Abstract
Metabotropic γ-aminobutyric acid receptors (GABABRs) play a critical role in inhibitory synaptic transmission in the hippocampus but the ontogeny of their subunit synthesis and synaptic localisation has not been determined. Here we report the distributions and developmental profiles of GABAB1 and GABAB2 subunits in cultured rat embryonic hippocampal neurons. Limited levels of GABAB1 and GABAB2 immunoreactivity were present at 3 days in vitro (DIV). At 7 DIV, when baclofen-evoked inwardly rectifying K+ channel-mediated responses first appear in the cells, there was a more widespread expression within the soma and proximal dendrites. Levels of the K+ channel GIRK 1 were relatively constant at all time points suggesting channel availability does not limit the appearance of functional GABABRs. At 14 DIV the staining displayed a punctate dendritic distribution and near maximal GABABR-mediated electrophysiological responses were obtained. About half of the puncta for each GABABR subunit in dendrites co-localised with the synaptic marker SV2a suggesting that these subunits are at or very near to synapses. Interestingly, at all ages strong GABABR immunoreactivity was also present in the nuclei of neurons. These results provide an important developmental baseline for future studies aimed at investigating, for example, the trafficking and functional regulation of these receptors.
Keywords: GABA, Hippocampus, Culture, GIRK
1. Introduction
GABABRs mediate the late phase of GABAergic inhibitory transmission in the CNS. They have been relatively well characterised pharmacologically and the cDNAs encoding them have been cloned (for reviews, see Marshall et al., 1999; Couve et al., 2000; Bowery et al., 2002; Calver et al., 2002). The main effects of GABABR activation are the regulation of cAMP via pertussis toxin-sensitive G-proteins leading to a decrease in Ca2+ and an increase in K+ conductances (Misgeld et al., 1995). The reduced Ca2+ conductance is mainly associated with presynaptic P/Q and N-type channels while the effects on K+ conductance have been attributed mainly to G-protein-activated inward-rectifier potassium (GIRK) channels and are post-synaptic (Luscher et al., 1997). The subunit composition of native GIRK channels in hippocampal neurons is not yet known, but the single-channel properties of GIRK1/GIRK2 channels (Velimirovic et al., 1996) resemble those of G-protein-gated channels in neurons (Grigg et al., 1996; Leaney, 2003).
Functional GABABRs comprise heterodimers of GABAB1 and GABAB2 subunits (Kaupmann et al., 1998). GABAB1 contains the ligand-binding domain (Malitschek et al., 1999) whereas GABAB2 couples to the G-protein (Robbins et al., 2001). The two subunits associate, in part, via coiled-coil domains in their cytoplasmic C-termini and this assembly is necessary to overcome GABAB1 retention in the endoplasmic reticulum via an RSRR motif, proximal to the coiled-coil domain of GABAB1 (Margeta-Mitrovic et al., 2000; Pagano et al., 2001).
A number of splice variants of GABAB1 have been identified (reviewed in Bowery et al., 2002). GABAB1a has an N-terminal sequence of 163 amino acids while GABAB1b has an alternative 47 amino acid N-terminus. Both variants are expressed in brain but have different developmental profiles. GABAB1a predominates before birth whereas GABAB1b is more highly expressed in adults (Fritschy et al., 1999). Another isoform, GABAB1e, is a truncated protein that terminates prior to the first transmembrane domain. This protein can heterodimerise with GABAB2 but does not form functional receptors (Schwarz et al., 2000). In addition, some alternative splice variants have been identified in only one species. GABAB1c* occurs only in humans and is highly expressed in fetal brain (Calver et al., 2000; Martin et al., 2001). GABAB1c, GABAB1d, and GABAB1f variants have been cloned from rat brain cDNA libraries but it remains to be established whether any of these other variants can form functional receptors on heterodimerisation with GABAB2. Significantly, GABAB1 knock-out mice show no pre- or post-synaptic GABABR-mediated responses and also display a marked down-regulation of GABAB2 subunit expression suggesting that GABAB1 is required in all functional GABABRs (Pagano et al., 2001; Prosser et al., 2001).
