on medication | 5 (16) |
| Community referrals | 110 (100) |
| Word of mouth | 35 (32) |
| Hospital medical or psychiatric services | 16 (15) |
| Hospital detoxification inpatient unit | 15 (14) |
| Other community addiction treatment programs | 11 (10) |
| Methadone treatment programs (MTP) | 9 (8) |
| Internet buprenorphine provider locator | 5 (5) |
| Other or unknown referral source | 19 (17) |
Table 2.
Baseline Demographics and Drug Use, Jail- vs. Community-Referrals
| Jail referrals (n=32) n (%) |
Community referrals (n=110) n (%) |
Fischer’s exact test P |
|
|---|---|---|---|
| Male | 31 (97) | 86 (78) | 0.01 |
| Age in years, mean (range) | 41 (21–52) | 42 (25–67) | 0.6 |
| Race/Ethnicity | |||
| African American | 6 (19) | 14 (13) | |
| Hispanic | 21 (66) | 37 (34) | 0.002 |
| White, non-Hispanic | 5 (15) | 58 (53) | 0.002 |
| Insurance status | |||
| Medicaid | 12 (38) | 72 (65) | 0.003 |
| Private insurance | 5 (15) | 25 (23) | |
| No insurance | 15 (47) | 13 (12) | 0.003 |
| Employed | 3 (8) | 28(25) | 0.04 |
| Homeless* | 3 (10) | 7 (6) | |
| Substance use, last 7 days or at arrest | |||
| Heroin | 32 (100) | 72 (65) | 0.001 |
| Injection drug use | 14 (45) | 30 (27) | .09 |
| Other opioids, non-prescribed | 2 (7) | 35 (32) | 0.03 |
