Abstract
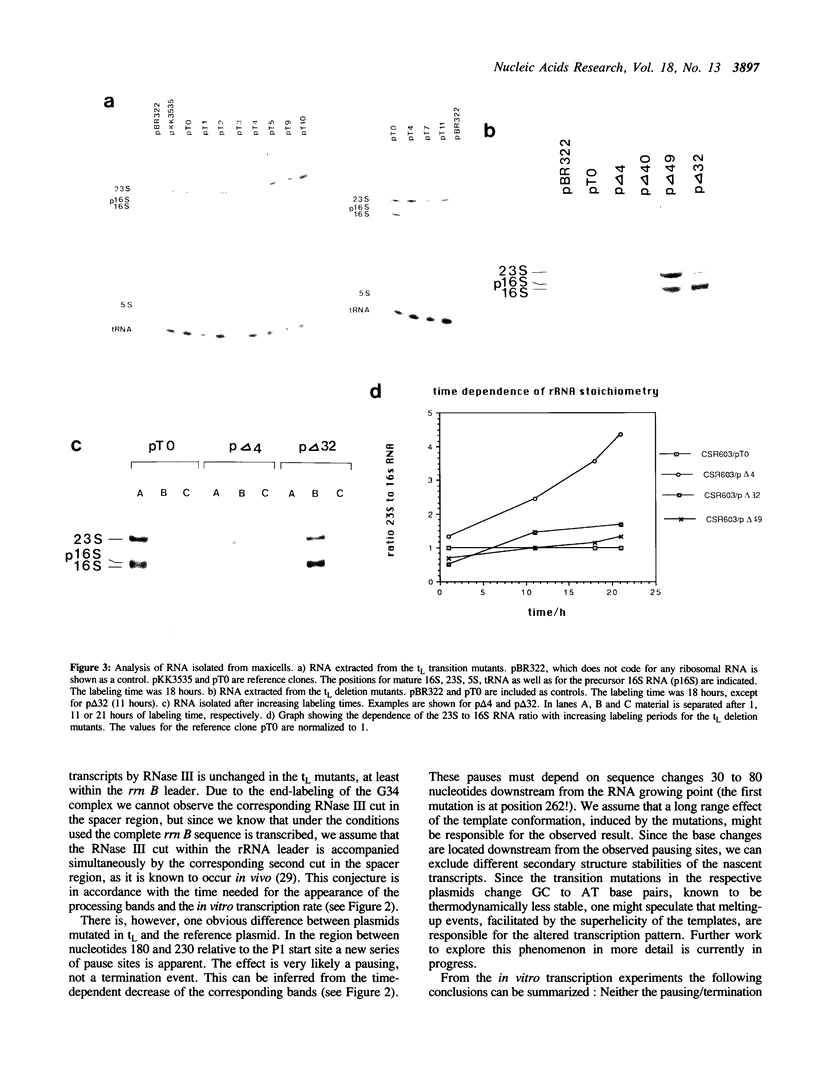
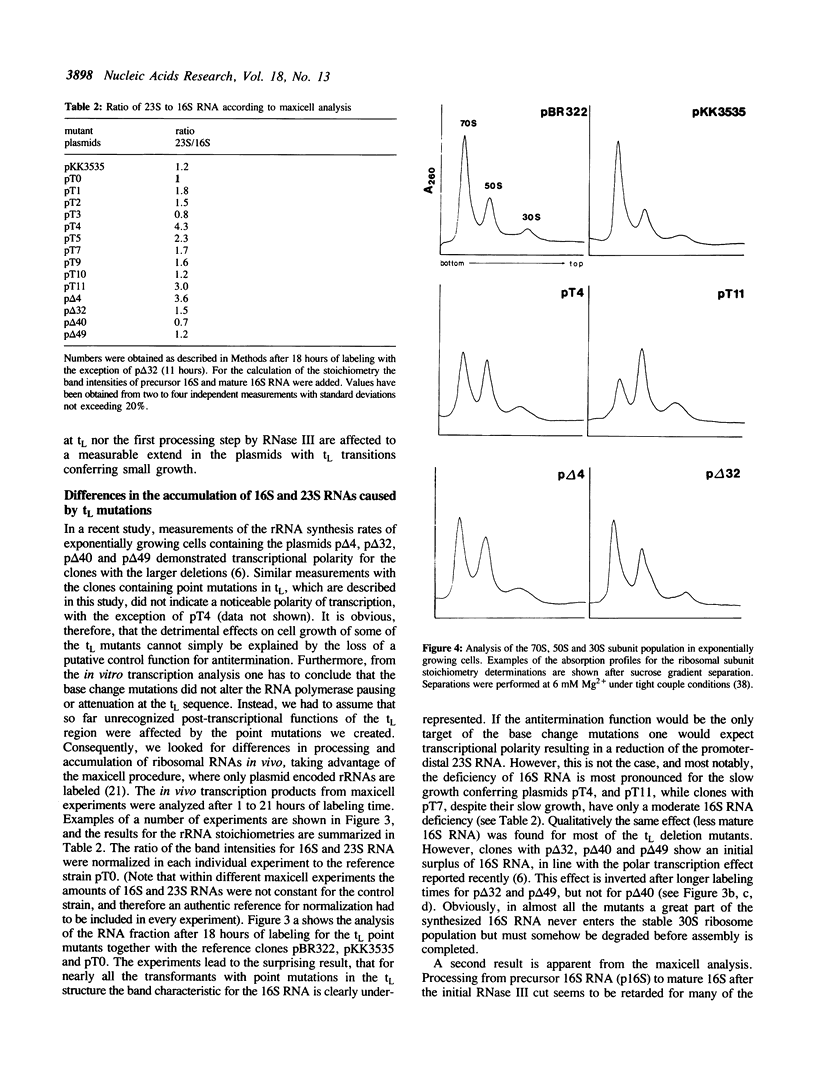
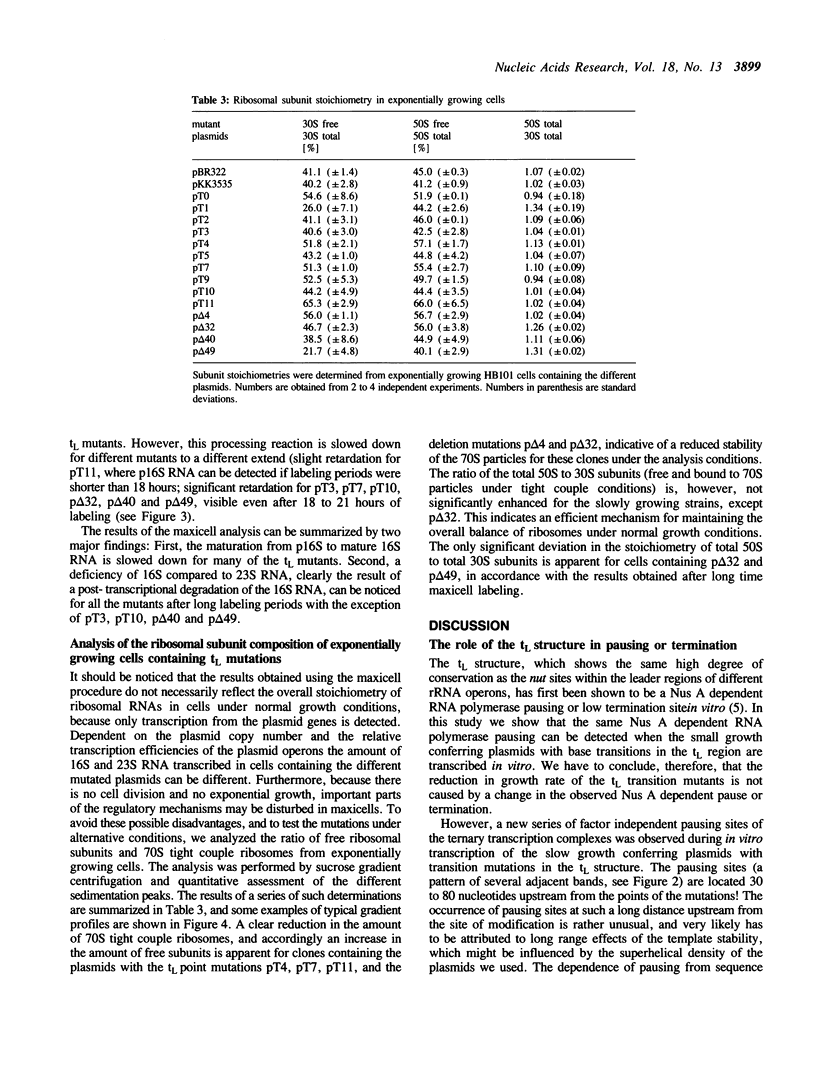
We have investigated a series of mutations within a plasmid encoded E. coli ribosomal RNA leader region. The mutations are localized within a structure known as tL, which has been shown to mediate RNA polymerase pausing in vitro, and which is assumed to have a control function in rRNA transcription antitermination. The effects of the mutated plasmids were analyzed by in vivo and in vitro experiments. Some of the base change mutations led to severely reduced cell growth. As opposed to previous results obtained with mutants where the tL structure has been deleted in part or totally, the tL base change mutations did not result in polar transcription in vivo, rather they revealed a general reduction in the amount of the promoter proximal 16S versus the distal 23S RNA. The deficiency of the 16S RNA, which was most pronounced for some of the slowly growing transformants, can only be explained by a post-transcriptional degradation. In addition, many mutants showed a defective processing after the initial RNase III cut. In line with these results a quantitative analysis of the ratio of ribosomal subunits and 70S tight couple ribosomes showed a reduced capacity to form stable 70S particles for the slowly growing mutants. Together, these findings indicate an important function of the tL structure in post-transcriptional events like processing of rRNA precursors and correct assembly of 30S subunits.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Hatfield G. W. Effects of promoter strengths and growth conditions on copy number of transcription-fusion vectors. J Biol Chem. 1984 Jun 25;259(12):7399–7403. [PubMed] [Google Scholar]