GABABRs have been implicated in the development of some neuronal pathways. For example, GABAB1 and GABAB2 subunits are present in the rat neocortex from embryonic day 14 (E14) suggesting that functional GABABRs are present during prenatal development in vivo (Lopez-Bendito et al., 2002). Furthermore, it has been reported that during corticogenesis in the rat CNS cortical plate cells release GABA which acts as a chemoattractant for GABABR-containing ventricular zone neurons migrating from germinal regions (Behar et al., 2001). GABA and the selective GABABR agonist baclofen both stimulate Xenopus retinal ganglion cell neurite outgrowth in culture and GABABR antagonists applied to the developing optic projection in vivo cause a dose-dependent shortening of the optic nerve (Ferguson and McFarlane, 2002).
GABABRs are well expressed in the mature rat hippocampus (Bischoff et al., 1999) but little is known about any role they may play during hippocampal development or their characteristics in cultured neurons. Cultured hippocampal neurons are a widely used model system for the study of the synthesis, trafficking and function of many neuronal receptors. These cultures are highly amenable to imaging and functional assays and can be transfected with exogenous cDNAs. For example, hippocampal neurons have been used extensively to investigate directed protein transport (Silverman et al., 2001) and neuroreceptor trafficking under basal and stimulated conditions (e.g. AMPARs; Lissin et al., 1999; Noel et al., 1999; Passafaro et al., 2001). As a first step towards fully exploiting this cell culture model to study GABABRs, here we provide immunocytochemical data on developmental and distribution profiles of GABAB1 and GABAB2 subunits and electrophysiological data on the development of functional receptors.
2. Materials and methods
2.1. Cell culture
Hippocampal cultures were prepared as previously described (Hirbec et al., 2003). Briefly, hippocampi from E18 rats were dissected, recovered by enzymatic digestion with trypsin, and dissociated through a Gilson pipette. The cells were then plated onto 22 mm glass coverslips coated with poly-l-lysine in 35 mm petri dishes. Cultures were maintained at 37 °C in a 95% O2, 5%CO2-humidified incubator and used 3°28 days after plating.
2.2. Immunocytochemistry
For total GABAB1a,b, GABAB2 and SV2a immunolocalisation, cells were washed with warm HEPES-buffered saline (HBS, components in mM: NaCl, 119; KCl, 5; CaCl2, 2; MgCl2; 2; HEPES, 25; d-glucose, 30; pH 7.2), fixed in warm 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for 10 min at room temperature, and permeabilised for 5 min in 0.1% Triton X-100. Coverslips were incubated overnight at 4 °C with primary antibodies diluted in 5% BSA (Sigma) in HBS. We tested a range of available antibodies for their suitability. Specifically we found that none of the following antibodies worked well in our system; N-terminal directed GABAB1 antibodies from Eurogentec, Santa Cruz, and Chemicon; N-terminal directed GABAB2 antibodies from Eurogentec and Chemicon and a GABAB2 antibody from BD Biosciences. Successful immunostaining was achieved, however, with C-terminal directed antibodies from Chemicon. For double staining, cells were incubated with guinea pig anti-GABAB1a,b antibody (Chemicon; 10 ng ml−1) or GABAB2 (Chemicon, 30 ng ml−1) and rabbit anti-synaptic vesicle protein 2a (SV2a, Chemicon; 10 μgml−1). Primary antibodies were visualized with appropriate fluorochrome conjugated secondary antibodies (Molecular Probes: Alexa green 488 goat anti-rabbit, Alexa red 568 goat anti-guinea pig and goat anti-mouse, 10 μgml−1) in HBS with 5% BSA. Negative control staining using only the secondary antibodies was including for every experiment.