| Cocaine | 17 (52) | 23 (21) | 0.003 |
| Heavy alcohol (≥5 drinks/occasion) | 9 (29) | 21 (19) | .3 |
| Benzodiazepines, non-prescribed | 0 (0) | 28 (25) | 0.001 |
| Current smoking | 27 (90) | 85 (80) | 0.6 |
| Opioid treatment history | |||
| Methadone treatment program (MTP), current | 0 (0) | 9 (8) | 0.2 |
| MTP, ever | 24 (75) | 67 (61) | 0.1 |
| Buprenorphine treatment, ever | 11 (34) | 60 (55) | 0.07 |
Treatment Retention
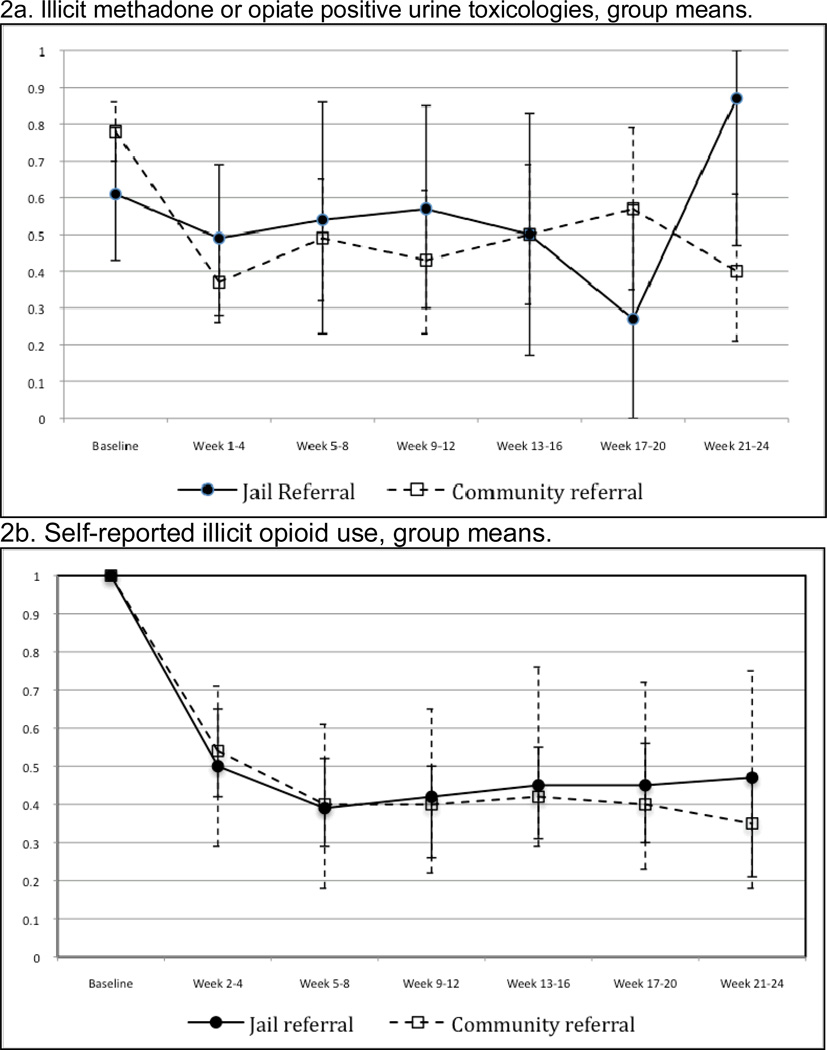
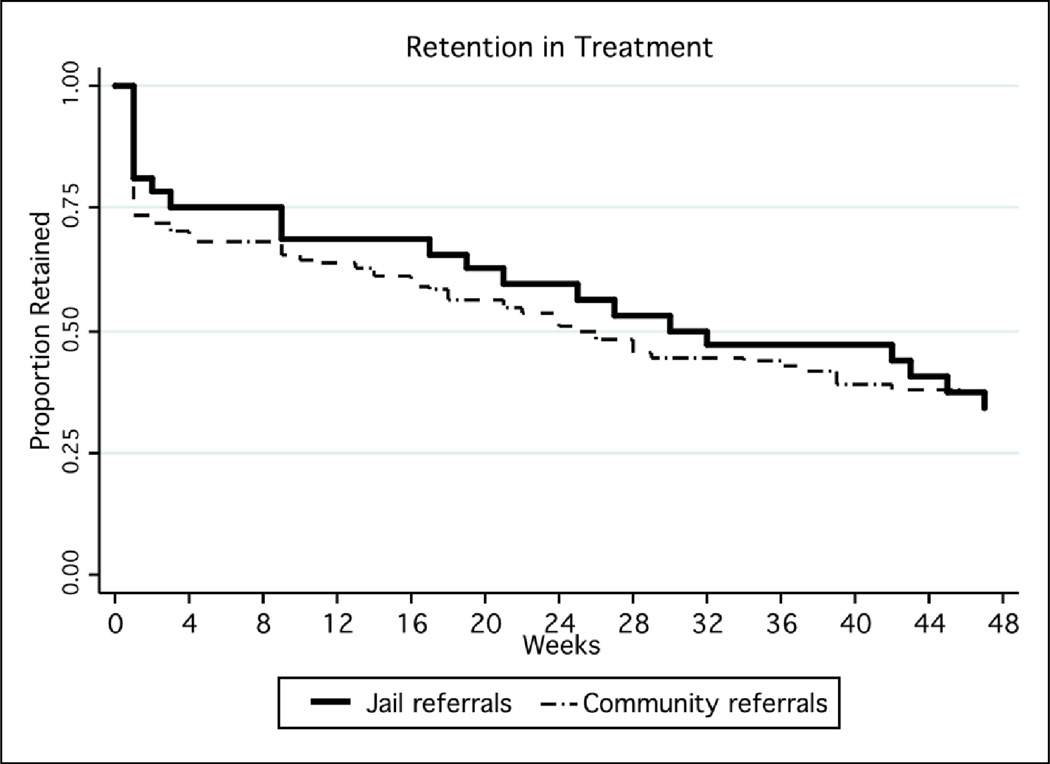
Treatment retention over time was similar between groups (Figure 2). The mean treatment duration was 34 weeks (95%CI 29–39); 37(26–48) among post-release patients, 33 (27–39) among community referrals. For inactive patients, the mean observed time in treatment (time to drop-out) was 21 (12–30) weeks post-release; 17 (12–21) weeks in community referrals. Urine toxicology and self-reported heroin and other opioid use indicated ongoing opioid use in a significant proportion of patients, with no differences between groups (Figure 3a and 3b). Mean days per week using opioids decreased from 7 days per week at pre-arrest/induction visit to 1 day per week at week 12 (among 92 of 142 patients retained through week 12).