- Aksoy S., Squires C. L., Squires C. Evidence for antitermination in Escherichia coli RRNA transcription. J Bacteriol. 1984 Jul;159(1):260–264. doi: 10.1128/jb.159.1.260-264.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E., Lund E., Tokimatsu H., Rabson A. B., Calvert P. C., Reynolds F., Zahalak M. Processing of the 5' end of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3598–3602. doi: 10.1073/pnas.75.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N., Friesen J. D. Isolation of "relaxed" mutants of Escherichia coli. J Bacteriol. 1968 Feb;95(2):729–731. doi: 10.1128/jb.95.2.729-731.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Hayes F., Vasseur M. Processing of the 17-S Escherichia coli precursor RNA in the 27-S pre-ribosomal particle. Eur J Biochem. 1976 Jan 15;61(2):433–442. doi: 10.1111/j.1432-1033.1976.tb10037.x. [DOI] [PubMed] [Google Scholar]
- Holben W. E., Prasad S. M., Morgan E. A. Antitermination by both the promoter and the leader regions of an Escherichia coli ribosomal RNA operon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5073–5077. doi: 10.1073/pnas.82.15.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Sirdeskmukh R., Schlessinger D. Nucleolytic processing of ribonucleic acid transcripts in procaryotes. Microbiol Rev. 1986 Dec;50(4):428–451. doi: 10.1128/mr.50.4.428-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Levin J. R., Chamberlin M. J. Mapping and characterization of transcriptional pause sites in the early genetic region of bacteriophage T7. J Mol Biol. 1987 Jul 5;196(1):61–84. doi: 10.1016/0022-2836(87)90511-0. [DOI] [PubMed] [Google Scholar]
- Levin J. R., Krummel B., Chamberlin M. J. Isolation and properties of transcribing ternary complexes of Escherichia coli RNA polymerase positioned at a single template base. J Mol Biol. 1987 Jul 5;196(1):85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- Lindahl L. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J Mol Biol. 1975 Feb 15;92(1):15–37. doi: 10.1016/0022-2836(75)90089-3. [DOI] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Genetics and sequence analysis of the pcnB locus, an Escherichia coli gene involved in plasmid copy number control. J Bacteriol. 1989 Mar;171(3):1254–1261. doi: 10.1128/jb.171.3.1254-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Bortner S., Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986 Nov;205(2):285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Spacer transfer RNAs in ribosomal RNA transcripts of E. coli: processing of 30S ribosomal RNA in vitro. Cell. 1977 Jun;11(2):247–262. doi: 10.1016/0092-8674(77)90042-3. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Ruiz A. A., Godson G. N. Promotion, termination, and anti-termination in the rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli K-12. Mol Gen Genet. 1984;195(3):391–401. doi: 10.1007/BF00341439. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Skripkin E. A., Kagramanova V. K. A putative internal promoter in the 16 S/23 S intergenic spacer of the rRNA operon of archaebacteria and eubacteria. FEBS Lett. 1987 Jul 27;219(2):269–273. doi: 10.1016/0014-5793(87)80233-8. [DOI] [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- Noll M., Noll H. Translation of R17 RNA by Escherichia coli ribosomes. Initiator transfer RNA-directed binding of 30 S subunits to the starting codon of the coat protein gene. J Mol Biol. 1974 Nov 5;89(3):477–494. doi: 10.1016/0022-2836(74)90477-x. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Transcriptional block caused by a negative supercoiling induced structural change in an alternating CG sequence. Cell. 1985 Jan;40(1):129–137. doi: 10.1016/0092-8674(85)90316-2. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Botstein D. Directed mutagenesis with sodium bisulfite. Methods Enzymol. 1983;100:457–468. doi: 10.1016/0076-6879(83)00073-7. [DOI] [PubMed] [Google Scholar]