2.3. Microscopy
Images were captured using a Leica TCS laser scanning confocal system attached to a DM RBE epi-fluorescence microscope (Leica, Heidelberg, Germany). The 488 and 568 nm laser bands of a Kr–Ar laser were used for dual dye excitation and FITC/TRITC filters for fluorescence emission. There was no bleed through between the channels under the conditions used and the same physical parameters were used for all the taken images. Images were processed using Adobe Photoshop 5.0. Quantification of the co-localisation of GABAB receptors and presynaptic markers was done by counting the number of the puncta per 100 μm length of dendrite for each of the antibodies within given fields.
2.4. Western blotting
Primary embryonic rat hippocampal neurons were used at 03, 07, 14, and 21 DIV for preparing protein and Western blot analysis were performed to determine the level of protein GABAB1a,b, GABAB2. Briefly, dishes of cultured neurons were washed twice with warm HBS. They were then scraped off the plate and incubated with lysis buffer for 60 min at 4 °C. After centrifugation, the lysate supernatants were suspended in standard SDS sample buffer and warmed at 37 °C for 5 min. SDS–PAGE was performed on 7.5% or 10% polyacrylamide gels, and proteins were transferred onto polyvinylidene difluoride membrane. Tris-buffered saline with 1% Tween-20 (TBS-T) and 5% nonfat dry milk was used for blocking and for the dilution of primary and secondary antibody. For detection of GABAB1a,b, GABAB2 proteins, we used polyclonal anti-GABAB1a,b or GABAB2 antibody (Chemicon) and for GIRK channels we used anti-GIRK1 (Santa Cruz) and GIRK2 (Santa Cruz and Chemicon). The bound antibodies were detected using rabbit anti-guinea-pig HRP-conjugated IgG (Sigma, 1:10000 dilution) or anti-goat HRP-conjugated IgG (Sigma, 1:10000 dilution) with enhanced chemiluminescence. Quantitation of the blots was performed by measuring the band intensity using the public domain ImageJ program in the NIH image software and the level of protein normalised to the amount of protein of 03 DIV.
2.5. Biotinylation assays
High-density hippocampal neuron cultures (1 × 106 cells/dish) were washed twice with HBS, pH 7.4, incubated for 30 min at 4 °C with 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce) dissolved in HBS containing 2 μM TTX . After incubation, cells were washed twice with HBS, pH 7.4, incubated in 50 mM NH4Cl for 15 min and scraped into lysis buffer (0.5 M NaCl; 1% Triton, 1 mM EDTA in PBS, pH 7.4, supplemented with protease inhibitors), sonicated briefly and centrifuged for 15 min at 13,500 rpm. The supernatants were mixed with 100 μl of streptavidin beads (Sigma) and rotated overnight at 4 °C. The beads were washed three times with lysis buffer and the biotinylated protein was recovered by resuspension in SDS sample buffer for 5 min at 37 °C, subjected to SDS–PAGE and Western blotting. Interleaved controls blots using antibody against the intracellular protein tubulin (Santa Cruz) were performed to verify that only surface proteins were biotinylated.
2.6. Electrophysiology
Recordings were obtained from pyramidal neurons maintained in culture for 4 days to 4 weeks. Petri dishes containing cultured cells were perfused at 1–2 ml min−1 with HBS supplemented with either 2-amino-5-phoshonopentanoic acid (D-APV, 50 μM) or L-689 560 (5 μM) to block NMDA receptors; NBQX (2 μM) to block AMPA and kainate receptors; picrotoxin (100 μM) to block GABAA receptors and tetrodotoxin (TTX, 500 nM) to eliminate action potentials. The extracellular solution was applied via rapid perfusion from a computer controlled sewer pipette allowing complete change of the perfusing solution in less than 20 ms. Current–voltage analysis was performed by application of 10 μM baclofen 4 s at different holding potentials ranging from −110 to 0 mV in 10 mV steps. Cell capacitance and resistance characteristics were recorded prior to each agonist application. Response magnitude at −90 mV was measured in pA and normalised. All statistics are presented as mean ± SEM.