Figure 2.
a–b. Rates of urine toxicologies positive for illicit methadone or opiates and self-reported illicit opioid use: group means through week 24
2a. Urine toxicology results display the proportions (group means) of individuals with any urines positive for opioids among during the corresponding 4-week intervals. Baseline results represents initial urine toxicology results in primary care buprenorphine treatment for all participants (community referrals and post-release jail referrals).
2b. Opioid use data indicate the proportions (group means) of patients reporting any illicit or non-prescribed opioid use during the corresponding 4-week intervals. Baseline results represent current use (last 7 days) at the initial primary care buprenorphine visit among community referrals, or pre-arrest use among jail referrals.
*Error bars represent 95% confidence intervals for group mean estimates.
DISCUSSION
Buprenorphine maintenance delivered in a public hospital primary care setting appears a feasible mechanism for engaging and retaining persons released from jail in effective, office-based treatment for opioid dependence. Post-release patients appear similar to community-referred individuals in terms of treatment retention and opioid abstinence vs. on-going, problematic use. Generally, retention in buprenorphine treatment is highly effective in limiting rates of on-going opioid use.(1,16) This analysis extends from the main trial data of a randomized comparison of jail-to-community buprenorphine to methadone maintenance in NYC, and mirrors other results a single-arm buprenorphine re-entry cohort in Puerto Rico as well as analysis of a large community-based clinical trial, all of which recent studies document the feasibility buprenorphine at re-entry. As in the San Juan cohort, post-release patients in this study were seen in a general medical practice. Extrapolating from the methadone literature and years of experience, longitudinal improvements to health, criminal justice outcomes and related costs can be expected to parallel the expansion of re-entry buprenorphine treatment.
Post-release buprenorphine maintenance was not universally beneficial in the short term, as roughly half of those induced to buprenorphine in jail and referred to our program did not report for an initial post-release visit, and, once in our practice, many struggled to achieve sustained opioid abstinence. While strategies for maximizing these good clinical outcomes among re-entry buprenorphine patients are needed, rates of retention and opioid use were essentially the same as in community-recruited participants, and, in the related NYC jail-based randomized trial, were equivalent or better than those observed among methadone-treated participants.(5) Familiar barriers to successful re-entry, including lack of housing, poor social support, unemployment, lack of or intermittent health insurance, and co-morbid cocaine, alcohol and sedative-hypnotic dependence, all likely impacted this post-jail cohort’s ability to remain in treatment.
From a public health and safety perspective, however, it is also likely that even ‘a little’ post-release opioid agonist maintenance goes a long way. Due to the relatively long-half-lives of buprenorphine and methadone, discharging persons from jail on an average maintenance dose of buprenorphine ‘covers’ the individual for a few days from opioid withdrawal symptoms. Participants in this study were able to present to clinic up to 5 days after release with only mild opioid withdrawal symptoms and prior to any relapse to heroin use. In terms of overdose prevention, buprenorphine’s long half-life insures opioid tolerance is high and remains so during the week of release while the agonist activity of heroin and other opioids are partly blocked, thus reducing the substantial risk of accidental overdose known to occur immediately post-release upon return to previous levels of opioid use.(17,18) Further, while in buprenorphine treatment, participants universally report reduced opioid and intravenous drug use from pre-arrest baseline. From a harm reduction perspective and based on self-report, persons exposed to buprenorphine treatment for any period appear to achieve meaningful reductions in illicit opioid ingestion, both in terms of the frequency and quantity of use, during that period and immediately afterwards.