- Siehnel R. J., Morgan E. A. Unbalanced rRNA gene dosage and its effects on rRNA and ribosomal-protein synthesis. J Bacteriol. 1985 Aug;163(2):476–486. doi: 10.1128/jb.163.2.476-486.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Processing pathway of Escherichia coli 16S precursor rRNA. Nucleic Acids Res. 1989 Feb 25;17(4):1649–1663. doi: 10.1093/nar/17.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Dahlberg A. E. Site-directed mutagenesis of ribosomal RNA. Analysis of ribosomal RNA deletion mutants using maxicells. J Mol Biol. 1982 Aug 15;159(3):417–439. doi: 10.1016/0022-2836(82)90292-3. [DOI] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowiak C., Reynolds R. L., Chamberlin M. J., Wagner R. The tRNAGlu2 gene in the rrnB operon of E. coli is a prerequisite for correct RNase III processing in vitro. Nucleic Acids Res. 1988 Aug 25;16(16):7885–7899. doi: 10.1093/nar/16.16.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Burgess R. R. Escherichia coli RNA polymerase binding and initiation of transcription on fragments of lambda rifd 18 DNA containing promoters for lambda genes and for rrnB, tufB, rplC,A, rplJ,L, and rpoB,C genes. Gene. 1979 Aug;6(4):331–365. doi: 10.1016/0378-1119(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zacharias M., Wagner R. Deletions in the tL structure upstream to the rRNA genes in the E. coli rrnB operon cause transcription polarity. Nucleic Acids Res. 1987 Oct 26;15(20):8235–8248. doi: 10.1093/nar/15.20.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias M., Wagner R. Functional characterization of a putative internal promoter sequence between the 16S and the 23S RNA genes within the Escherichia coli rrnB operon. Mol Microbiol. 1989 Mar;3(3):405–410. doi: 10.1111/j.1365-2958.1989.tb00185.x. [DOI] [PubMed] [Google Scholar]