3. Results
During this study, we investigated as many as possible anti-GABABR subunit antibodies for their suitability for immunocytochemistry using cultured neurons. Ideally, we aimed to have a library of antibodies raised in different species that recognized extracellular and intracellular domains of the two GABABR subunits that would allow simultaneous comparison of localisations and relative proportions of the surface expressed and total populations of each of the subunits. Of the antibodies tested, only two guinea pig polyclonal antibodies, both of which recognize intracellular C-terminal epitopes, gave sufficiently consistent and reproducible immunofluorescent staining to be used in our system (comparison data not shown).
In all subsequent experiments fixed, permeabilised hippocampal neurons were double labeled for GABAB1a,b or GABAB2 and for SV2a. At the earliest age measured, 3 DIV (Fig. 1A–F), GABAB1a,b subunits were detected but expression was restricted to the cell soma, including the nucleus, with no labeling in developing neurites. The distribution of GABAB2 was more widespread with limited labeling in the developing processes and a few puncta were observed. At this immature stage prior to widespread synapse formation, a broadly similar pattern of diffuse labeling was observed for SV2a.
Fig. 1.
Distributions at 3 DIV. (A) A representative neuron fixed at 3 DIV stained with anti-GABAB1a,b antibody (10 ng ml−1). (B) The same neuron stained with anti-SV2a antibody. (C) Phase contrast image. (D) a representative neuron fixed at 3 DIV stained with anti-GABAB2 antibody (20 ng ml−1). (E) SV2a labeling. (F) Phase contrast image. Scale bars 10 μm.
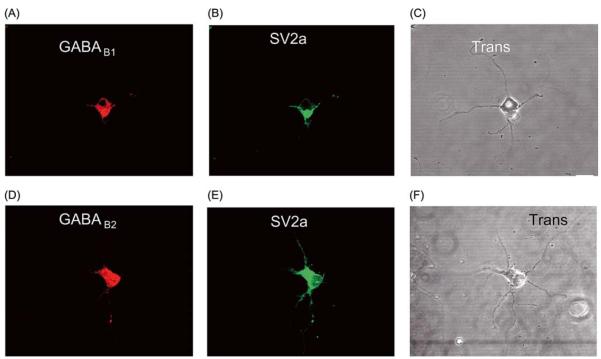
One week after plating, both GABAB1a,b and GABAB2 were still mainly concentrated in the cell body (Fig. 2A,E) with only small amounts of diffuse labeling present in proximal regions of the neurites. SV2a staining was more extensively distributed throughout the processes but did not yet show a clear synaptic-like localisation. These data suggest that under our culture conditions few synapses were formed at this developmental time point in these cells (Fig. 2B,F). Again, it was notable that immunoreactivity for both GABAB1a,b and GABAB2 was present in the nucleus.
Fig. 2.
Distributions at 7 DIV. Distribution of GABAB1a,b and GABAB2 subunits (A, E) and pre-synaptic marker SV2a (B, F) in hippocampal neurons in culture fixed at 7 DIV. Yellow in panels (C) and (G) demonstrates co-localisation. Phase contrast image images are shown in (D) and (H). Scale bar 10 μm.
At 14 DIV, a marked change in the distribution of GABAB1a,b and GABAB2 was observed with strong, punctate staining throughout the neuronal processes (Fig. 3A,D). SV2a staining was also clearly punctate suggesting that by 14 DIV synapses are established. Quantitation of the puncta revealed ~55% co-localisation of GABAB1a,b and GABAB2 with SV2a (Table 1).
Fig. 3.
Distributions at 14 DIV. GABAB1a,b (A), GABAB2 (D) and SV2a (B, E) in hippocampal neurons in culture fixed at 14 days after plating. The yellow in (C) and (F) represents the co-localisation of GABAB1a,b, GABAB2 and SV2a, respectively. Boxed regions are shown at higher magnification. Scale bar 10 μm.