There are important limitations of this study. Uninsured post-release patients received free buprenorphine medication, which allowed much higher treatment retention vs. uninsured community referrals, though insured patients in both groups had the least barriers to both visits and medication supply. Post-release patients were previously induced onto buprenorphine in a controlled environment, and represented patients new to community treatment but already on a stable buprenorphine dose vs. community referrals, who were new to treatment and undergoing buprenorphine induction. We estimate a lower proportion of the entire jail-based trial’s buprenorphine, a proportion of whom were not referred to our clinic or were referred but did not matriculate, continued in community treatment at 6 months compared to the community referrals in this analysis initiating treatment at our clinic. The primary care buprenorphine clinic protocols, including telephone-based support, weekly-to-monthly visits, and no ancillary counseling or group requirements, may have kept patients who persisted with regular opioid use in care longer than at a buprenorphine program which quickly refers patients with continued use to more intensive care or methadone maintenance. A moderate-intensity model may have struck some balance of supportive but not overwhelming care that appealed to jail-released patients experienced in traditional community opioid treatment, including methadone maintenance, residential treatment, or intensive outpatient.
In conclusion, primary care buprenorphine-naloxone maintenance appears a feasible model of re-entry opioid treatment, particularly among urban jail-released populations. Primary care treatment models are likely highly adaptable to the dissemination of new, effective opioid and other addiction pharmacotherapies to high-risk, underserved patients, including re-entry populations.
Figure 1.
Treatment Retention: Jail- vs. Community-Referrals to Primary Care Buprenorphine Treatment
*Treatment retention was measured as the period from the baseline visit though the last week of the last active buprenorphine prescription.
Acknowledgements
The authors wish to thank Andrew B. Wallach MD and Valerie Perel MD, Bellevue Hospital Center staff, Jason Hershberger MD and the NYC Department of Health and Mental Hygiene, and Ms. Nadina Santana-Correa for their support of this study.
Role of Funding Source
Funding for this study was provided in part by a grant from the New York City Department of Health and Mental Hygiene and the New York City Health and Hospitals Corporation and R21-DA020583.
Footnotes
Conflict of Interest
The authors declare they have no conflict of interests. Drs. Lee and Gourevitch receive extramural grants from Cephalon and Alkermes Inc. supporting studies of alcohol treatment.
References
- 1.Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: A Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- 2.Fiellin DA, Kleber H, Trumble-Hejduk JG, McLellan AT, Kosten TR. Consensus statement on office-based treatment of opioid dependence using buprenorphine. J Subst Abuse Treat. 2004;27(2):153–159. doi: 10.1016/j.jsat.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Reynaud-Maurupt C, Caer Y, Escaffre N, Gagneau M, Galinier A, Marzo JN, Meroueh F. High-dose buprenorphine substitution during incarceration. Presse Med. 2005;34:487–490. [PubMed] [Google Scholar]
- 4.Garcia CA, Correa GC, Viver AD, et al. Buprenorphine-naloxone treatment for pre-release opioid-dependent inmates in Puerto Rico. J. Addict. Med. 2007;(1):126–132. doi: 10.1097/ADM.0b013e31814b8880. [DOI] [PubMed] [Google Scholar]
- 5.Magura S, Lee JD, Hershberger J, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99(1–3):222–230. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magura S, Rosenblum A, Lewis C, Joseph H. The effectiveness of in-jail Methadone Maintenance. J Drug Issues. 1993;23(1):076–099. [Google Scholar]
- 7.Tomasino V, Swanson AJ, Nolan J, Shuman H. The Key Extended Entry Program KEEP: a methadone treatment program for opiate-dependent inmates. Mt. Sinai J. Med. 2001;68:14–20. [PubMed] [Google Scholar]
- 8.Dolan KA, Shearer J, MacDonald M, Mattick RP, Hall W, Wodak AD. A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system. Drug Alcohol Depend. 2003;72:59–65. doi: 10.1016/s0376-8716(03)00187-x. [DOI] [PubMed] [Google Scholar]
- 9.Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91:220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyman B. The Hospital Secure Unit. In: Puisis M, editor. Clinical Practice in Correctional Medicine. St. Louis, MO: Mosby; 1998. [Google Scholar]
- 11.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, Barry DT, O'Connor PG, Schottenfeld RS. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham C, Giovanniello A, Sacajiu G, et al. Buprenorphine treatment in an urban community health center: what to expect. Fam Med. 2008;40(7):500–506. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. J Gen Intern Med. 2009;24(2):226–232. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison-a high risk of death for former inmates. N. Engl. J. Med. 2007;356:157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addiction. 2003;98:185–190. doi: 10.1046/j.1360-0443.2003.00264.x. [DOI] [PubMed] [Google Scholar]