Table 1.
Quantitative analysis of the distribution and synaptic localisation of GABABR subunit puncta in neurons at 14 and 21 DIV. Regions of dendrite were selected at random and the immunoreactive puncta counted. A puncta was defined as a spot of immunofluorescence showing at least twice the background level of fluorescence. Puncta was classed as co-localised if greater than 50% of the pixels were overlapping. The data are the mean and SEM taken from at least three different fields in three separate cell culture preparations
| Puncta per 100 μm | Puncta co-localised with SV2a per 100 μm |
Percentage of GABABR subunit puncta co-localised with SV2a |
Percentage of SV2a puncta co-localised with GABABR subunit |
|
|---|---|---|---|---|
| 14 DIV | ||||
| GABAB1 | 30 ± 1.7 | 19 ± 0.6 | 56 ± 1.1 | – |
| SV2a | 38 ± 3.5 | – | – | 49 ± 5.2 |
| GABAB2 | 29 ± 1.1 | 14 ± 0.6 | 49 ± 4.1 | – |
| SV2a | 38 ± 2.3 | – | – | 50 ± 2.3 |
| 21 DIV | ||||
| GABAB1 | 36 ± 2.9 | 18 ± 0.6 | 67 ± 4.6 | – |
| SV2a | 56 ± 6.3 | – | – | 45 ± 1.1 |
| GABAB2 | 39 ± 3.5 | 25 ± 2.3 | 71 ± 1.7 | – |
| SV2a | 63 ± 2.3 | – | – | 42 ± 1.1 |
At 21 DIV, the staining for GABAB1a,b and GABAB2 remained highly punctate in the dendrites and the number of puncta increased (Fig. 4A,D; Table 1). Staining for SV2a was diminished in the soma and more pronounced in the dendrites. There was approximately the same degree of co-localisation for each of the subunits with synaptic marker (Fig. 4C,F; Table 1) despite an increase of ~50% in the number of synaptic puncta per 100 μm dendrite.
Fig. 4.
Distributions at 21 DIV. GABAB1a,b (A), GABAB2 (D) and SV2a (B, E) in hippocampal neurons in culture fixed at 21 days after plating. The yellow in (C) and (F) represents the co-localisation of GABAB1a,b, GABAB2 and SV2a, respectively. Boxed regions are shown at higher magnification. Scale bar 10 μm.
In parallel experiments, we determined the developmental profile of functional GABABRs. In initial experiments, the nature and specificity of baclofen-evoked K+ channel-mediated responses were determined at 10–20 DIV (Fig. 5B,C). In all cells, a robust baclofen-induced inward current was observed at −90 mV that was blocked by the specific GABABR antagonist CGP 55845. The reversal potential of the baclofen-evoked current was −75 mV, which correlates well with the potassium equilibrium potential (EK = −78 mV) and the responses displayed decreased slope conductance at depolarized membrane potentials characteristic of inwardly rectifying channels (Fig. 5).
Fig. 5.
Activation of current by baclofen applied to cultured rat embryonic hippocampal CA1 neurons (10–20 DIV). (A) Typical inward current elicited at a holding potential of −90 mV by application of 10 μM baclofen, with 5 mM external K+ and 110 mM internal K+. (B) Reversible antagonism of response with CGP 55845 (n = 4), normalised responses. (C) Individual traces of reversible GABAB antagonism. 10 μM baclofen, closed bars. 1 mM CGP 55845, open bars. Cells held at −90 mM. (D) Current–voltage relationship of the baclofen activated current. Current was measured during 4 s baclofen pulse at manually varied membrane potentials. Data points are expressed as the mean ± SEM of the normalised responses (n = 6). Reversal potentials for baclofen-induced current were approximately −78 mV as calculated using the Nernst equation.
Consistent with the immunostaining data, no GABABR reliable responses were detected until about 7 DIV. Baclofen-evoked responses were detected in ~90% of all pyramidal cells tested at 14 DIV but were variable between individual cells. Responses were normalised to cell size by adjusting the current to cell capacitance showing that the response magnitude increases with DIV (Fig. 6).
Fig. 6.
Developmental profile of GABAB receptor induced currents in cultured rat embryonic neurons. (A) Time course of DIV versus response magnitude. Responses were variable and increased in magnitude until after 2 weeks with inward potassium currents reaching approximately 150 pA. (B) Data normalised to the cell capacitance as an indication of cell size.
To exclude the possibility that the appearance of functional postsynaptic GABABRs was limited by the availability of suitable K+ channel proteins, we assessed the developmental expression levels of GIRK proteins by Western blot analysis. We tested antibodies against both GIRK1 and GIRK2, but, despite repeated experiments, we could not detect GIRK2 in our cultures (data not shown). Our results with the anti-GIRK1 antibody show that there is robust expression of this subunit at 3 DIV and that the amount of protein remains relatively constant at all of the time points measured (Fig. 7A). In contrast, the total levels of expression of both GABAB1 and GABAB2 increase during development (Fig. 7B,C). These results suggest that it is the availability of GABABR subunits rather than the effector channels that dictate the developmental profile of functional GABABRs. We also looked at the surface expression of GABABRs using biotinylation assays. In these experiments, the GABAB1 antibody did not give consistent results, probably because it is not sufficiently sensitive in Western blots. The developmental profile of GABAB2 surface expression from the biotinylation assays was consistent with the immunocytochemical localisation and electrophysiological data showing a marked increase between 7 and 14 DIV (Fig. 7D).
Fig. 7.
Immunochemical identification of the developmental profile of GABAB1a,b, GABAB2 receptors and the K+ channel GIRK1. (A) Proteins from rat embryonic hippocampus (30 μg/lane) of 03, 07, 14 and 21 DIV were subjected to SDS–PAGE and Western blotting with GIRK1 antibody. The graph shows results of four separate experiments normalised to the protein level of 03 DIV (mean ± SD). (B) The same experiment was performed twice for GABAB1a,b and (C) three times for GABAB2. (D) Biochemical analysis of the GABAB2 subunit on the cell surface during development by biotinylation assays on hippocampal neuron cultures at 03, 07, 14 and 21 DIV. Three hundred micrograms of protein were used for cell surface labeling and 10% of the total input was loaded as input control.
4. Discussion
Cultured cells are readily manipulated and are ideally suited to immunocytochemistry and confocal imaging techniques, but the development and cellular localisations of GABABRs in these cells have not previously been characterised. In this report, we have analysed the ontogeny of GABAB1 and GABAB2 subunits separately and determined their co-localisation with synaptic markers. It was not possible to compare directly the distributions of GABAB1 and GABAB2 subunits because of the lack of suitably specific high affinity antibodies raised in different species that work well in this system.
The developmental changes in the distributions of GABAB1 and GABAB2 subunits showed a similar pattern. Each of the proteins was restricted to the intracellular regions of the cell soma at early time points up to 7 DIV despite the fact that neurites were formed. Since no extracellular epitope-directed antibodies are available for GABABR subunits, we were unable to determine the degree of surface expression. Nonetheless, qualitative assessment of the images of cells fixed prior to 7 DIV appears to have most, if not all, GABABR immunoreactivity in the neuronal cell body.
Between 7 and 14 DIV, there was a dramatic redistribution of the subunits. GABAB1 and GABAB2 displayed a more widespread, increasingly punctate pattern throughout the processes with ~30 puncta per 100 μm length of dendrite for both subunits. Approximately, half of the puncta for each subunit present in dendrites co-localised with SV2a. It is important to emphasize, however, that this includes both intracellular and surface expressed GABABR subunits.
By 21 DIV, there was a further increase in the number of puncta per 100 μm length of dendrite for both subunits that paralleled the increase in synapses defined by SV2a. It should be noted that light microscopy cannot resolve pre- and postsynaptic GABAB1 and GABAB2 immunoreactivity. At the electron microscope level GABABRs have been shown to be present at both loci, possibly with the splice variant GABAB1a being predominantly postsynaptic and GABAB1b being predominantly presynaptic (Fritschy et al., 1999). Therefore, the co-localisation observed here will include both pre- and postsynaptic GABABRs.
In optical sections through the cell using the confocal microscope, we consistently observed high levels of both GABAB1 and GABAB2 immunoreactivity in the cell nuclei of the embryonic hippocampal cultures. We (Vernon et al., 2001) and others (Nehring et al., 2000; White et al., 2000) have previously reported that GABAB1 binds strongly to the transcription factor ATF4 via coiled-coil domains present in both proteins. Thus, one possible explanation for the presence of GABABRs in the nucleus is that they may co-translocate with ATF4 and play some role in gene regulation.
The imaging results suggested that GABABRs were unlikely to be available for agonist activation in neurons until about 7 DIV. We, therefore, tested for the presence of functional postsynaptic GABABRs on cells at different ages using electrophysiology and bath application of the agonist baclofen. No robust GABABR responses were detected prior to 7 DIV. Between 7 and 10 DIV, there was a rapid development of GABABR-mediated currents and these increased to a maximum at the third week after plating. These functional results are consistent with the confocal imaging data and indicate that full surface targeting of heterodimers does not occur until 2 weeks after plating. Consistent with our data, GABAB1 deficient mice appear normal at birth but approximately 10–14 days after birth the animals start to develop an epilepsy phenotype that resulted in premature death in the C57B1/6J genetic background (Prosser et al., 2001).
It has been reported recently using postnatal cultured hippocampal neurons that robust K+ currents mediated by GIRK channels composed of GIRK1 and GIRK2 subunits are present at 7 DIV (Leaney, 2003). To assess whether the availability of GIRK channels was likely to be a limiting factor to the appearance of the functional GABABRs in our cells, we did GIRK1 and GABABR subunit Western blots of cells maintained in culture for 3, 7, 14 and 21 days. In contrast to GABAB1 and GABAB2 which both showed an increase in expression levels during cell development and maturation, the levels of GIRK1 protein were relatively constant across all of the time points with similar amounts of protein at 3 and 21 DIV. Surface biotinylation assays of GABAB2 showed a marked increase in membrane localisation of this subunit, which is essential for functional GABABRs, concurrent with the increase in total GABAB1 and GABAB2 expression between 7 and 14 DIV. Taken together, these data suggest that the synthesis, heterodimerisation and trafficking of GABABR subunits to the dendritic membrane dictate the developmental appearance of functional GABABRs.
The growth cones of Xenopus retinal ganglion cell axons express GABABRs and grow alongside the GABAergic cells. In culture, the GABABR agonist baclofen stimulates retinal ganglion cell neurite outgrowth and in vivo the GABABR antagonist CGP54626 causes a dose-dependent shortening of the optic projection suggesting that GABABRs are involved in development of the Xenopus optic tract (Ferguson and McFarlane, 2002). In contrast, our data indicate that rat hippocampal neurons in culture do not express functional GABABRs until ~7 DIV and suggest that these receptors are unlikely to play a significant role in early neuronal processes in this cell type.
Using recombinant receptors in heterologous expression systems, previous studies have showed that functional receptors are formed when GABAB1 and GABAB2 are expressed together (Kaupmann et al., 1998). However, it was not clear whether the availability alone of these two subunits was sufficient for the cell surface expression of GABABRs neurons. During development in vitro in our hippocampal cultures, the expression of both subunits increases several fold from 3 DIV onwards. In contrast, the appearance of functional receptors at the cell surface occurs somewhat later at around 7 DIV. These results suggest that in cultured neurons the development and maturation of functional GABABRs require more than just the availability of the individual subunits.
Acknowledgements
We are grateful to the MRC and the Wellcome Trust for financial support. We also thank Graham Collingridge, Zafar Bashir and Lucy Cotton for helpful discussions and electrophysiological advice.
References
- Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb. Cortex. 2001;11:744–753. doi: 10.1093/cercor/11.8.744. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J. Comp. Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Calver AR, Medhurst AD, Robbins MJ, Charles KJ, Evans ML, Harrison DC, Stammers M, Hughes SA, Hervieu G, Couve A, Moss SJ, Middlemiss DN, Pangalos MN. The expression of GABA(B1) and GABA(B2) receptor subunits in the cNS differs from that in peripheral tissues. Neuroscience. 2000;100:155–170. doi: 10.1016/s0306-4522(00)00262-1. [DOI] [PubMed] [Google Scholar]
- Calver AR, Davies CH, Pangalos M. GABA(B) receptors: from monogamy to promiscuity. Neurosignals. 2002;11:299–314. doi: 10.1159/000068257. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol. Cell. Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Ferguson SC, McFarlane S. GABA and development of the Xenopus optic projection. J. Neurobiol. 2002;51:272–284. doi: 10.1002/neu.10061. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Grigg JJ, Kozasa T, Nakajima Y, Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J. Neurophysiol. 1996;75:318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, Collingridge GL, Henley JM. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Leaney JL. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur. J. Neurosci. 2003;18:2110–2118. doi: 10.1046/j.1460-9568.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J. Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairen A, Lujan R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur. J. Neurosci. 2002;15:1766–1778. doi: 10.1046/j.1460-9568.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Malitschek B, Schweizer C, Keir M, Heid J, Froestl W, Mosbacher J, Kuhn R, Henley J, Joly C, Pin J-P, Kaupmann K, Bettler B. The N-terminal domain of GABAB receptors is sufficient to specify agonist and antagonist binding. Mol. Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors—the first 7TM heterodimers. Trends Pharmacol. Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Martin SC, Russek SJ, Farb DH. Human GABA(B)R genomic structure: evidence for splice variants in GABA(B)R1 but not GABA(B)R2. Gene. 2001;278:63–79. doi: 10.1016/s0378-1119(01)00678-3. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolinek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog. Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- Nehring RB, Horikawa HP, El Far O, Kneussel M, Brandstatter JH, Stamm S, Wischmeyer E, Betz H, Karschin A. The metabotropic GABAB receptor directly interacts with the activating transcription factor 4 (ATF-4) J. Biol. Chem. 2000;275:35185–35191. doi: 10.1074/jbc.M002727200. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prezeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol. Cell. Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, Pangalos MN. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, Maki RA. Characterization of gamma-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J. Biol. Chem. 2000;275:32174–32181. doi: 10.1074/jbc.M005333200. [DOI] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Jareb M, Burack MA, Vogt L, Sonderegger P, Banker G. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA. 2001;98:7051–7057. doi: 10.1073/pnas.111146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velimirovic BM, Gordon EA, Lim NF, Navarro B, Clapham DE. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Lett. 1996;379:31–37. doi: 10.1016/0014-5793(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Vernon E, Meyer G, Pickard L, Dev K, Molnar E, Collingridge GL, Henley JM. GABA(B) receptors couple directly to the transcription factor ATF4. Mol. Cell. Neurosci. 2001;17:637–645. doi: 10.1006/mcne.2000.0960. [DOI] [PubMed] [Google Scholar]
- White JH, McIllhinney RA, Wise A, Ciruela F, Chan WY, Emson PC, Billinton A, Marshall FH. The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc. Natl. Acad. Sci. USA. 2000;97:13967–13972. doi: 10.1073/pnas.240452197. [DOI] [PMC free article] [PubMed] [Google Scholar]